Rhinopoma microphyllum (Brunnich, 1782)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6421029 |
|
DOI |
https://doi.org/10.5281/zenodo.6612336 |
|
persistent identifier |
https://treatment.plazi.org/id/860EC844-5716-FFEF-FF1D-F484BCF4FB7B |
|
treatment provided by |
Plazi |
|
scientific name |
Rhinopoma microphyllum |
| status |
|
1. View Plate 12: Rhinopomatidae
Greater Mouse-tailed Bat
Rhinopoma microphyllum View in CoL
French: Grand Rhinopome / German: Grose Mausschwanzfledermaus / Spanish: Rhinopoma grande
Other common names: Larger Rat-tailed Bat
Taxonomy. Vespertilio microphyllus Brunnich, 1782 .
Arabia and Egypt. Restricted by K. F. Koopman in 1975 to “ Giza, Egypt.”
Rhinopoma macrophyllumis the type species of the genus. Separate specific status formerly claimedfor the Indian form kinnear: has been disproved. All local forms only show minute differences in morphological characteristics and almost no variation in cytochrome-b. Nevertheless, possible subspecific status of particular allopatric populations diversified mostly in their mean bodysize is in some instances supported by a phylogeographic signal in mtCR. Six subspecies have been recognized in the past: nominotypical microphyllum from north-western Africa and western Middle East; sumatrae named by O. Thomas in 1903 from Sumatra; kinneari by R. C. Wroughton in 1912 from South Asia; tropicalis by D. Kock in 1969 from the African Sahel; harrison: by D. A. Schlitter and A. F. DeBlase in 1974 from eastern Middle East and south-western Afghanistan; and asiriensis by I. A. Nader and Kock in 1982 from western and southern Arabian Peninsula; their taxonomic validity and distributional status arestill a matter ofdiscussion, and a taxonomic revision is needed. The recent report from Lombok is obviously based on misidentification (of the Chaerephon plicatus). Here considered as monotypic.
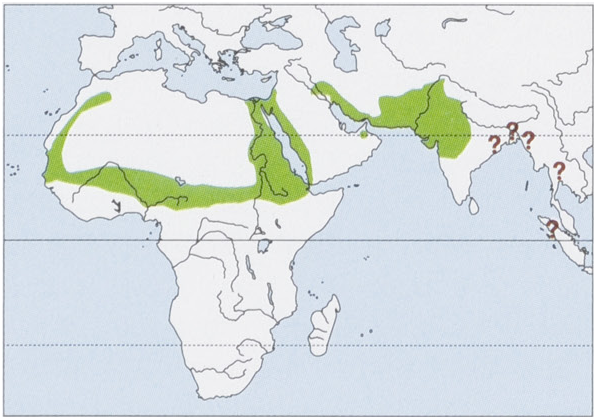
Distribution. Confirmed from NW Africa, the Sahel, and Nile Valley, E through the Middle East and WArabia to NW& C India (E to E Madhya Pradesh, 80° E); with possible distribution spots in E & SE India (indicated byfour mostlyhistorical records). Reportedly common in Bangladesh (yet disproved by recent reports) and retained on species lists of Myanmar, Thailand, and Sumatra without being supported by anyrecent record. View Figure
Descriptive notes. Head-body 78-81 mm, tail 48-85 mm, ear 19-22 mm, hindfoot 13-17 mm, forearm 57-74 mm; weight 14-40 g. The Greater Mouse-tailed Batis largest species of Rhinopoma , with tail generally shorter than forearm. Nasal muzzle padis broadly trapezoidal, dorsal margin is variable, and noseleafis indistinct. Pelage is fine, silky, and paler on ventral side, sometimes whitish; hairs are unicolored. Pelage varies from dull gray to tawny or reddish brown; all three colors reportedly appear simultaneously in the Indian colonies. Compared with other species of Rhinopoma , tail of the Greater Mouse-tailed Bat is distinctly shorter and sometimes thicker in appearance (92% of forearm on average vs. 116-137% in other species); hindlegs are similar, with tibia lengths 19-8-30 mm (37% of forearm on average vs. 45-46% in other species). Skull is large (greatest lengths of skull 18-21-8 mm and length of upper tooth row [C'-M’] 6-6-8 mm) and robust, clearly larger than in other species of Rhinopoma . Sagittal crest is very high (up to 4 mm in frontal region) and continuous over entire dorsum of braincase, connecting massive protuberant supraoccipital crest that extends laterally to somewhat weaker lambdoid crests. Nasal swellings are relatively small and low compared with other species of Rhinopoma , anteriorly not exceeding level of mesial margin of canine alveolus. Rostrum and interorbital constriction appear narrower in dorsal view. Dorsum of rostrum is flat but surrounded by distinct pentagonal crest distally tapered into sharp ridges extending to anterior edge of sagittal crest and forming triangular groove in between. V-shaped palatal incision terminates behind level of distal M’ margins. C' is particularly large, with distal elongation ofits cutting edge. Talon of upper fourth premolar (P*) is relatively small, rounded with low cingular wall, and without palato-mesial cusp. Major axis of P* has pronounced distal and mesial extensions, the latter expanding in distinct separate cusp obvious in lateral view. M' and M* are robust with broad protocones, and talons are rounded butrelatively small with low cingular wall showing no hypoconal undulation. A considerably reduced M? lacks even rudimental metacrista. Lower incisors are massive and bilobated; C Is particularly robust, nearly square on section, without labial cingulum but particularly pronounced cingular ridge uprising along lingual wall of tooth up to small mesial cusp; and P, is distinctly tricuspid, very large, larger than P,, and situated transversally from lingual to labial margins of the tooth row. P, is broad with short but broad distal basin and lingually turned mesial cutting edge forming thus distinct lingual groove at mesial one-third of crown—characteristic also shared with the Muscat Mouse-tailed Bat ( R. muscatellum ) and the Hadramaut Mouse-tailed Bat ( R. hadramauticum ). Lower molars are robust, but M,is distinctly more reduced that in other species of Rhinopoma ; M; trigonid is considerably smaller than M, or M, trigonids; entoconid crest of M, is low, unlike other species; M, talonid length does not exceeds trigonid length. Baculum as in other species of Rhinopoma is small (c. 1 mm) and shaped like elongated conus. Chromosomal complement has 2n = 42 and FN = 66, identical in Jordan and India, with eight metacentric pairs of chromosomes, five submetacentric, seven acrocentric, and medium-sized metacentric X-chromosome.
Habitat. Arid regions with sparse vegetation, typically with precipitation below 300 mm, including rocky desert countries and semi-desert habitats in southern parts of the distribution, from sea level in Egypt up to elevations of 1200 m in Morocco, Afghanistan, and Gujarat ( India). The Greater Mouse-tailed Bat roosts in rocky crevices, small caves, mines, underground tunnels, wells, old monuments and buildings, and spacious caves (large colonies). It tolerates low humidity and daylight illumination.
Food and Feeding. Occasional diet samples formerly reported from Mauritania, Rajasthan, Israel, and Iran contained beetles ( Scarabaeidae , Tenebrionidae , and Curculionidae ) as prevailing diet components (up to 80%), supplemented with Hymenoptera , Formicidae, Isoptera , Lepidoptera , and Orthoptera , including nymphal stages of desert locust (Schistocerca gregaria). Considerable seasonal variation was demonstrated in the Rajasthan Desert: in post-monsoon (October-November) and winter (December—February), individuals particularly ate beetles (40% and 44%, respectively), and in summer (March—June) and particularly monsoon period (July-September), diets had a pronounced drop in diversity (with disappearance of Dictyoptera and Neuroptera and decreased representation of Coleoptera , Lepidoptera , and Hymenoptera , common in other periods) but a dominant representation of winged termites (Odontotermes obesus, Microtermes obessi, and Anacanthotermes sp.). Detailed investigations in Israel demonstrated a lack of foraging activity in winter (November—March), and an opportunistic selection of diverse prey available in May after arriving at summer roosts, characterized by predominance of beetles ( Tenebrionidae , Carabeidae, Scarabaeidae , and Curculionidae ) and Lepidoptera (30%), which later disappeared from diets. In June and early July, beetles continued to dominate diets, accompanied by increasing contribution of smaller winged ants and Heteroptera. Large winged ants ( Camponotus fellah and C. sanctus) increased rapidly in diets at the beginning ofJuly, and by the end July to September when leaving summer roosts, they made up more than 90% of diets. Female preference for large winged ants was obvious from beginning of June until the end ofJuly when mostly smaller winged ants were preferred. The rapid increase in frequencies of large-winged ants in diets of males during late July and August was closely correlated with rapid accumulation of fat reserves and increase in body weight (25-39 g on average).
Breeding. Female Greater Mouse-tailed Bats give birth to a single young per year, and mating occurs in March-April prior to sexual segregation in summer. A detailed study on breeding biology on Greater Mouse-tailed Bats in Jodhpur, India showed that initiation of spermatogenesis and increase of testes size of adult males were coincident with their migration to the winter roost in late September and early October. Interstitial cell secretion is evident in testes during December, and spermatozoa are shed from February onward. Testes regress in size, and spermatogenesis is arrested in warmer weather from the end of April until mid-October. Onset of estrus is sudden and is coincident with migration from winter roosts. Births occur in July, and gestation is estimated at c.123 days. Postpartum females pass into a period of long anestrus until the next breeding season. Young are weaned at 4-6 weeks old. Female reproductive tract is bilaterally functional, and pregnancy can occur on either side of the uterus. The single Graafian follicle destined to rupture in March is already well developed in winter. After ovulation, an extroverted corpus luteum is formed, which does not persist until pregnancy ends. Fertilized ovum divides in fallopian tube and until it reaches uterus where it implants mesometrially; it is still a segmenting morula. Birth occurs by head presentation, and the entire process takes ¢.20-25 minutes. Placentophagia normally does not occur. Immature appearance of the reproductive system ofjuvenile females during breeding season suggests that they do not reach sexual maturity until their second year of life. Timing of reproduction is roughly the same in Rajasthan; data from Punjab suggest about one-week delay in timing of particular stages similarly as those from Israel, where mating takes place in April and births appear mostly at beginning ofJuly.
Activity patterns. Activity of the Greater Mouse-tailed Bat is restricted in winter at least in the northern part of the distribution. In Israel, it spent five winter months (November-March) in torpor in large winter colonies, preferably occupying heated geothermal caves with rather stable temperatures of ¢.20°C and almost 100% relative humidity. In winter roosts, individuals are in torpor continuously without periodical arousal, do not leave roosts to forage, and live entirely on fat reserves. Their skin temperatures remain c.1-3°C above ambient cave temperatures; outside temperatures are 4-22°C. Average torpid metabolic rate was 0-14 ml O,/g/h, and breathing cycle was prolonged, up to 16 minutes. Greater Mouse-tailed Bats differ in these parameters from Arabian Mouse-tailed Bats ( R. cystops ) in the same winter colony and are more sensitive to lower temperatures—a switch to normothermia appears with a temperature drop to 16°C. A shallow hypothermia during daytime resting also can be attained in summer roosts. Studies in Israel reported considerable differences between males and females relative to daytime resting and nightly activity patterns: lactating females were normothermic and had longer foraging bouts than non-lactating females and males, which perform shallow daytime torpor (body temperatures of 29-32°C) and short foraging bouts at night. Evening emergence of summer colonies is accompanied by increased activity about one hour prior to departure. It starts c.10 minutes after sunset at light intensities of 1-10 Ix, and in large colonies,it proceeds with separated groups of tens to hundreds of individuals. A complex study of nightly activity in Israel using on-board recording of diverse activity variables demonstrated foraging in compact groups, bouts of flying activity up to five hours in females and up to 90 km spentflying during a nightly foraging bout. As in other congeners, multtharmonic QCF with maximum energy at second harmonic, long pulses (9-15 milliseconds) of constant frequency at searching flight, with shortening and increasing bandwidth when approaching an obstacle. Individualspecific frequencies (second harmonics) are 27-31 kHz. Field records show relatively little variation among particular populations, with mean end frequency of 25-7 kHz (range 25-2-26-7 kHz) and mean peak frequency of 28 kHz (27-29 kHz) in Jordan, mean end frequency of 25-3 kHz (25-25-5 kHz) and mean peak frequency of 27-1 kHz (26-8-27-7 kHz) in Iran, and end frequencies of23-1-25-2 kHz and peak frequencies of 28-2-31-2 kHz in Algeria. Detailed analyses from Israel (peak frequency 28 kHz) determined detection ranges for small to large prey (3-18 mm) to be 2-6-5 m at 20 dB and 5-5-14 m at 0 dB and found no influence of light intensity on echolocation variables. On-board recording of echolocation behavior of foraging individuals in Israel showed that spectral shifts recorded in groups of foraging Greater Mouse-tailed Bats did not show patterns predicted for spectral jamming avoidance (individually-specific reduction of frequency bandwidth) but instead an increased bandwidth, shortened duration, and increased repetition rate, simply features characterizing approach of an obstacle.
Movements, Home range and Social organization. The Greater Mouse-tailed Bat has considerable seasonal differences in roost occupancy, patterns of sexual segregation, and way of life. In most of its distribution, winter is survived in torpor, often in mass winter colonies. Regular seasonal movements occur. An exceptional case of a banded individual that moved 900 km was reported in south-western India. Homing experiments with 108 Greater Mouse-tailed Bats in Rajasthan showed early returns in 73% of them released from 14 km away from their home roosts, 50% from 20 km, and 9% from 45 km. Compared with other species of Rhinopoma , the Greater Mouse-tailed Bat tends to form large colonies (up to tens of thousands), assembled in compact clusters with close contact among individuals. This is particularly true for winter colonies in which other local species of Rhinopoma are often admixed. In contrast to other species of Rhinopoma , the Greater Mouse-tailed Bat also forms large, sexually segregated colonies in summer. In Israel, largest summer colonies of males are estimated at 3000-6000 individuals, and the largest female colonies at 5000-10,000 individuals. A colony of 20,000 individuals was reported in a cave in the Mesopotamian Plain of Iran. Most records generally report smaller colonies of tens to a few hundred individuals. In northern Israel with enormous densities of Greater Mouse-tailed Bats, summer sexual segregation 1s accompanied by distant separation of male and female roosts and their feeding grounds: males occur at higher elevations. In late August, non-reproductive males leave their roosts and are occasionally observed in southern female roosts in September—October. Females and juveniles disappear from breeding colony roosts in early September, and roosts are completely abandoned by late October. In India, Greater Mouse-tailed Bats regularly reuse spring and autumn roosts, but some reports suggest that some populations use the same roost in summer and winter.
Status and Conservation. Classified as Least Concern on The IUCN Red List. The Greater Mouse-tailed Bat is widespread and common with no major conservation threats. Yet in some regions (particularly Sahel and West Africa) the species is known only from a few single records and is apparently quite rare.
Bibliography. ACR (2017), Adam & Hubert (1972), Advani (1981a, 1982a), Akmali et al. (2011), Amr (2000), Anand Kumar (1965), Anderson (1902), Aulagnier (2013g), Banerjee & Karim (1986), Bates & Harrison (1997), Benda & Gaisler (2015), Benda & Vallo (2017), Benda, Abi Said et al. (2016), Benda, Andreas et al. (2006), Benda, Dietz et al. (2008), Benda, Faizolahi et al. (2012), Benda, Lucan et al. (2010), Benda, Ruedi & Aulagnier (2004), Bhatnagar (1994), Boonman et al. (2013), Brosset (1962a), Cvikel, Berg et al. (2015), Cvikel, Levin et al. (2015), DeBlase (1980), DeBlase et al. (1973), Dieuleveut et al. (2010), Dobson (1878), Dookia & Singh (2017), Ellerman & Morrison-Scott (1951), Fajri et al. (2018), Felten (1962), Gaisler (1970), Gaisler et al. (1972), Gaur et al. (1980), Ghalib et al. (2007), Gopalakrishna (1986), Gopalakrishna & Badwaik (1987), Handa & Kaur (1979), Harrison (1963a), Harrison & Bates (1991), Hill (1977), Hulva, Horaéek & Benda (2007), Judas et al. (2018), Kangoyé etal. (2015), Khajuria (1988), Kock (1969d), Kock et al. (2001), Koopman (1975, 1994), Lall (1986), Largen et al. (1974), Lay (1967), Levin, Ar et al. (2012), Levin, Plotnik et al. (2015), Levin, Roll et al. (2013), Levin, Yom-Tov & Barnea (2009), Levin, Yom-Tov, Barnea & Huchon (2008), Levin, Yom-Tov, Hefetz & Kronfeld-Schor (2013), Loumassine et al. (2017), Madkour (1978), Molur et al. (2002), Nader & Kock (1982), Pearch et al. (2001), Poulet (1970), Purohit & Senacha (2004), Qumsiyeh (1985), Qumsiyeh & Baker (1985), Qumsiyeh & Jones (1986), Rahman, Perveen, Rauf, Salim, Ali & Khattak (2015), Rahman, Perveen, Rauf, Salim, Khan et al. (2015), Roberts (1977 1997), Sandhu (1988), Schlitter & DeBlase (1974), Schlitter & Qumsiyeh (1996), Schmidt & Joermann (1983), Shahabi et al. (2017), Sharifi & Hemmati (2002), Sharifi et al. (2014), Shayer (2015b), Simmons (2005), Singwi & Lall (1983), Sinha (1976, 1980, 1981a, 1981b), Thomas (1903b, 1913a), Van Cakenberghe & De Vree (1994), Wason (1978), Wassif & Madkour (1963), Whitaker & Yom-Tov (2002), Wroughton (1912, 1918b).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Rhinopoma microphyllum
| Don E. Wilson & Russell A. Mittermeier 2019 |
Vespertilio microphyllus
| Brunnich 1782 |