Centaurium erythraea, Rafn, Rafn
|
publication ID |
https://doi.org/10.1016/j.phytochem.2018.07.015 |
|
DOI |
https://doi.org/10.5281/zenodo.10514678 |
|
persistent identifier |
https://treatment.plazi.org/id/604887FB-FFEB-A144-606E-0C95FFCAFC6A |
|
treatment provided by |
Felipe |
|
scientific name |
Centaurium erythraea |
| status |
|
2.1. Targeted UHPLC-MS/MS analysis of SG in C. erythraea View in CoL methanol extracts
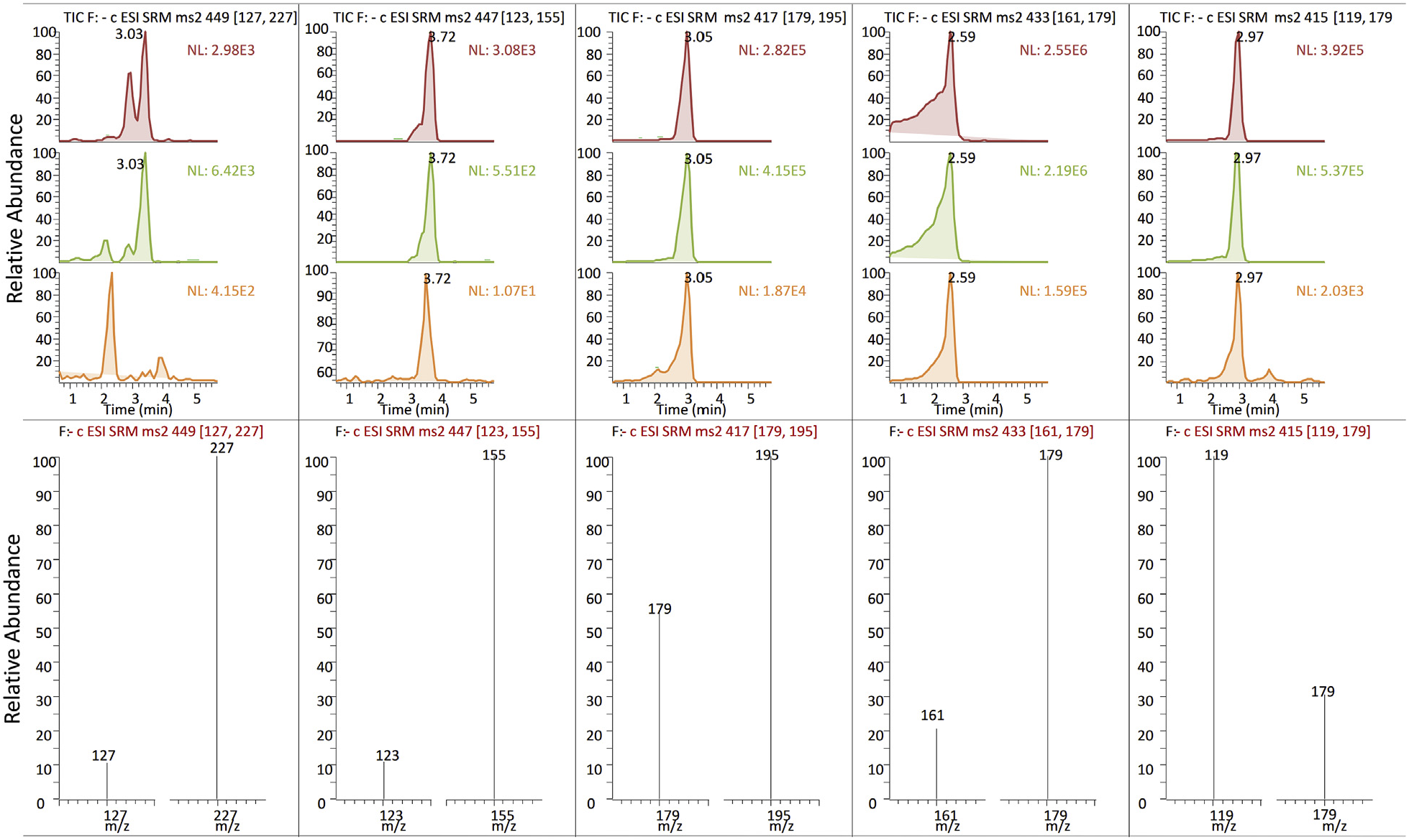
UHPLC-qqqMS quantitative analysis of C. erythraea methanol extracts was targeted towards one iridoid (loganin – 1) and four secoiridoid (secologanin – 2, sweroside – 3, swertiamarin – 4 and gentiopicrin – 5) compounds, which were unambiguously assigned, based on their retention times, MS, MS 2, and UV spectra, and comparisons with the reference standards. Molecular ions of targeted secoiridoids were visible as adducts of acetic acid which was used as a mobile phase, as previously described in Banjanac et al. (2017). Following PIS experiments and analysis of MS 2 spectra (Table S1), two diagnostic fragments of each compound were utilized in SRM experiment for the accurate quantification of targeted compounds ( Fig. 2 View Fig ).
Iridoid glucoside loganin ( 1), which was eluted at Rt = 3.05 min, showed a pseudomolecular ion [M + CH 3 COOH− H] − at m/z 449. Diagnostic MS 2 fragments of 1 were [M− H− C 6 H 10 O 5] − at m/z 227, resulting from the loss of glucose, and [ 1,4 F] − at m/z 127 ( Sun et al., 2015). According to Sun et al. (2015), 1,4 F − ion and [3-oxoMAQ–H] − ion are generated in MS 3 spectrum of 1, due to the cleavage of pyran ring stemming from isomerization of hemiacetal group. Secologanin ( 2) with a pseudomolecular ion [M + CH 3 COOH− H] − at m/z 447 was eluted at Rt = 3.72 min, and it yielded daughter ions [M− C 6 H 10 O 5 −H 2 O− CO−24−H] − at m/z 155 via RDA cleavage, and [M− C 6 H 10 O 5 −H 2 O− CO− C 2 O 2 −H] − at m/z 123. A pseudomolecular ion [M + CH 3 COOH− H] − at m/z 417 visible as a peak at Rt = 3.05 min was assigned to sweroside ( 3), and it generated product ion [M− H− C 6 H 10 O 5] − at m/z 195 by the loss of a glucose unit, and [C 6 H 12 O 6 −H] − at m/z 179, corresponding to deprotonated glucose. Swertiamarin ( 4), which was eluted at Rt = 3.59 min, showed pseudomolecular ion [M + CH 3 COOH− H] − at m/z 433, and its main diagnostic fragments were: [C 6 H 12 O 6 −H] − at m/z 179, corresponding to deprotonated glucose, and [C 6 H 12 O 6 −H− H 2 O] − at m/z 161 that was produced from the glycosyl ion. Gentiopicrin ( 5) exhibited an acetic acidadducted ion [M + CH 3 COOH-H] − at m/z 415 visible in UHPLC/MS 2 chromatogram as a peak at Rt = 2.59 min, and its MS 2 spectrum revealed diagnostic fragments: [C 6 H 12 O 6 −H] − at m/z 179, corresponding to deprotonated glucose, and [M− C 6 H 10 O 5 −H 2 O− C 2 O 2 −H] − at m/z 119, resulting from the loss of glucose, H 2 O and C 2 O 2 moieties. Identification of targeted compounds in samples was additionally confirmed by UHPLC/DAD analysis, by comparing the UV spectra with those of the reference standards. UHPLC/DAD data, including Rt and λ max values are presented in Table S1. The average UHPLC-MS chromatogram as well as the UHPLC-UV chromatogram of C. erythraea methanolic extracts is characterized by a predominant peak attributed to the presence of 4, the main component of the leaves (chromatograms not presented). Compounds 3 and 5 are less abundant. These findings are in accordance with some previous studies ( Banjanac et al., 2017; Bo ž unovi ć et al., 2018; Đ or đ evi ć et al., 2017; Š iler et al., 2014, 2012; Stefkov et al., 2014; van der Sluis, 1985). Loganin has also been previously reported for C. erythraea ( Đ or đ evi ć et al., 2017), as well as secologanin ( Đ or đ evi ć et al., 2017; Takagi and Yamaki, 1982).
2.2. Identi fi cation of SG biosynthetic pathway genes in the transcriptome of
C. erythraea aerial parts
For the purpose of elucidating the biosynthetic pathway responsible for the synthesis of SG, we have taken advantage of the transcriptome resource available for the aerial parts of C. erythraea . Previously unknown candidates for all genes potentially responsible for the synthesis of secologanin have been identified by screening this transcriptome database for candidate genes by performing a BLAST search with already characterized MIA pathway genes from Catharanthus roseus . These included geranyl diphosphate synthase ( GPPS), geraniol synthase ( GES), geraniol-8-oxidase ( G8O), 8-hydrohygeraniol oxidoreductase ( 8HGO), iridoid synthase ( IS), iridoid oxidase ( IO), 7-deoxyloganetic acid glucosyltransferase ( 7DLGT), two candidates for 7-deoxyloganic acid hydrolase ( 7DLH1 and 7DLH2), loganic acid O-methyltransferase ( LAMT), and secologanin synthase ( SLS). All of the selected candidates have shown very high similarity (above 70%) to the corresponding MIA biosynthetic genes present in the NCBI database. Additionally, two candidate CPR genes necessary for the activity of cytochrome P450s were retrieved. The gene-specific primers were successfully designed for the expression analysis of the selected genes, which was confirmed by regular PCR and dissociation curve analysis after the real-time PCR run. The identity of the retrieved candidates was confirmed by sequencing PCR products acquired by amplification using primers designed for qPCR analysis. Herewith reported for the first time for C. erythraea , these newly identified genes encoding probable rate-influencing enzymes in the SG biosynthetic pathway improve the knowledge of the molecular mechanisms underlying the SG biosynthesis and chemical diversity found in this species. Understanding the biogenetic pathways in plants is the first step towards achieving improved production of specialized metabolites. The only way to overcome the rate limiting steps of a targeted pathway is to elucidate their intrinsic regulation. Two distinctive attempts at upregulation have been performed, that can be described as “push” and “pull” strategies. The two strategies are focused on overexpressing either the starting steps of a pathway or the terminal ones. Both strategies were shown to have their own advantages and disadvantages, depending on the specific pathway that has been investigated. Pathways leading to the production of various specialized metabolites have been elucidated in plants so far using biochemical and genetics approaches. Good examples are tropane biosynthetic pathway in Hyoscyamus niger ( Zhang et al., 2004) , investigation of the factors that regulate carotenoid biosynthesis in tomato fruits ( Liu et al., 2015) and flavonoid biosynthesis in both the model plant Arabidopsis thaliana and a range of crop species, including tomato, maize, rice, and bean (See Tohge et al., 2017 for comprehensive review).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |