Leptogorgia cofrini, Breedy, Odalisca & Guzman, Hector M., 2005
|
publication ID |
https://doi.org/ 10.5281/zenodo.170963 |
|
publication LSID |
lsid:zoobank.org:pub:635890AD-D871-435C-8562-5F654043253A |
|
DOI |
https://doi.org/10.5281/zenodo.6265019 |
|
persistent identifier |
https://treatment.plazi.org/id/6C1D1726-FFA1-8767-FEF5-FD29FBFB9004 |
|
treatment provided by |
Plazi |
|
scientific name |
Leptogorgia cofrini |
| status |
sp. nov. |
Leptogorgia cofrini View in CoL , sp. nov.
( Figs. 1–6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 )
Material examined. Holotype: UCR 398A, preserved, Islas Tortugas, Gulf of Nicoya, Costa Rica, 1.5 m, J. Cortés, 18 July 1985.
Paratypes: MCZ 62065, 2 specimens, preserved, Isla Tolinga, Gulf of Nicoya, Costa Rica, 2 m, O. Breedy, 21 August 2000; UCR 398B, as the holotype; UCR 1048, dry, Isla Canal Afuera, Gulf of Chiriquí, Panama, 3–5 m, H.M. Guzman, 10 December 2001; UCR 1319, dry, Islote, Gulf of Chiriquí, down to 11 m, H.M. Guzman, 20 April 2002; UCR 1401, 2 specimens, dry, Islote Frijol, Gulf of Chiriquí, 1–15 m, H.M. Guzman, 24 April 2002; UCR 1446, dry, Isla Otoque, Gulf of Panama, Panama, 1–5 m, H. M. Guzman, 9 May 2002; UCR 1519, 3 specimens, UCR 1532, preserved, Cabo Matapalito, Península de Osa, Costa Rica, 10 m, O. Breedy, 12 March 2004; UCR 1521, 2 specimens, preserved, Isla Jicarita SW, Gulf of Chiriqui, 15–20 m, H.M. Guzman, 19 April 2002; UCR 1522, preserved, Isla Barca, Gulf of Chiriqui, 3–9 m, H.M. Guzman, 18 April 2002; UCR 1526, preserved, eastern Islas Negritos, Gulf of Nicoya, 11 m, O. Breedy, 21 November 2002; UCR 1529, 9 specimens, preserved, western Islas Negritos, 11 m, O. Breedy, 21 November 2002; UCR 1531, 3 specimens, preserved, Archipiélago Murciélago, Costa Rica, 3–18 m, O. Breedy, 2 December 2003; UCR 1533, preserved, Bahía Salinas, Costa Rica, 10 m, O. Breedy, 9 July 2002; UCR 1569, 5 specimens, preserved, Roca Prosper, Gulf of Chiriquí, 3–15 m, H.M. Guzman, 11 December 2002; UCR 1570, 3 specimens, preserved, Cabeza de Mono, Bahía Culebra, Costa Rica, 9 m, E. Ruiz, 24 May 1997; UCR 1571, preserved, Cabeza de Mono, 10 m, O. Breedy, 27 June 1997; UCR 1572, 3 specimens, preserved, Archipiélago Murciélago, 15 m, O. Breedy, 16 October 1999; UCR 1573, 2 specimens, preserved, Isla del Caño, Costa Rica, 20 m, O. Breedy, 13 September 1996.
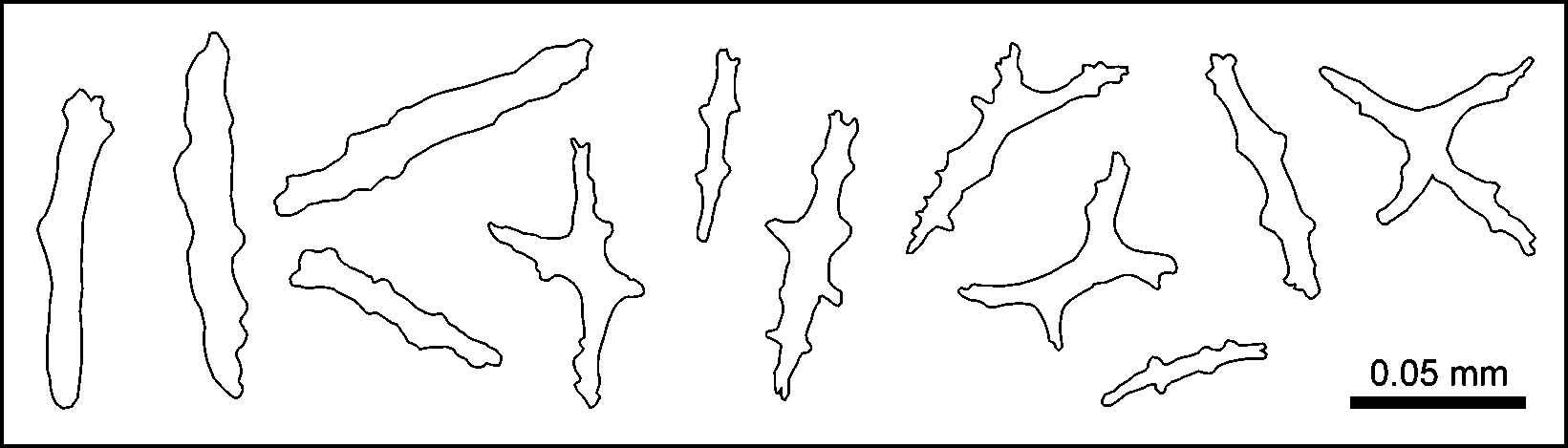
Diagnosis. Dwarf, white colonies, up to 7 cm in length, and 5 cm in width. Axis cylindrical. Growth form upright, branching abundant, and bushy, with a single stem reaching up to 3 mm in height before branching, or multiple stems (up to 4). Anastomosis absent. Polyps sparsely placed all around branches, fully retractile. Sclerites colourless, and mostly capstans up to 0.09 mm in length, spindles few and up to 0.12 mm in length, and long anthocodial rods up to 0.14 mm in length.
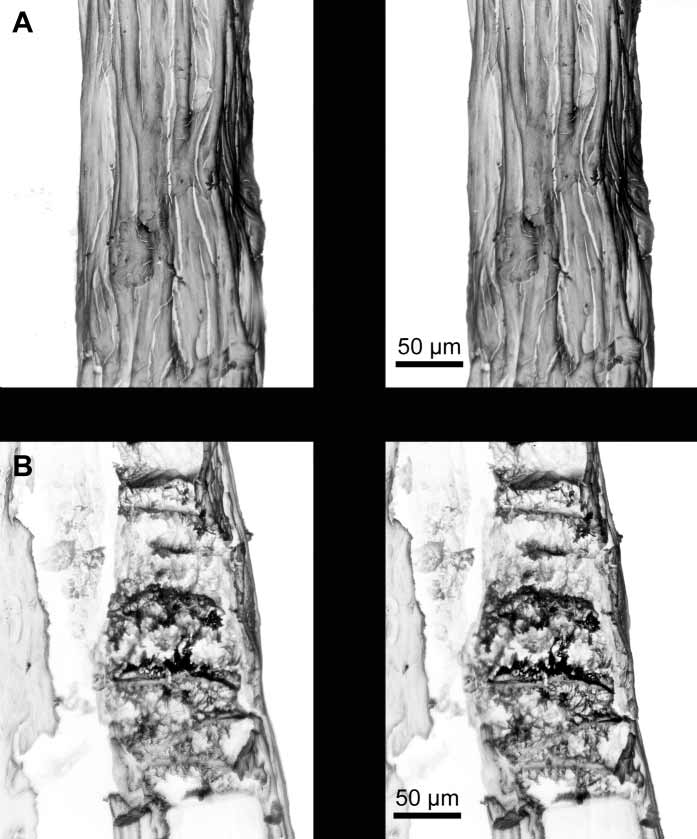
Description. The holotype is a small, bushy, white colony 3.4 cm in height and 3.0 cm in width, arising from a laminar holdfast covered by coenenchyme but devoid of polyps ( Fig. 1 View FIGURE 1 B). When it was alive, the holdfast spread over a rocky substrate, and other colonies were growing in close proximity ( Fig. 1 View FIGURE 1 A). There are three main stems arising from a small holdfast producing profuse irregular branching in many directions. The main stems are 1.5–2.0 mm in diameter, and the terminal twigs about 1.0 mm. Terminal twigs are pointed, up to 15 mm in length, and curved at the ends. Polyps are colourless, and are sparsely distributed on all sides, fully retractile into the coenenchyme, which is almost flat around the apertures ( Fig. 1 View FIGURE 1 B–C). Sclerites of the coenenchyme are colourless ( Fig. 1 View FIGURE 1 D). The few longer ones are tuberculate spindles, some slightly curved, up to 0.12 mm in length and 0.04 mm in width, with warts in girdles. The shorter ones are blunt tuberculate capstans, 0.09 mm in length, and 0.04 mm in width, with two whorls of complex tubercles and terminal clusters ( Figs. 1 View FIGURE 1 D, 2A). A small number of crosses are also present, up to 0.07 by 0.07 mm in size ( Fig. 2 View FIGURE 2 A, bottom left). The anthocodiae mostly contain long, narrow, somewhat flattened rods, up to 0.14 mm in length, and 0.01 mm in width, with some lobelike marginal projections, and also, smaller rods with branching projections ( Figs. 2 View FIGURE 2 B, 3). The anthocodial rods are arranged vertically below the polyp tentacles. The combination of long anthocodial rods, abundant large capstans, and a low occurrence of spindles are distinct characteristics of the new species ( Fig. 1 View FIGURE 1 D).
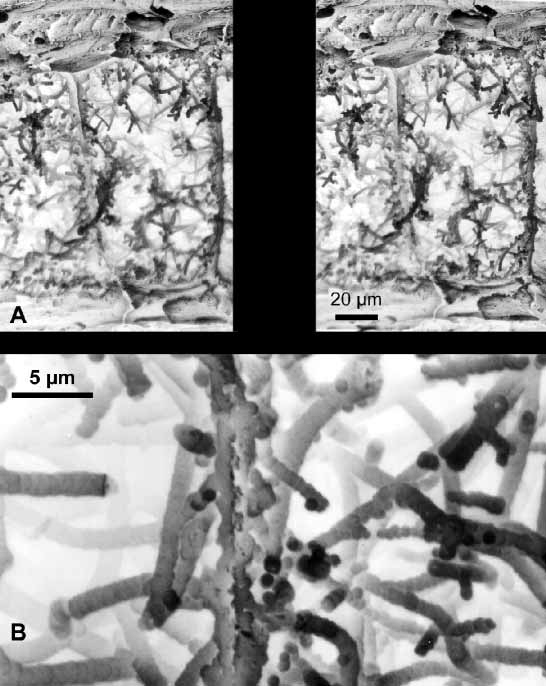
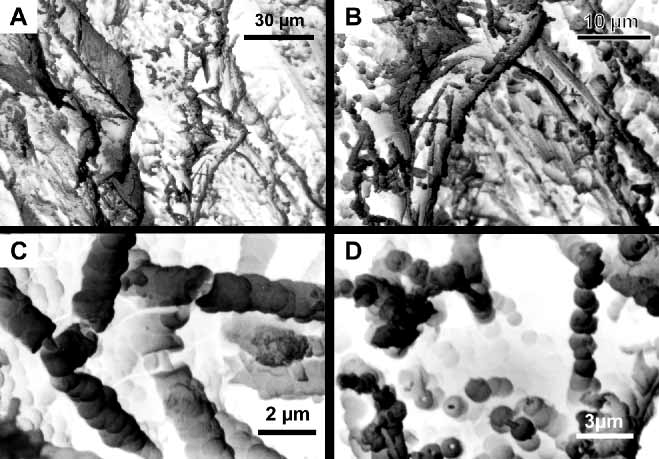
Axis and holdfast. The axis of the terminal branches is pale yellow, with a clearly visible narrow white chambered central core, becoming darker amber in the larger branches and main stems. Layers of mineralized gorgonin, the axial cortex, surround the central core. After maceration in sodium hypochlorite, the axis shows longitudinal strands of CHAp, leaving dark grooves where gorgonin was removed ( Fig. 4 View FIGURE 4 A). This arrangement of mineralized strands has been observed in other species of Leptogorgia ( Lewis et al. 1992, Bayer 2000, Bayer & Macintyre 2001). The chambers of the axial core of L. cofrini sp. nov. are filled with organic filaments mineralized with CHAp ( Figs. 4 View FIGURE 4 B, 5A–B). The filaments are coated with microspheres of CHAp that fuse to produce branching extensions that partially anastomose. Microspheres that are isolated, or have different degrees of fusion are also found ( Fig. 5 View FIGURE 5 ). In this new species the meshwork of filaments is not dense, anastomosis is open, and mineralization consists of mostly large microspheres (up to 0.90 µm).
The holdfast consists of thin layers of gorgonin with mineralized loculi ( Fig. 6 View FIGURE 6 A). Loculi are filled with organic filaments ( Fig. 6 View FIGURE 6 B–C) that are also mineralized. Longitudinal fractures of the surface expose the filaments coated with microspheres of CHAp fused to form columnlike arrangements ( Fig. 6 View FIGURE 6 C–D). After partial removal of the organic matter by maceration in sodium hypochlorite, some microspheres show a hollow core where the organic filaments were dissolved ( Fig. 6 View FIGURE 6 D), thus, a concentric deposition process around the filaments has occurred.
Etymology. This species is named in honor of Dr. David A. Cofrin, a physician, philanthropist and visionary scienceenthusiast who has contributed to the advancement of research in biology. Dr. Cofrin's interest in the rise of the Isthmus of Panama and its influence over the last 12 million years on the evolution of life’s diversity in the Americas is encouraging the development of extensive research on marine biology and paleobiology.
Habitat. The new species was found inhabiting shallow waters, from 1 m to 25 m in depth on rocky communities exposed to strong waves and currents. It is very common between 10 and 15 m where it appears in patches together with other octocorals species, but being the dominant species.
Distribution. Various localities along the Pacific coast of Costa Rica and Panama, under contrasting oceanographic and hydrological conditions (e.g., upwelling and nonupwelling regimes).
Remarks. Leptogorgia cofrini sp. nov. is allied to a group of Leptogorgia that could be called the Leptogorgia alba Duchassaing & Michelotti, 1864 group. They all are white with various branching patterns and different abundances of sclerite types. Excluding Leptogorgia styx Bayer, 2000 , that was properly described and characterised, the rest of this group needs revision and redescription. However, Leptogorgia cofrini sp. nov. presents a characteristic small size, branching pattern, and sclerites that clearly differentiate it. The arrangement of CHAp in layers along the axis of L. cofrini sp. nov. matches L. styx ( Bayer 2000) , but the CHAp mineralization of the filaments in the core chambers showes some similarity to that found in Leptogorgia cardinalis ( Bayer, 1961) by Bayer & Macintyre (2001), having a looser mesh of filaments, and larger microspheres.
| MCZ |
Museum of Comparative Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Octocorallia |
|
Order |
|
|
Family |
|
|
Genus |