Acanthosaura phuketensis, Pauwels, Olivier S. G., Sumontha, Montri, Kunya, Kirati, Ul, Awat Ni Tik, Samphanthamit, Phamon, Wood, Perry L. & Grismer, Lee L., 2015
|
publication ID |
https://doi.org/10.11646/zootaxa.4020.3.4 |
|
publication LSID |
lsid:zoobank.org:pub:893ACB72-A8EF-4330-BA59-5D88F46B88D0 |
|
DOI |
https://doi.org/10.5281/zenodo.5619893 |
|
persistent identifier |
https://treatment.plazi.org/id/915C87B5-9513-FF93-DAC0-FDF08DEAF801 |
|
treatment provided by |
Plazi |
|
scientific name |
Acanthosaura phuketensis |
| status |
sp. nov. |
Acanthosaura phuketensis View in CoL sp. nov.
Figures 1–12 View FIGURE 1 a View FIGURE 2 View FIGURE 3 a View FIGURE 4 a
Acanthosaura armata: Boettger 1887: 38 View in CoL .
Acanthosaura cf. crucigera View in CoL : Chan-ard et al. 1999: 90, left. Acanthosaura crucigera: Grossmann & Tillack 2000: 30 –31, 36, 38. Acanthosaura crucigera: Pauwels et al. 2000: 132 –133.
Acanthosaura crucigera: Pauwels et al. 2002: 27 View in CoL .
Acanthosaura crucigera: Manthey 2008: 24 View in CoL : RA00044-4, RA00046-3; 25: RA00047-4. Acanthosaura cf. crucigera: Sumontha et al. 2012: 69 .
Acanthosaura cf. crucigera: Sumontha et al. 2015: 110 View in CoL .
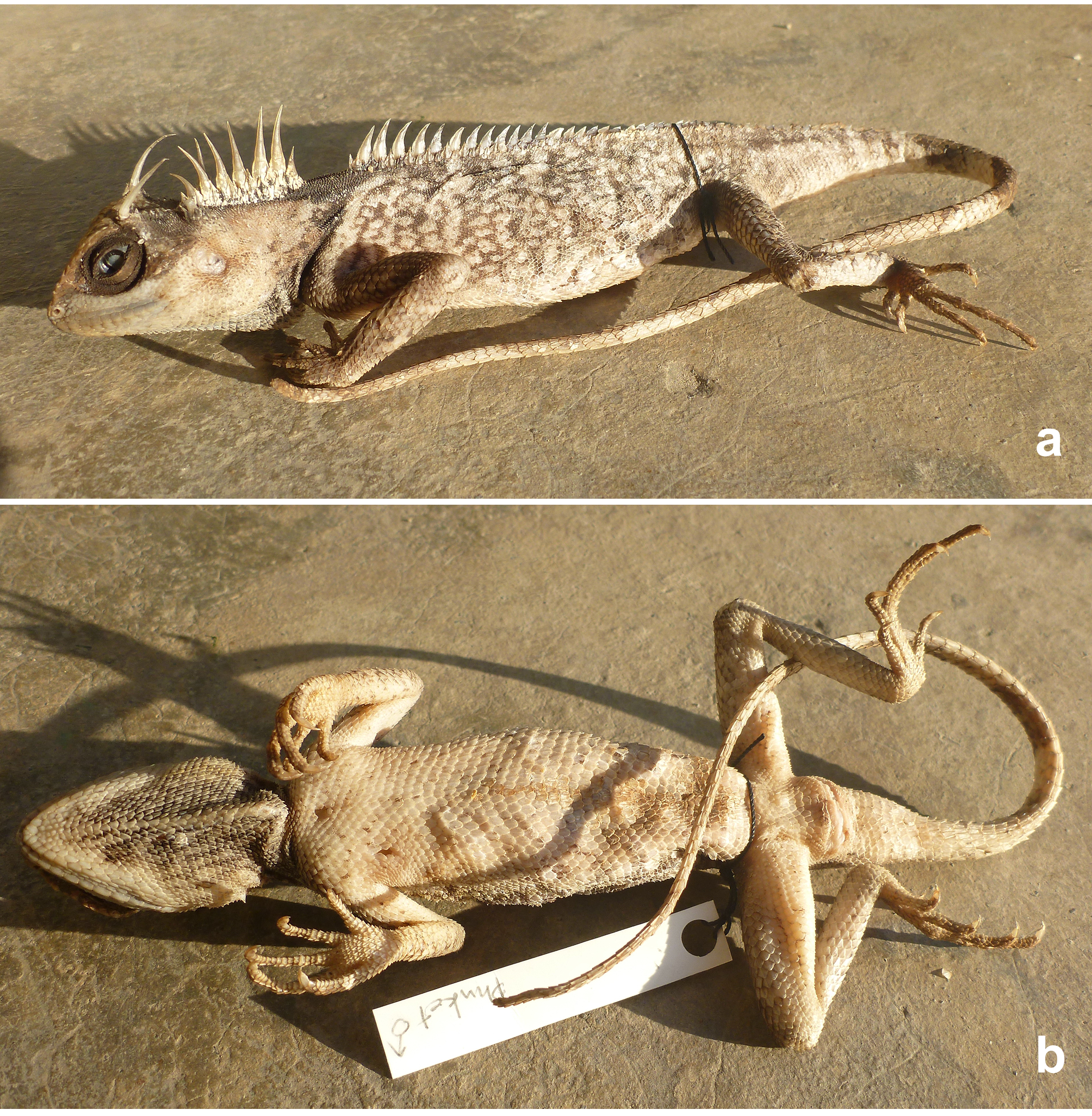
Holotype. Ethanol-preserved whole adult male individual, THNHM 22542 (formerly Montri Sumontha’s field number MS 560) from Ban Bangrong (coordinates UTM UPS ca. 47 p0433130 0 888891 = 0 8o 02.465N 98 o 23.588 E; altitude ca. 41 m asl), Thalang District, Phuket Island, Phuket Province, southwestern Thailand. Collected by Awat Nitikul on 2 June 2008.
Paratypes. Five ethanol-preserved whole individuals, one adult male (hemipenes everted) IRSNB 2681 (formerly IRSNB 15141) from Phang-Nga Wildlife Breeding Station, Muang District, Phang-Nga Province, southwestern Thailand, collected by O. S. G. Pauwels on 26 July 1998, and two adult females PSUZC-RT 2012.5 and THNHM 22543 (formerly MS 559 and MS 225 respectively) and two subadult males, PSUZC-RT 2012.6 and QSMI 1166 (formerly MS 558 and MS 557 respectively) from the same locality, collecting date and collector as for the holotype.
Diagnosis. A medium-sized species (maximum SVL 123.5 mm) with a single long cylindrical spine above posterior margin of eye; large spine on occiput between tympanum and nuchal crest; tympanum naked, roundish, large; moderately developed gular pouch, black in females; scales on flanks randomly intermixed with medium and large scales; nuchal crest present and strongly developed; 12-17 scales in the diastema between the nuchal and vertebral crests; vertebral crest strongly developed, composed of enlarged, pointed scales beginning at shoulder region and continuing till first 6th of tail while regularly decreasing in size; tail 1.4–1.7 times SVL; black collar present; black eye patch present, extending posteriorly to reach the nuchal crest.
Description of the holotype. Adult male. SVL 123.5 mm. TaL 205.6 mm, tail complete. Head length 31.4 mm; head moderately long (HL/SVL 25%), somewhat narrow (HW/SVL 18%), not tall (HD/HL 0.59), triangular in dorsal and lateral profile. Snout short (SL/HL 35%); interorbital and frontal regions and rostrum wide (RW/RH 238%), steepy sloping anteriorly. Canthus rostralis prominent, forming a large projecting shelf extending above eye, composed of 14/14 enlarged scales; shelf terminates with a notch anterior to the postorbital spine. Rostral moderate in size, rectangular, bordered laterally by first supralabials and posteriorly by five smaller scales; nasal concave, surrounded by 1/1 prenasal anteriorly and 3/3 postnasals posteriorly; seven scales between nasal scales; supranasals somewhat elongate; enlarged scales above orbit weakly keeled; no enlarged row of keeled scales below orbit. Eye relatively large (EYE/HL 20%), orbit very large (ORBIT/HL 33%). Prefrontal and frontal scales slightly keeled and larger than scales between orbit and supralabials; scales on occiput weakly keeled; no enlarged parietal eyespot. Extremely elongate epidermal spine above posterior margin of eye, posteriorly recurved, surrounded by 6/ 5 enlarged scales. Suborbitals small, slightly keeled, extending by a row of 6/6 enlarged keeled scales from below posterior margin of eye to anterior margin of tympanum, increasing in size posteriorly. Elongate epidermal spine on lateral margin of nape, anteriorly recurved (the left one broken near its base and healed), surrounded by a rosette of 5/5 much shorter spiny scales, themselves surrounded by a row of pointed scales. Tympanum exposed, oblong, about as wide as tall, surrounded by small conical scales. SL 11/11, rectangular, slightly decreasing in size posteriorly; mental squarish above, becoming triangular below, larger than 1st pair of IL; 2 postmentals of comparable size, widely in contact behind mental; 4 scales contacting the mental; chinshields enlarged, extending posteriorly past angle of jaw, separated from infralabials by one scale row anteriorly and three at angle of jaw; IL 12/12, rectangular, scales in center of series largest; gulars sharply keeled and spinose with 3 enlarged midventral rows. Dewlap very slightly extensible, gular pouch moderate. Nuchal crest composed of 7 very elongate, lanceolate scales bordered on each side by 2–3 rows of enlarged, spinose scales; nuchal crest followed by a diastema of 17 scales at base of nape. Dorsal body crest very developed, extending from posterior margin of diastema onto first sixth of tail; vertebral crest composed of enlarged, epidermal, laterally compressed, spinose scales, bordered by a single row of smaller paravertebral spinose scales; vertebral crest tapers slightly to base of tail, then fades progressively. Body moderately developed, triangular in cross-section. Dorsal scales small, medium-sized and large mixed without regular pattern, with keels projecting posteriorwards; scales of pectoral region and abdomen larger than dorsals, keeled, more or less arranged in transverse rows; keeled scales anterior to vent not enlarged. Limbs relatively long; dorsal and ventral scales of forelimbs keeled, spinose, about the same size. Five digits on manus; subdigital scales keeled, subdigital lamellae under 4th finger 17/16. Scales of hind limbs keeled and spinose; postfemoral scales small, interspersed with larger spinose scales. Five digits on pes; subdigital scales keeled, subdigital lamellae under 4th toe 22/23. Tail length 1.7 times SVL, tail covered with keeled spinose scales, keels on subcaudals directed posteriorly; subcaudals much longer than supracaudals; base of tail 14.3 mm wide.
Variation. Morphometrical and meristic data for the type series are shown in Table 1 View TABLE 1 . All type specimens show 2 postmentals. They also all show a nuchal crest composed of 7 lanceolate scales, although the size of these scales is much larger in the holotype /adult males than in the adult female or subadults. The throat of the two adult females is blackish, while it is beige with black longitudinal reticulations in the adult male holotype.
Distribution. Within Phuket Island, Acanthosaura phuketensis sp. nov. was recorded so far only from the type locality and nearby in Khao Phra Thaeo Non-hunting Area ( Fig. 13–14 View FIGURE 13 View FIGURE 14 ), but it probably occurs in other remaining forested areas of the island. Chan-ard et al. (1999: 90) illustrated two individuals, including an adult male, that they identified as Acanthosaura cf. crucigera , from Khao Lak National Park in Phang-Nga Province. That same adult male and an adult female were illustrated by Grossmann & Tillack (2000: 31) under Acanthosaura crucigera . One of the individuals already illustrated by Chan-ard et al. (1999: 90) plus an additional individual from Khao Lak were illustrated by Manthey (2008: 24). Khao Lak park is situated along the sea on the mainland, at ca. 65 km north of the type locality of Acanthosaura phuketensis sp. nov. Based on the coloration and scalation characteristics visible on these photographs, these individuals are referrable to Acanthosaura phuketensis sp. nov. The phylogeny presented by Kalyabina-Hauf et al. (2004) and Ananjeva et al. (2008) groups three A. crucigera samples from Takua Pa District with a sample (IRSNB 15141, here renumbered IRSNB 2681 and designated paratype of A. phuketensis sp. nov.) from ‘ Thai Muang’ (in fact Muang) District, all from Phang-Nga Province. On the map these authors provided, the Takua Pa locality is erroneously shown as if it was located on Phuket Island. Manthey (2008: 25) also showed the photograph of an adult male from Khao Sok in Surat Thani Province. The mention of A. cf. crucigera from the type locality of Cyrtodactylus ranongensis Sumontha, Pauwels, Panitvong, Kunya & Grismer, 2015 by Sumontha et al. (2015) in Suk Samran District in Ranong Province is referrable to A. phuketensis sp. nov. The known localities of Acanthosaura phuketensis sp. nov., spreading over Phuket, Phang-Nga, Surat Thani and Ranong provinces, are all located on the Phuket Range, a 200 km long continuation of the Tenasserim Range. The range of the new species is surrounded by other populations of the crucigera group with shorter horns, as is well illustrated through the photographs from various populations and localities presented by Manthey (2008).
Natural history. Acanthosaura phuketensis sp. nov. is primarily an arboreal species, as shown on published in situ photographs ( Chan-ard et al., 1999; Grossmann & Tillack, 2000; Manthey 2008) and according to our personal field observations. The Phuket type specimens were collected at night while they were asleep on large trees in mature secondary forest. However this species also regularly descends on the ground. Pauwels et al. (2000) found by day an individual under a stone in the dry bed of a stream, a few meters from another individual found active on the ground, in mature secondary forest. This diurnal species can be locally abundant; many individuals were observed in mature secondary forest at the type locality, in primary forest in Khao Phra Thaeo Non-hunting Area, and in primary and mature secondary forest in Phang-Nga Wildlife Breeding Station. At the type locality it was observed by us (AN, KK, MS, OSGP, PS) in syntopy with 23 species of squamates, namely Cnemaspis phuketensis Das & Leong and C. vandeventeri Grismer, Sumontha, Cota, Grismer, Wood, Pauwels & Kunya, 2010 , Cyrtodactylus brevipalmatus (Smith) , C. oldhami (Theobald) , C. phuketensis Sumontha, Pauwels, Kunya, Nitikul, Samphanthamit & Grismer, 2012 (Gekkonidae) , Bronchocela cristatella (Kuhl) , Calotes emma Gray (Agamidae) , Ahaetulla fasciolata (Fischer) and A. prasina (Boie) , Boiga cyanea (Duméril, Bibron & Duméril) and B. cynodon (Boie) , Dendrelaphis caudolineatus (Gray) , D. cyanochloris (Wall) , D. formosus (Boie) , D. haasi Van Rooijen & Vo g el a n d D. striatus (Cohn) , Ptyas carinata (Günther) and P. f u s c a (Günther) ( Colubridae ), Aplopeltura boa (Boie) , Pareas carinatus (Boie) (Pareatidae) , Python brongersmai Stull (Pythonidae) , Trimeresurus phuketensis Sumontha, Kunya, Pauwels, Nitikul & Punnadee, 2011 , Tropidolaemus wagleri Wagler (Viperidae) , and with four species of anuran amphibians, Ingerana tasanae (Smith) , Limnonectes blythii (Boulenger) (Dicroglossidae) , Hylarana eschatia (Inger, Stuart & Iskandar) (Ranidae) and Nyctixalus pictus (Peters) (Rhacophoridae) . In Suk Samran District in Ranong Province it has been observed in secondary forest at the proximity of Bronchocela cristatella , Calotes emma (Agamidae) , Cyrtodactylus brevipalmatus and C. ranongensis , Gehyra mutilata (Wiegmann) , Gekko gecko (Linnaeus) , Hemidactylus frenatus (Schlegel) , H. garnotii Duméril & Bibron and H. platyurus (Schneider) , Ptychozoon lionotum Annandale (Gekkonidae) , Ahaetulla mycterizans (Linnaeus) and A. prasina , Boiga dendrophila (Boie) and B. drapiezii (Boie) (Colubridae) , Rhabdophis nigrocinctus (Blyth) , Xenochrophis trianguligerus (Boie) (Natricidae) , Pareas carinatus (Pareatidae) and Malayopython reticulatus (Schneider) ( Sumontha et al., 2015) . The species seems to be strictly sylvicolous, and was never found in highly degraded secondary forest nor in plantations.
Figure 9 View FIGURE 9 shows a subadult male Acanthosaura phuketensis sp. nov. eating a large leaf-colored grasshopper (Orthoptera). In captivity, A. phuketensis sp. nov. accepts meal worms, crickets, woodlice and earthworms ( Pauwels et al., 2000 and unpubl. obs.). The abdominal cavity of the paratype IRSNB 2681 contained numerous nematodes.
Etymology. The specific epithet is an adjective in reference to Phuket Island and Phuket Range, on which the type locality lies. We suggest the following common names: Kingkakhaownaam Phuket ( Thai), Phuket Horned Tree Agamid (English), Acanthosaure de Phuket (French), Phuket-Nackenstachler ( German), Phuketstekelnekagame (Dutch).
Comparisons to other species. Table 2 shows a comparison of morphometric and meristic data for all currently recognized species of Acanthosaura and A. phuketensis sp. nov. It is mostly based on the interspecific comparison table provided by Wood et al. (2010, Table 5) and additional data from Hallermann (2000; only data for lectotype A. fruhstorferi (Werner) included), Ananjeva et al. (2011) and Bobrov (2013). However, it is to be noted that Wood et al. (2010) wrongly defined the character ‘ORBIT’ used in their comparison table, as the ‘eye diameter, measured from the posterior to the anterior edge of the eye’, while they actually meant and measured the orbit diameter, not the eye diameter. About the same table presented by Wood et al. (2010), it should also be noted that there was a lapsus calami regarding the ratio TaL/SVL for A. coronata , erroneously indicated as varying from ‘0.06–1.01’, while the corrected variation has been used in our Table 2. A close re-examination of the material regarded by Wood et al. (2010) to represent A. crucigera led to separate populations from western Thailand and at and near its type locality in Myanmar from those from the Kra Isthmus area based on coloration and morphometrical characteristics, as well as on molecular data (Wood et al., in prep.). Only the northern populations are actually referrable to A. crucigera , showing, similarly to the type material, a relatively low nuchal crest, a low dorsal crest, and no contact between the black eye patch and the nuchal crest in both sexes, among other characteristics. The morphological variation for A. crucigera that was presented by Wood et al. (2010) in their interspecific comparison table has consequently been reviewed to include only what we regard here as true A. crucigera (see Appendix: Material examined). Acanthosaura phuketensis sp. nov. can be differentiated from A. armata by its higher maximal length of the postorbital spines (11.8 vs. 9.9 mm), its much higher number of scales in the diastema between the nuchal and the dorsal crests (12–17 vs. 1–8), a lower number of infralabials (10–12 vs. 12–15) and the presence of a black eye patch (vs. absence). It can be distinguished from A. bintangensis by having a larger tympanum (TD/HD 0.22–0.33 vs. 0.16), much longer postorbital spines (PS 4.6–11.8 vs. 1.9–4.2 mm, and PS/HL 0.23–0.38 vs. 0.07–0.19), longer spines in the nuchal crest (NSL/HL 0.21–0.39 vs. 0.17–0.21), longer spines in the dorsal crest (DS 2.3–8.3 vs. 1.8–2.2 mm, and DS/HL 0.11–0.26 vs. 0.08–0.09), more ventral scales (57–67 vs. 51–55), less subdigital lamellae on the fourth finger (15–17 vs. 23), less subdigital lamellae on the fourth toe (21–24 vs. 26–28), much longer occipital spines (OS 2.6–9.5 vs. 1.2–2.6 mm, and OS/HL 0.13–0.30 vs. 0.10–0.11), less scales surrounding the occipital spine (4–5 vs. 6–7), more scales between the fifth canthals (12–13 vs. 10–11), presence of a light knee patch (vs. absence), less developed gular pouch (GP 0–2 vs. 3–4) and absence of an enlarged row of keeled scales below orbit (vs. presence). From A. brachypoda , A. phuketensis sp. nov. can be differentiated by a larger tympanum (TD/HD 0.22–0.33 vs. 0.21), much longer postorbital spines (PS/HL 0.27– 0.28 in the two adult female paratypes of A. phuketensis sp. nov. vs. 0.11 in the adult female holotype of A. brachypoda ), longer spines in the nuchal crest (NSL/HL 0.23 and 0.30 in the two adult female paratypes of A. phuketensis sp. nov. vs. 0.16 in the adult female holotype of A. brachypoda ), much longer spines in the dorsal crest (DS/HL 0.11 and 0.14 in the two adult female paratypes of A. phuketensis sp. nov. vs. 0.06), a distinctly higher number of scales in the diastema between the nuchal and the dorsal crests (12–17 vs. 7), less subdigital lamellae on the fourth finger (15–17 vs. 18), a much longer occipital spine (OS/HL 0.19 and 0.24 in the two adult female paratypes of A. phuketensis sp. nov. vs. 0.03), and less scales between the nasals (NS 7–8 vs. 9). The character DIAS is given both as 4.5 and 1.9 mm in the original description of A. brachypoda , which is based on a single specimen, so this character cannot be used here for comparison. The methodology provided for measuring FOREL and HINDL in the original description of A. brachypoda is insufficiently described to allow verifying that these measurements were taken according to our methodology, so we could not use these characters here; CS, NCS, NR, NSCSL, NSSLC and NSSOS were not provided in the original description of A. brachypoda . A. phuketensis sp. nov. can be distinguished from A. capra (Günther, 1861) based on a generally larger tympanum (TD/HD 0.22–0.33 vs. 0.21–0.23), shorter spines in the nuchal crest (NSL/HL 0.21–0.39 vs. 0.42–0.43), slightly higher maximal length of dorsal crest spines (8.3 vs. 6.8 mm), much higher number of scales in the diastema between nuchal and dorsal crests (12–17 vs. 4–7), presence of occipital spines (vs. absence), lower number of scales between the nasals (7–8 vs. 9), higher number of scales between the fifth canthals (12–13 vs. 9), generally more scales between the seventh supralabial and the sixth canthal (11–14 vs. 9–11) and a notably less developed gular pouch (GP 0–2 vs. 3– 4). From A. cardamomensis , it can be separated based on a much lower maximal length of dorsal crest spines (8.3 vs. 14.2 mm, with a sudden decrease in size before dorsum mid-length in male A. phuketensis sp. nov., posterior to dorsum mid-length in male A. cardamomensis ), a generally higher number of scales in the diastema separating the nuchal and the dorsal crests (12–17 vs 6–15), a lower maximal length of occipital spines (9.5 vs. 13.3 mm, and OS/ HL 0.13–0.30 vs. 0.24–0.56), a generally less-developed gular pouch (GP 0–2 vs. 1–4); another character to separate them is based on coloration: in male A. phuketensis sp. nov. the black eye patch extends posteriorly to reach the nuchal crest, while it never does in A. cardamomensis (see species’ description and photographs in Wood et al. 2010 and all photographs of A. cf. crucigera from Cardamom Mountains [= A. cardamomensis ] in Manthey 2008). Acanthosaura phuketensis sp. nov. is distinguishable from A. coronata (Günther, 1861) based on a larger tympanum (0.22–0.33 vs. 0.14–0.17 mm), presence of postorbital spines, occipital spines, of nuchal and dorsal crests (vs. absence), higher number of subdigital lamellae on the fourth finger (15–17 vs. 13–14), higher number of subdigital lamellae on the fourth toe (21–24 vs. 17–19), longer tail (TaL/SVL 1.4–1.7 vs. 0.6–1.0), higher number of scales between the fifth canthals (12–13 vs. 8–11), higher number of scales from the fifth canthal to the fifth supralabial (8–10 vs. 5–6), lower number of scales between the nasals and the rostrals (1–2 vs. 3–4), generally higher number of scales between the seventh supralabial and the sixth canthal (11–14 vs. 6–11), presence of a black, diamond shaped, nuchal collar (vs. absence) and presence of a black eye patch (vs. absence). From A. crucigera , it is distinguished by a larger tympanum (TD/HD 0.22–0.33 vs. 0.14–0.21), a higher maximal length of postorbital spines (11.8 vs. 7.8 mm), a distinctly higher maximal length of occipital spines (9.5 vs. 4.9 mm), a higher maximal length of nuchal crest spines (12.2 vs. 8.9 mm), a higher maximal length of dorsal crest spines (8.3 vs. 5.5 mm); although several morphometrical characters of both species are overlapping (see Table 2), an easy character to separate them is based on coloration: in male A. phuketensis sp. nov. the black eye patch extends posteriorly to reach the nuchal crest, while it never does in A. crucigera (see species’ original description by Boulenger 1885). From A. lepidogaster (Cuvier, 1829) , A. phuketensis sp. nov. can be separated based on its much longer postorbital spines (4.6–11.8 vs. 1.5–2.5 mm, PS/HL 0.23–0.38 vs. 0.06–0.11), much higher maximal length of nuchal crest spines (4.1–12.2 vs. 2.9–3.4 mm, NSL/HL 0.21–0.39 vs. 0.12–0.15) and of occipital spines (9.5 vs. 3.4 mm), its much higher maximal length of dorsal crest spines (8.3 vs. 2.7 mm, DS/HL 0.11–0.26 vs. 0.07–0.12), its generally lower number of subdigital lamellae on the 4th finger (15–17 vs. 17–19), higher number of scales between the fifth canthals (12–13 vs. 7–10), higher number of scales between the seventh supralabial and the sixth canthal (11–14 vs. 10) and lower number of scales in contact with the mental (4 vs. 5). A. phuketensis sp. nov. can be separated from A. nataliae Orlov, Nguyen & Nguyen, 2006 by its much lower maximal length of postorbital spines (11.8 vs. 17.8 mm), nuchal crest spines (12.2 vs. 23.8 mm, NSL/HL 0.21–0.39 vs. 0.58) and dorsal crest spines (8.3 vs. 17.7 mm, DS/HL 0.11–0.26 vs. 0.44), a longer diastema between nuchal and dorsal crests (3.6–7.6 vs. 2.5 mm, DIAS /SVL 0.05–0.08 vs. 0.04) with more scales (12–17 vs. 10), presence of occipital spines (vs. absence), lower number of scales between the fifth canthals (12–13 vs. 14), lower number of scales between the seventh supralabial and the sixth canthal (11–14 vs. 16), presence of a black, diamond shaped, nuchal collar (vs. absence), presence of light knee patch (vs. absence) and much lesser development of gular pouch (GP 0–2 vs. 4). From A. titiwangsaensis , it can be distinguished by its larger tympanum (TD/HD 0.22–0.33 vs. 0.17–0.20), its much longer postorbital spines (4.6–11.8 vs. 3.3–4.4 mm, PS/HL 0.23–0.38 vs. 0.14–0.18), its much longer occipital spines (2.6–9.5 vs. 1.8–2.3 mm, OS/HL 0.13–0.30 vs. 0.09–0.10), its much higher maximal length of nuchal crest spines (12.2 vs. 4.4 mm, NSL/HL 0.21–0.39 vs. 0.11–0.18), its much longer dorsal crest spines (2.3– 8.3 vs. 1.7–2.1 mm, DS/HL 0.11–0.26 vs. 0.07–0.09), its higher number of ventrals (57–67 vs. 47–57), its lower number of subdigital lamellae on 4th finger (15–17 vs. 20–21), lower number of scales bordering the rostral scale (5–8 vs. 9), lower number of scales bordering the mental (4 vs. 5), presence of light knee patch (vs. absence) and lesser development of gular pouch (0–2 vs. 2–4).
......continued on the next page
TABLE 1. Morphometrical (in mm) and meristic data for the type series of Acanthosaura phuketensis sp. nov. For character abbreviations see material and methods. Paired meristic characters are given left / right. NA = not applicable.
| Holotype THNHM 22542 Adult male | Paratype Paratype IRSNB 2681 PSUZC-RT Adult male 2012.5 Adult female | Paratype THNHM 22543 Adult female | Paratype QSMI 1166 Subadult male | Paratype PSUZC-RT 2012.6 Subadult male | |
|---|---|---|---|---|---|
| SVL TaL | 123.5 205.6 | 96.3 118.4>101.2 189.8 | 100.8 145.0 | 69.2 107.0 | 74.2 113.2 |
| TaL/SVL TBW | 1.7 14.3 | NA 1.6 12.1 14.5 | 1.4 9.2 | 1.5 5.5 | 1.5 5.4 |
| HL HW | 31.4 22.8 | 26.9 29.6 18.0 21.2 | 27.4 19.1 | 19.7 14.4 | 23.0 14.7 |
| HD | 18.6 | 14.8 18.6 | 16.3 | 10.9 | 12.3 |
| SL ORBIT | 11.0 10.5 | 10.2 10.1 9.7 11.2 | 9.3 9.0 | 6.8 6.6 | 8.3 7.3 |
| EYE TD | 6.3 4.7 | 6.5 4.7 3.2 4.3 | 4.0 3.6 | 3.7 3.5 | 3.7 4.0 |
| TD/HD TN | 0.25 0 | 0.22 0.23 0 0 | 0.22 0 | 0.32 0 | 0.33 0 |
| PS PS/HL | 11.8 0.38 | 9.1 8.0 0.34 0.27 | 7.7 0.28 | 4.6 0.23 | 6.5 0.28 |
| NSL NSL/HL | 12.2 0.39 | 9.1 6.8 0.34 0.23 | 8.1 0.30 | 4.1 0.21 | 6.5 0.28 |
| DS DS/HL | 8.3 0.26 | 6.5 3.3 0.24 0.11 | 3.9 0.14 | 2.3 0.12 | 2.5 0.11 |
| WNC DIAS | 2.9 7.6 | 2.4 2.4 4.5 6.5 | 2.0 7.6 | 1.4 3.6 | 1.7 3.8 |
| DIAS/SVL | 0.06 | 0.05 0.05 | 0.08 | 0.05 | 0.05 |
| DIASN FOREL | 17 38.8 | 13 12 33.5 42.9 | 13 33.9 | 15 22.3 | 14 27.5 |
| HINDL SUPRAL | 60.3 11/11 | 50.4 60.6 11/11 12/11 | 49.1 10/11 | 38.2 12/11 | 38.6 10/12 |
| INFRAL VENT | 12/12 66 | 11/11 12/12 57 59 | 10/10 57 | 11/12 67 | 11/11 58 |
| FI TO | 17/16 22/23 | 16/17 16/17 22/23 23/24 | 15/15 21/21 | 17/17 22/23 | 17/17 23/23 |
| OS OS/HL | 9.5 0.30 | 7.3 5.6 0.27 0.19 | 6.7 0.24 | 2.6 0.13 | 5.1 0.22 |
| NSSOS CS | 5/5 14/14 | 4/4 4/5 13/13 11/12 | 4/4 10/11 | 4/5 12/13 | 5/5 12/12 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Acanthosaura phuketensis
| Pauwels, Olivier S. G., Sumontha, Montri, Kunya, Kirati, Ul, Awat Ni Tik, Samphanthamit, Phamon, Wood, Perry L. & Grismer, Lee L. 2015 |
Acanthosaura cf. crucigera : Sumontha et al . 2015 : 110
| Sumontha 2015: 110 |
Acanthosaura crucigera :
| Sumontha 2012: 69 |
| Manthey 2008: 24 |
Acanthosaura crucigera : Pauwels et al . 2002 : 27
| Pauwels 2002: 27 |
Acanthosaura cf. crucigera
| Grossmann 2000: 30 |
| Pauwels 2000: 132 |
| Chan-ard 1999: 90 |
Acanthosaura armata :
| Boettger 1887: 38 |