Charadrahyla sakbah, Jiménez-Arcos & Calzada-Arciniega & Alfaro-Juantorena & Vázquez-Reyes & Blair & Parra-Olea, 2019
|
publication ID |
https://doi.org/10.11646/zootaxa.4554.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:81247C5A-18DD-4B13-B40E-EE0E0AB0D15C |
|
DOI |
https://doi.org/10.5281/zenodo.5933977 |
|
persistent identifier |
https://treatment.plazi.org/id/650987BF-272C-FFFF-CBEA-FA29D5E5FD37 |
|
treatment provided by |
Plazi |
|
scientific name |
Charadrahyla sakbah |
| status |
sp. nov. |
Charadrahyla sakbah View in CoL sp. nov.
Jiménez-Arcos, Calzada-Arciniega, Alfaro-Juantorena, Blair & Parra-Olea
Mixteca Cloud-forest Treefrog, Rana arborícola de la Mixteca
Holotype. ( Fig. 2A View FIGURE 2 in life; Fig. 4A, B View FIGURE 4 in preservative). IBH 30989. Adult male. Río Chite ku'e (Río de las Mil Cascadas), San Isidro Paz y Progreso, Santa Maria Yucuhiti, Oaxaca, Mexico. 1390 mts. 17.074902° N, 97.834758° W, 26 January 2018. Collected by Víctor H. Jiménez Arcos and Liz A. Alfaro Juantorena. GoogleMaps
Paratypes. IBH 30990 ( Fig. 2B View FIGURE 2 ) , IBH 30991. Adult males. IBH 30992, IBH 30993, IBH 30994. Adult females ( Fig 2 C, D, E View FIGURE 2 ). All bearing identical locality data as the holotype. 26 January 2018. Collected by Víctor H. Jiménez-Arcos and Rafael Alejandro Calzada Arciniega .
Diagnosis. A large member of the genus Charadrahyla with females showing larger SVL (mean 84.04 mm; range 81.15–85.75 mm) than males ( 70.07 mm; 67.91–73.21 mm). Charadrahyla sakbah differs for all other species of the genus by the presence of the following character combination: (1) axillary membrane present in male and female adults ( Fig. 3A, B View FIGURE 3 ), (2) hypertrophied webbings between toes I and II in adult males ( Fig. 3C, D View FIGURE 3 ), (3) one conical tubercle on either side of the vent in females and males ( Fig. 2F View FIGURE 2 ), (4) nuptial excrescences in adult males ( Fig. 3E View FIGURE 3 ), (5) vocal slits absent, and (6) females with snout dorsal profile rounded and males acuminate.
Comparison with other species. Character states of Charadrahyla sakbah in parentheses. Specimens of C. taeniopus ( Günther 1901) , C. chaneque ( Duellman 1961) , C. nephila ( Mendelson & Campbell 1999) and C. esperancensis ( Canseco-Márquez, Ramírez-González & González-Bernal 2017) lack axillary membrane (present), lack conical tubercles in both sides of the vent (present) and have normal webbings between toes I and II in adult males (hypertrophied). Moreover, these species are distributed in the Atlantic versant. Charadrahyla taeniopus has been recorded in localities in the states of Hidalgo, Veracruz, and Puebla ( Duellman 2001). Charadrahyla chaneque is restricted to the east of the Isthmus of Tehuantepec in Oaxaca and Chiapas ( Duellman 2001). Charadrahyla nephila is recorded in the Sierra de Juarez and Sierra Mixe ( Mendelson & Campbell 1999), whereas C. esperancensis is restricted to Sierra de Juarez ( Canseco-Márquez et al. 2017), both mountain systems located at northern Oaxaca.
The only adult female recorded in the literature of C. pinorum has a SVL of 34.6 mm and adult males range from 28.5 to 33.1 mm (81.15–85.75 and 67.91–73.21 mm females and males respectively), webbings between toes I and II normal (hypertrophied), vocal slits present (absent), male snout shape in dorsal profile rounded (acuminate), and tympanum not evident (visible). The adult females of C. juanitae have a SVL range from 37.6 to 39.8 and adult males from 27.7 to 35.8 mm (81.15–85.75 and 67.91–73.21 mm females and males respectively), webbings between toes I and II normal (hypertrophied), nuptial excrescences underdeveloped (present in finger I and isolated patches in finger II), vocal slits present (absent), male snout shape in dorsal profile rounded (acuminate), and tympanum not evident (visible). Specimens of C. altipotens present normal webbings between toes I and II (hypertrophied), absent nuptial excrescences (present), yellow belly coloration in males (white to pale brown), and adult male dorsal coloration without blotches (darker blotches). Specimens of C. trux lack axillary membrane (present), conical tubercles on either side of vent absent (two conical tubercles present), vocal slits present (absent), and quadratojugals not articulating with the maxillaries (articulated) in five of nine specimens (see Adler & Dennis 1972). Charadrahyla tecuani adult males have SVL range from 52.5 to 57.8 mm ( 67.91–73.21 mm adult male), axillary membrane absent (present), vocal slits present (absent), and yellow belly coloration in males (white to pale brown). Table 1 summarizes the comparison of the main diagnostic characters among Charadrahyla species.
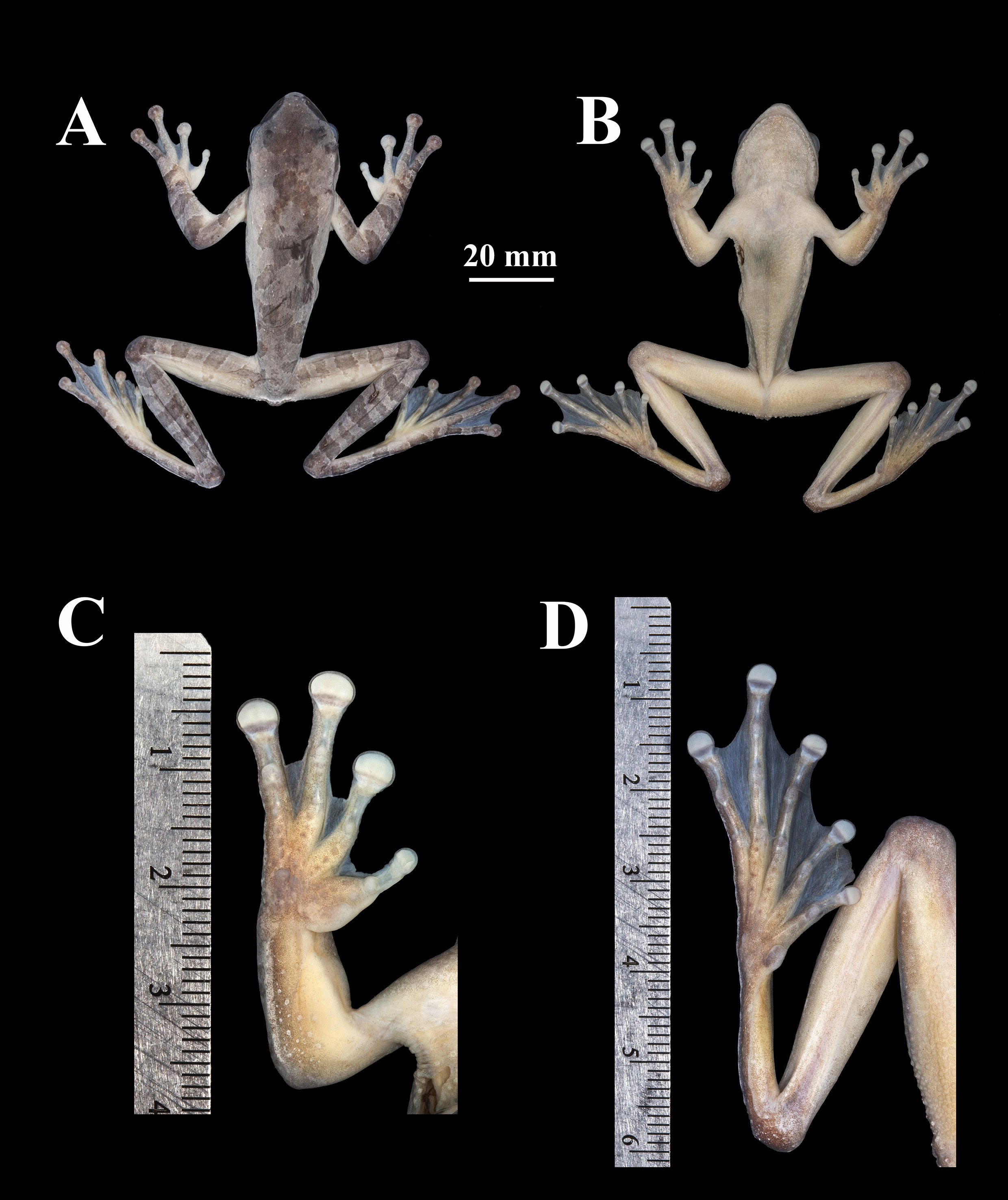
Description of holotype. Head wider than the body; body shape slender towards the cloaca ( Fig. 4A, B View FIGURE 4 ), SVL 73.21 mm, tibia length 39.98, foot length 33.61, head length 22.35, head width 21.66, diameter of tympanum 2.85, diameter of eye 6.85, interorbital distance 7.22, eye–tympanum distance 3.31. head slightly longer than wide; lateral profile rounded with snout sloping downward posterior to the nostril; snout shape in dorsal profile acuminate; canthus rostralis distinct and angular; loreal region concave; slightly protuberant nostril oval, which are directed posterolaterally; internarial region concave; vocal slits absent. Top of the head flat; interorbital region 33.33% of head width; eye diameter 31.63% of head width. Supratympanic fold distinct, thick, extending posteroventrally from posterior margin of orbit until anterior insertion of forearm; tympanum distinct, round; tympanic annulus distinguishable, obscured by supratympanic fold dorsally; width of tympanum 44.82% diameter of eye; width of tympanum 92.75% eye–tympanum distance.
Axillary membranes present extending slightly beyond the first third of the forearm (more visible in life); thoracic fold absent; dermal fold on wrist present. Fingers long and slender, with slight lateral fringes, bearing large rounded terminal discs, less developed on the Finger I; relative finger lengths: I <II <IV <III; discs on Fingers II, III, IV of similar size ( Fig. 4B View FIGURE 4 ), slightly wider than the tympanum; disc on Finger I smaller, width 62.54% width of tympanum. Subarticular tubercles large, diameter about two-third width of terminal disc on same finger, rounded, none bifid; supernumerary tubercules smaller than subarticular tubercules, rounded and distinguishable.
Hand webbing formula: I 2-2 ½ II 1 ¼-2 III 2-1 IV ( Fig. 4C View FIGURE 4 ). Tarsal fold absent; tibia length 54.61% SVL; foot length 45.91% SVL. Inner metatarsal tubercle distinct, large, ovoid, 2.1 times larger than subarticular tubercules; outer metatarsal small but distinct; subarticular tubercules distinct, elongated, elevated, diameter about one-half width of terminal disc on the same toe; supernumerary tubercles small, circular, arranged in row along axis of proximal portions of phalanges. Nuptial excrescences present, dark-colored with cone-shaped papillae (see Luna et al. 2018) ( Fig. 3E View FIGURE 3 ) Toes long and slender, bearing rounded: I <II <V <III <IV; terminal discs smaller than discs on fingers. Foot webbing formula: I ¼-½ II 1-2 III 1-2 ½ IV 2-1 V ( Fig. 4D View FIGURE 4 ). Webbings between toes I and II hypertrophied; in life the edge of the web rolled up dorsally forming a flap ( Fig. 3C View FIGURE 3 ), which can be extended ( Fig. 3D View FIGURE 3 ); was extended for preservation; larger and with irregular edges than the other webbings ( Fig. 4D View FIGURE 4 ). Cloacal opening directed posteroventrally at mid-level of thighs; conical tubercle on either side of vent, partially covered by distinctive vent sheath. Skin on dorsal surfaces smooth; skin on ventral surfaces distinctly granular; skin on flanks between forelimbs and hind limbs smooth.
Tongue cordiform, and slightly free in the back and less anteriorly. Vocal slits absent. Vomerine odontoids 6–5 on each side, left-right respectively, situated on transverse dentigerous process at anterior level of choanae, separated medially by a length slightly smaller than odontoid process; choanae subtriangular, widely separated. The quadratojugals ossified and in contact with the maxillaries (determined by radiographs; Adler & Dennis 1972)
Color. In life, dorsum of body and head, including lateral surfaces of head, are dark yellowish with golden tones ( Fig. 2A View FIGURE 2 ). A brown stripe begins almost near the nostrils, continues to the middle of the eye and surrounding it below, continuing through supratympanic fold. Dorsum with irregular dark brown blotch, extending up to twothirds of the back; the last third with brown spots. Dorsal surfaces of forearm dark yellowish, with distinct brown transverse bars, four in forearms; thighs yellow through the internal area towards the body up to the middle zone; the middle zone of the thighs, towards the tibia, greenish with transverse bars dark brown; discs on fingers III and IV darker that fingers. Flanks of the body yellow with three brown spots on each side. Venter of throat pale cream. Chest pale cream with smooth skin. Belly white in the middle; granular skin towards the area of the hindlimbs which are pale pink to yellowish; palpebral membrane clear on three-quarters and gray in the last quarter.
In preservative, dorsum of body and head, including lateral surfaces of head, are pale gray. A dark gray mask in the lateral surfaces of head that begins almost at the tip of the snout and continues until anterior insertion of forearm. Dorsum with irregular dark gray blotch, extending up to two-thirds of the back; the last third of the back with smaller spots. Dorsal surfaces of limbs pale gray with distinct dark gray transverse bars, four on forearm, three and five (left and right) on thigh, and six on tibia (both sides); discs of fingers III and IV darker than fingers, fingers and disc I and II whitish; flanks whitish with three irregular dark gray spots in each side ( Fig. 4A, B View FIGURE 4 ). Venter of throat whitish. Chest white with smooth skin. Belly light brown, granular. Undersurface of thighs white, granular area light brown; palpebral membrane clear on first three-quarters and gray in the last quarter.
1 Duellman (2001) describes slightly larger pigmented tubercles present immediately around the cloacal opening.
2 According to Adler & Dennis (1972), four of nine individuals is articulated in one or both sides with the maxillaries.
Variation. All the males show a lateral profile rounded with snout sloping downward posterior to the nostril and snout dorsal profile acuminate; in contrast females are bluntly rounded, in both lateral and dorsal profiles. The axillary membrane is more noticeable in life ( Fig. 3A, B View FIGURE 3 ) and in the females, possibly due to their larger body size. The three males ( holotype and two paratypes) show hypertrophied webbing. The nuptial excrescences are almost indistinguishable in the males, perhaps by the preservation process, but brown to black in life ( Fig. 3E View FIGURE 3 ). In the three males of the type series the nuptial excrescences correspond to dark-colored nuptial pads with cone-shaped papillae. The testes of two males ( paratypes: IBH 30990-91) were ovoid, white and not visibly granular (mean 8.48 mm; range 8.12–8.62 mm; 12.49; 12.32–12.66% of SVL). The females and males show a conical tubercle on either side of vent like the holotype ( Fig. 2F View FIGURE 2 ). In four specimens examined (IBH 30989, IBH 30991, IBH 30992 and IBH 30993) the quadratojugals are present, ossified and in contact with the maxilla. The variation in the proportions for females and males are given in Table 2.
Considerable color variations are present mainly in females. One female paratype (IBH 30992) shows a dorsal ground color yellow to greenish, with large irregular dark green blotches on the dorsum ( Fig. 2C View FIGURE 2 ). A second female paratype (IBH 30993) shows a dark cream to brownish dorsal ground color. Irregular blotches are also present, with greenish-brown tonalities ( Fig. 2D View FIGURE 2 ). The last female in the type series (IBH 30994) exhibits a dorsal ground color brownish-orange, and blotches with a chocolate color ( Fig. 2E View FIGURE 2 ). The three females show transverse bars in the limbs of a similar color to the dorsal blotches of each ( Fig. 2 C, D and E View FIGURE 2 ). A similar pattern was observed in one female not collected—the dorsal ground color was green with blotches and transverse bars a darker green ( Fig. 5A View FIGURE 5 ). All the females observed show lateral pattern of enlarged spots dark brown to black, like the female shown in Fig. 5A View FIGURE 5 . The throats in all female paratypes show irregular blotches dark brown to black. For males, the blotches are less conspicuous. One male (IBH 30990) shows a similar dorsal ground color in life as the holotype, but differs in that coloration is gray, blotches and transverse bars in limbs are darker gray, tibias and lower part of the back green with more intense yellow in the flanks ( Fig. 2B View FIGURE 2 ). The other paratype male (IBH 30991; not showed) presented a coloration almost identical to holotype. The three males show throats white in life and cream to light brown in preservative.
Etymology. The specific epithet is taken from the Mixteco language word “sa’bah” which mean “frog”. This is recognition to the San Isidro Paz y Progreso community for their conservation efforts towards their natural and cultural resources.
Distribution and natural history. Charadrahyla sakbah is only known from one locality in San Isidro Paz y Progreso, on the river Chite ku'e at 1390 m in elevation. This river is surrounded by cloud forest, presents several rocky outcrops, with steep slopes that generate numerous waterfalls. In some points the river flow extends to more than 10 m wide ( Fig 5B,C View FIGURE 5 ). The mean temperature in the area is 20°C and the rainfall annually is 1893.3 mm ( SMN 2018). The first specimen was found at night (00.10 hrs CST) on a rock wall about 1.8 m high and less than a 1 m from a waterfall. The air temperature at the time of discovery was 16.9°C with a relative humidity of 70%. Of the 12 observed individuals, 10 were found on a rocky-wall similar to the first specimen, one inside of a pool by the riverside, and one more in vegetation 2 m high from the substrate. The mean air temperature, considering all encounters, was 16.4°C (15.3-17.6) with 75% (70-80%) relative humidity. We recorded two other sympatric frogs, Ptychohyla leonhardschultzei (categorized as endangered by IUCN; Santos-Barrera et al. 2006) and Lithobates sierramadrensis , as well as the salamander Isthmura maxima (categorized as endangered by IUCN; IUCN SSC Amphibian Specialist Group 2016).
During collecting, females and males made low intensity “beeps”, similar to the warning sounds of other treefrogs (e.g. Agalychnis dacnicolor ; Duellman 2001). We observed that the color and tonality varied substantially within the same individual, perhaps due to the temperature or stress of the collection. Moreover, C. sakbah shows a marked sexual dimorphism in body size, snout shape, coloration, and hypertrophied webbings. For the other two species with hypertrophied webbings ( C. trux and C. tecuani ) the adult females are unknown. Therefore, Charadrahyla sakbah is the first species of the genus where the dimorphism in this trait is confirmed.
Considering the limited knowledge about reproductive biology in frogs of the genus Charadrahyla , we made a dissection of one of the female paratypes (IBH 30992) in order to determine the oocyte number and pigmentation. The oocytes number estimated was 750 in both parts of the oviduct. The pigmentation was yellow and we observed different developmental stages, but no mature oocyte. We found the size of the testes in C. sakbah (ranging 12.32– 12.66% of SVL) to be smaller than reported in C. taeniopus (without measurements but see Fig. 5 View FIGURE 5 in Duellman 1965), C altipotens (average of five males 20.65% of SVL; Duellman 1968), C. trux (average of two males 20.49% of SVL; Adler & Dennis 1972;) and C tecuani (23.2–24.1% of SVL; Campbell et al. 2009).
| IBH |
Universidad Nacional Autonoma de Mexico, Instituto de Biologia |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.