Hediste diadroma, Sato & Nakashima, 2003
|
publication ID |
https://doi.org/ 10.1046/j.1096-3642.2003.00059.x |
|
persistent identifier |
https://treatment.plazi.org/id/534787FA-FFB0-FFFD-9B4D-93D3FC40A0C2 |
|
treatment provided by |
Carolina |
|
scientific name |
Hediste diadroma |
| status |
sp. nov. |
HEDISTE DIADROMA View in CoL SP. NOV. ( FIGS 10 View Figure 10 , 16-27 View Figure 16 View Figure 17 View Figure 18 View Figure 19 View Figure 20 View Figure 21 View Figure 22 View Figure 23 View Figure 24 View Figure 25 , 38-43 View Figure 38 View Figure 39 View Figure 40 View Figure 41 View Figure 42 View Figure 43 )
Nereis japonica View in CoL : Kagawa, 1955: 11–16, fig. 5; Okada, 1960: 63–71, pls 1–4.
Neanthes japonica: Sun et al., 1980: 100–110 View in CoL , figs 1–3; Sato & Osanai, 1986: 263–270, figs 1–7; Qiu & Wu, 1993: 360–367, figs 1, 2.
Small-egg type of Neanthes japonica: Sato & Tsuchiya, 1987: 29–42 View in CoL , figs 1–5; Sato & Tsuchiya, 1991: 371– 382, figs 1, 2 and 4.
Small-egg form of Neanthes japonica: Sato & Ikeda, 1992: 299–307 , figs 4, 7.
Small-egg form of Hediste japonica: Sato & Masuda, 1997: 163–170 ; Sato, 1999: 129–143, figs 1–13.
Hediste japonica: Sato, 2001: 66–86 View in CoL , Rouse & Pleijel, 2001: pl. 5c.
Our previous studies ( Sato & Tsuchiya, 1991; Sato, 1999, 2000, 2001) showed the existence of three cryptic species (the small-egg, large-egg and Ariake forms) in Asian Hediste View in CoL ,andjudgedthefirsttobe H. japonicasensu stricto based on its original description ( Izuka, 1908), withoutexaminationofthetypematerial.However, having examined the type, this judgement is here revised; the Ariake form is identical to H. japonica View in CoL sensu stricto.
Neanthes japonica sensu Smith (1958) View in CoL collected from two localities of Japan seems to contain two or Type material
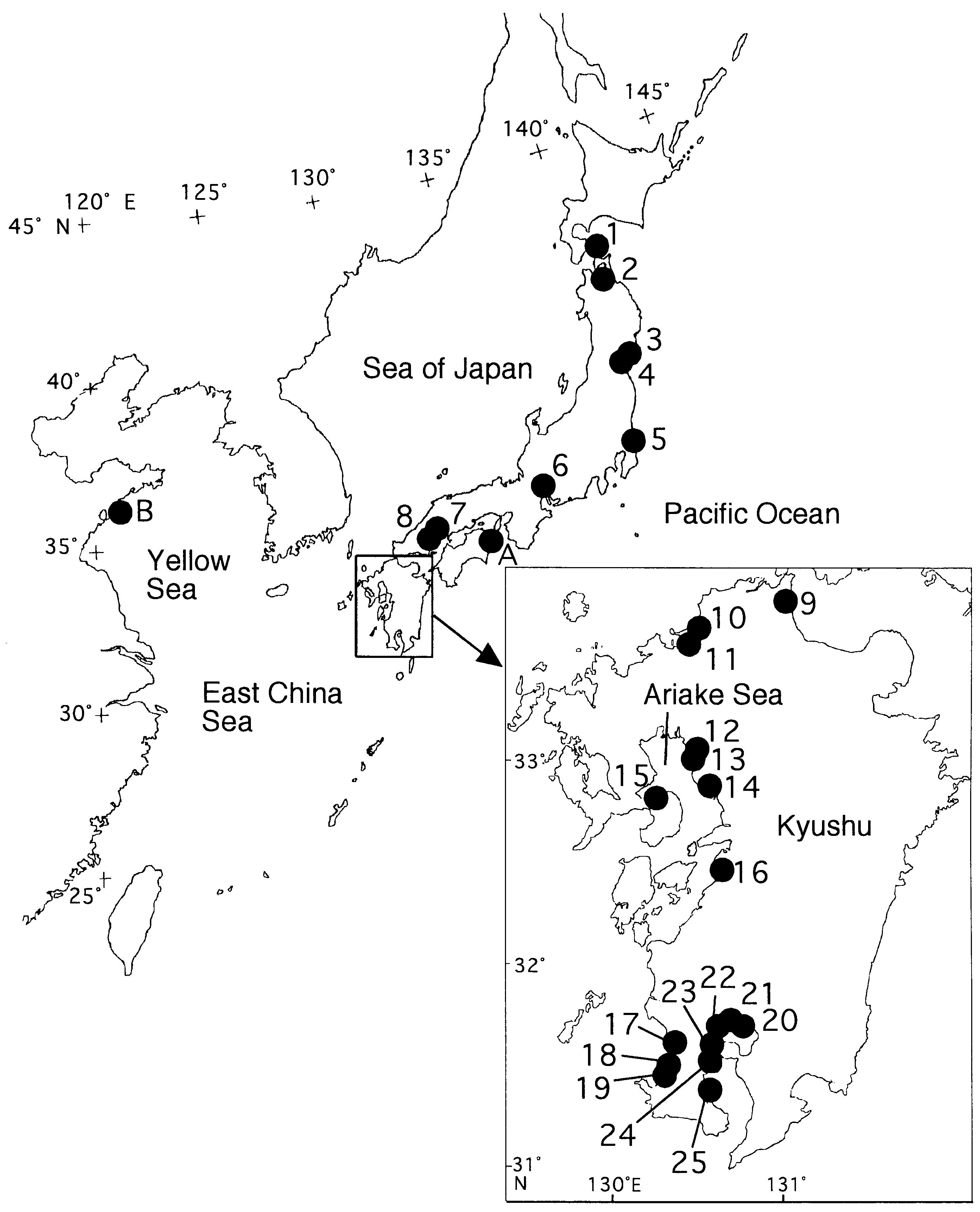
Holotype (NSMT-Pol-H 456): complete mature swarming female ( BL: 52 mm, BW: 2.8 mm, 94 setigers), mouth of Omoigawa River, Aira-cho , Kagoshima Prefecture, 25 February 1986, coll. M. Sato. Paratypes: mature swarming 1 male (ZIHU-2093) and 1 female (ZIHU-2092) ( BL: 37–50 mm, BW: 1.8–2.2 mm, 80–85 setigers), data as for holotype; mature swarming 5 males and 6 females ( BL: 42–65 mm, BW: 2.3–3.3 mm, 84–98 setigers), Omoigawa River, Aira-cho, Kagoshima Prefecture, 19 February 1988, coll. M. Sato (CMNH-ZW-1891, MNHN-Poly-1368, 1369, NSMT- Pol-P457, OMNH-Iv-4228, SMF-12067, 12068, USNM-1008438, 1008439, ZMUC-Pol-1504, 1507); mature swarming 1 female ( BL: 101 mm, BW: 4.6 mm, 88 setigers), Yahatagawa River , Itsukaichi-cho, Hiroshima Prefecture, 9 January 1982, coll. M. Sato (ZIHU-2094); mature swarming 2 males ( BL: 37 mm, BW: 2.4–2.5 mm, 84 setigers), Kyobashigawa, Otagawa River , Hiroshima-shi, Hiroshima Prefecture, 9 January 1981, coll. M. Sato (ZIHU-2095); mature swarming 5 females ( BL: 60–85 mm, BW: 3.3–4.7 mm, 78–105 setigers), Nanakitagawa River , Gamo, Sendai- 1 Total number on right and left sides of proboscis. shi, Miyagi Prefecture, 6 April 1981, coll. M. Sato (ZIHU-2097); mature swarming 2 males ( BL: 50– 65 mm, BW: 2.5–3.0 mm, 98 setigers), Usujiri, Minamikayabe-cho, Hokkaido, 17 June 1983, coll. K. Yokouchi (ZIHU-2096) .
Other material examined
Epitokes collected during reproductive swarming: Usujiri, Minamikayabe-cho , Hokkaido, 28 June 1983, coll. K. Yokouchi, 1 specimen . Nanakitagawa River, Gamo, Sendai-shi , Miyagi Prefecture, 6 April 1981, coll. M. Sato, 15 specimens ; 26 April 1994, coll. M. Ikeda, 52 specimens . Tonegawa River, Hagiwara , Chiba Prefecture, 23 March 1966, coll. unknown, 14 specimens . Fujimae, Shinkawa River, Nagoya-shi , Aichi Prefecture, 28 February 2002, coll. S. Sano et al. 12 specimens . Kyobashigawa, Otagawa River, Hiroshima-shi , Hiroshima Prefecture, 7–9 January 1981, coll. M. Sato, 13 specimens . Yahatagawa River, Itsukaichi-cho , Hiroshima Prefecture, 7 January 1981, coll. O. Kawamoto & M. Sato, 26 specimens ; 9 January 1982, coll. M. Sato, 5 specimens . Nakagawa River, Fukuoka-shi , Fukuoka Prefecture, 3, 4 March 1980, coll. M. Sato, 40 specimens . Amorigawa River, Hayato-cho , Kagoshima Prefecture, 21 February 1989, coll. M. Ikeda, 14 specimens . Byugawa River, Aira-cho , Kagoshima Prefecture, 21 February 1989, coll. M. Ikeda, 12 specimens ; 13 March 1994, coll. M. Sato, 40 specimens . Omoigawa River, Aira-cho , Kagoshima Prefecture, 25 February 1986, coll. M. Sato, 77 specimens ; 2 March 1987, coll. M. Sato, 1 specimen ; 4, 18 March , 17 April 1988, coll. M. Sato & M. Ikeda, 23 specimens ; 21, 22 February , 8, 9 March 1989, coll. M. Ikeda, 36 specimens ; 1 March 1991, coll. M. Sato, 11 specimens . Kotsukigawa River, Kagoshima-shi , 22 March 1985, coll. K. Kusamura, 1 specimen ; 25, 26 February 1986, coll. M. Sato et al. 6 specimens. Nagatagawa River, Taniyama, Kagoshima-shi , 20 February 1989, coll. M. Sato & M. Ikeda, 44 specimens ; 18, 20 February , 6 March 1992, coll. M. Sato, 30 specimens . Kaminokawa River , Higashi-ichiki-cho, Kagoshima Prefecture, 22 February 1989, coll. M. Sato, 2 specimens ; 12 February , 5 March 1992, coll. M. Sato, 10 specimens . Isakugawa River, Iriki-cho , Kagoshima Prefecture, 22 February 1989, coll. M. Ikeda, 7 specimens . Manosegawa River, Kaseda-shi , Kagoshima Prefecture, 22 February 1989, coll. M. Ikeda, 20 specimens ; 14 March 1994, coll. M. Sato, 3 specimens .
Epitokes obtained after rearing in laboratory: Kominatogawa River, Asadokoro , Aomori Prefecture, fixed 10 February 1984 after rearing for 2 months, coll. M. Sato, 1 specimen . Natorigawa River, Yuriage , Miyagi Prefecture, fixed 15 April 1980 after rearing for 5 months, coll. M. Sato, 9 specimens .
Immature specimens (atokes): Kusamigawa River, Kitakyushu-shi , Fukuoka Prefecture, 30 March 1998, coll. M. Sato, 13 specimens . Wajirogawa River, Fukuoka-shi , Fukuoka Prefecture, 31 March 1998, coll. M. Sato, 1 specimen . Domengawa River, Omutashi , Fukuoka Prefecture, 24 November 1998, 4 January 1999, coll. K. Ichimiya, 50 specimens . Omutagawa River, Omuta-shi , Fukuoka Prefecture, 24 November 1998, 4 January 1999, coll. K. Ichimiya, 11 specimens . A small creek at Kobe, Mizuho-cho , Nagasaki Prefecture, 28 July 1998, coll. M. Sato, 13 specimens . Kikuchigawa River, Tamana-shi , Kumamoto Prefecture, 14 September 1996, coll. A. Nakashima, 53 specimens . Maekawa River, Yatsushiro-shi , Kumamoto Prefecture, 25 August 1998, coll. K. Ichimiya, 22 specimens . Omoigawa River, Aira-cho , Kagoshima Prefecture, 24 November 1995 , 2 June, 28 September, 25 October , 25 November 1996, coll. A. Nakashima, 16 specimens ; laboratory-bred worms fixed 4–48 months after fertilization at 23 April 1988, 9, 17 April 1989, 28 March 1990, coll. M. Sato, 14 specimens. Mangrove forest (northern limit in Pacific) at Atagogawa River , Kiire-cho , Kagoshima Prefecture, 2 July 1996, coll. A. Nakashima, 7 specimens .
Diagnosis
Moderate number (10–20 in most cases) of paragnaths on both right and left sides of proboscis in group II. Homogomph falciger absent, heterogomph spinigers present in neuropodia. Neuropodial postchaetal ligule tapering to digitate lobe only in anterior setigers (up to setigers 15–25). At epitokous stage, delicate epitoke-specific sesquigomph spinigers added in all notopodial and neuropodial fascicles. Full-grown oocytes 130–170 Mm in diameter. Reproductive swarming in winter or early spring.
Description
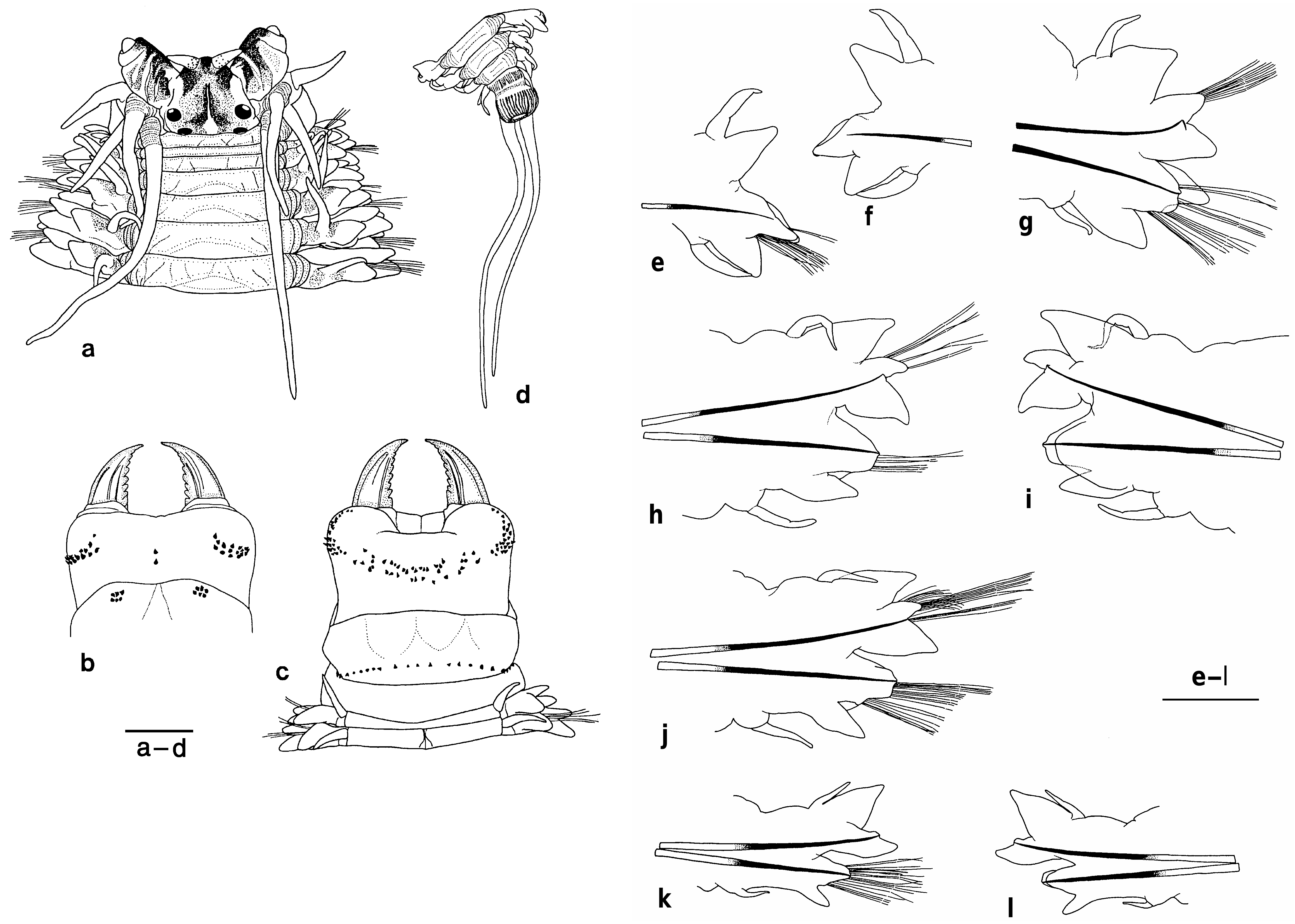
Body stout anteriorly, posteriorly gradually tapering toward pygidium ( Fig. 16a,d View Figure 16 ). Dorsum convex, venter relatively flat with longitudinal midventral groove. Colour in preserved specimens whitish cream with dark brown pigmentation on anterior dorsal surface; colour in life at sexually immature stage reddish brown.
Prostomium pyriform, broader than long, with pair of tapered antennae situated at anterior end. Pair of palps with massive palpophores about twice as long as antennae and short round palpostyles. Two pairs of round or oval eyes almost equal in size, arranged trapezoidally (the space between the anterior pair about 1.3 times as wide as that between the posterior pair). Longitudinal mid-dorsal groove present on anterior dorsum of prostomium. Partial dark pigmentation present on dorsal anterior surface of prostomium and palpophore ( Fig. 16a View Figure 16 ).
Peristomium nearly as long as following setigers, with 4 pairs of tentacular cirri of unequal length; posterior dorsal tentacular cirri longest, reaching back to setigers 6–12; anterior dorsal tentacular cirri next longest, reaching back to setigers 3–4.
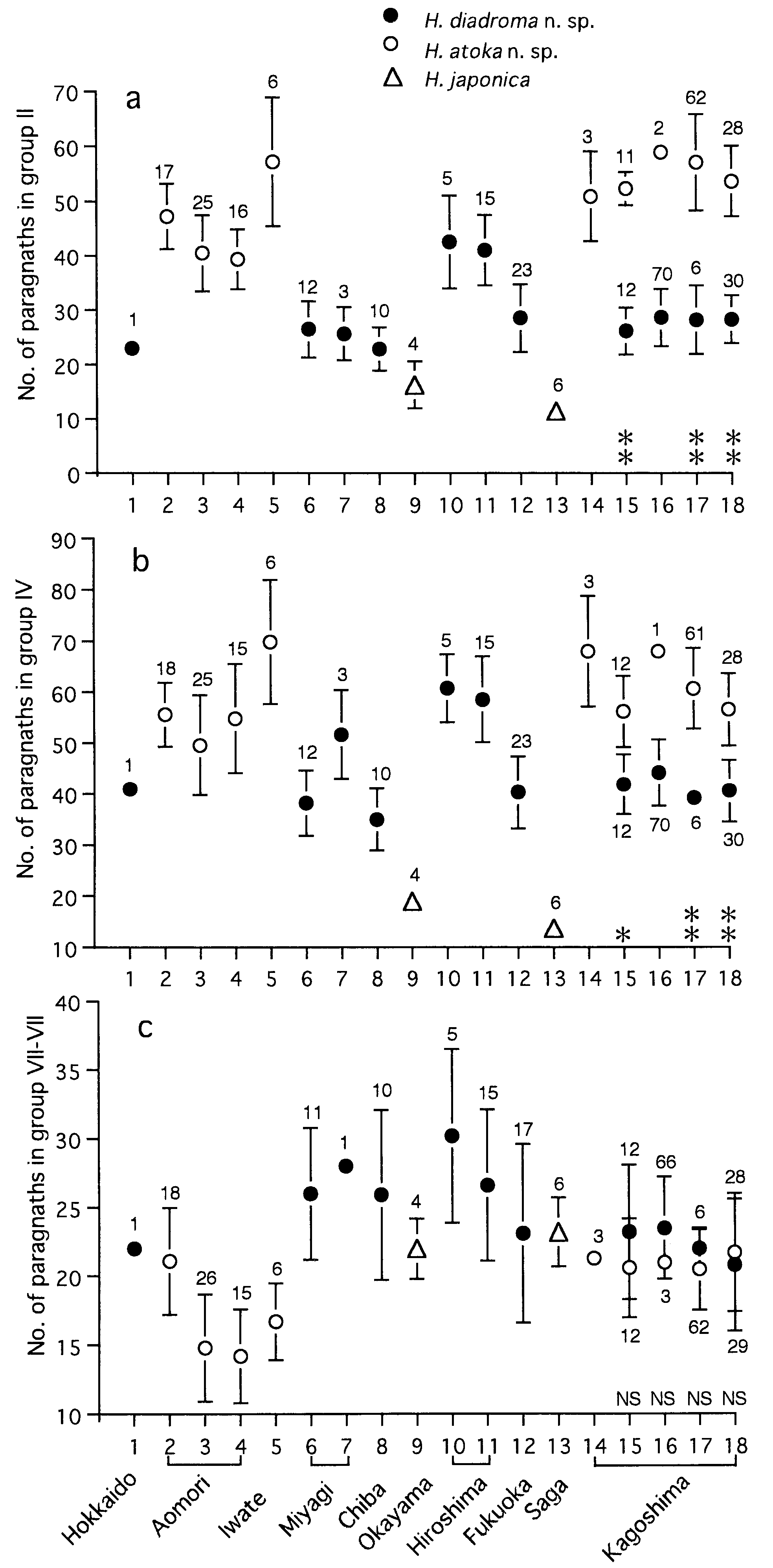
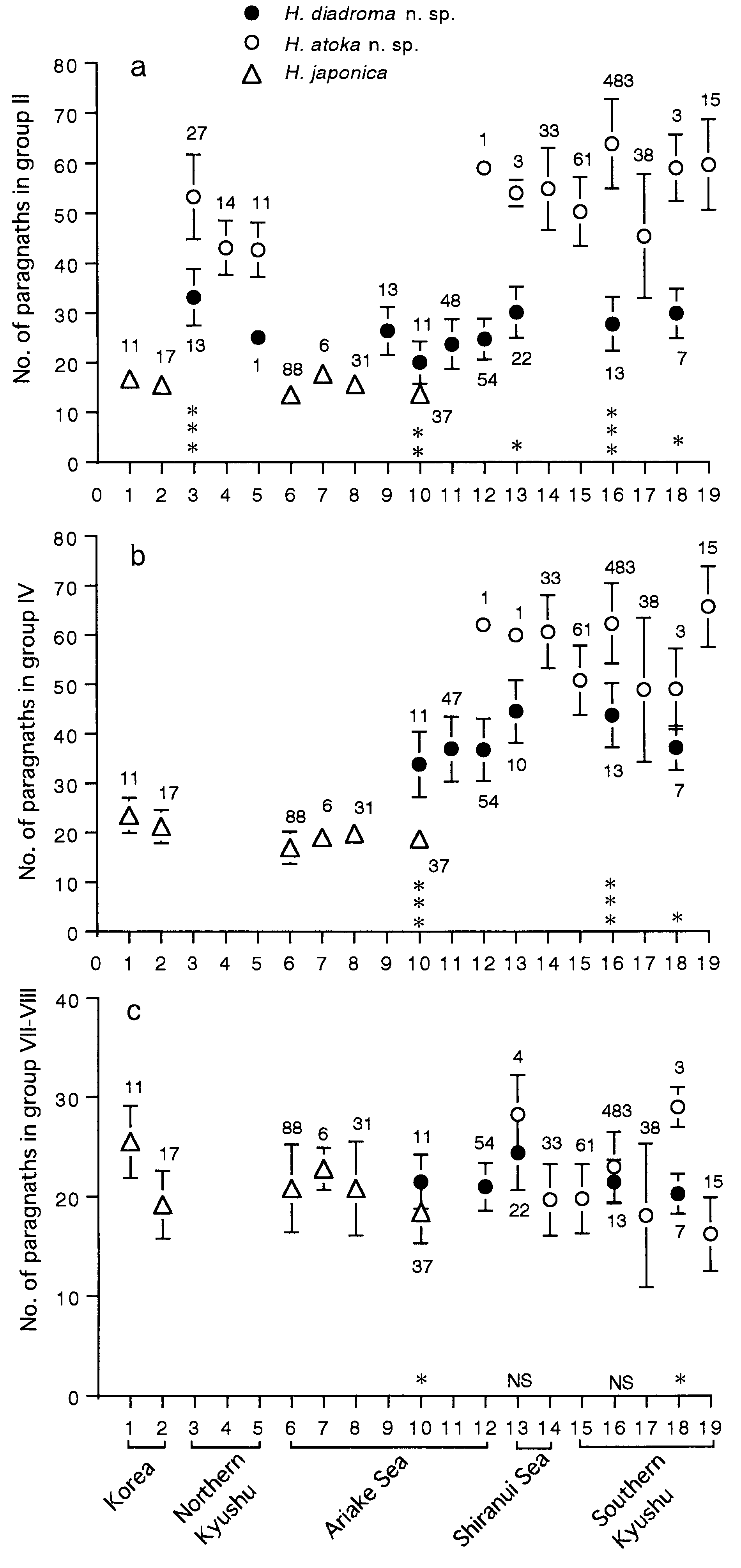
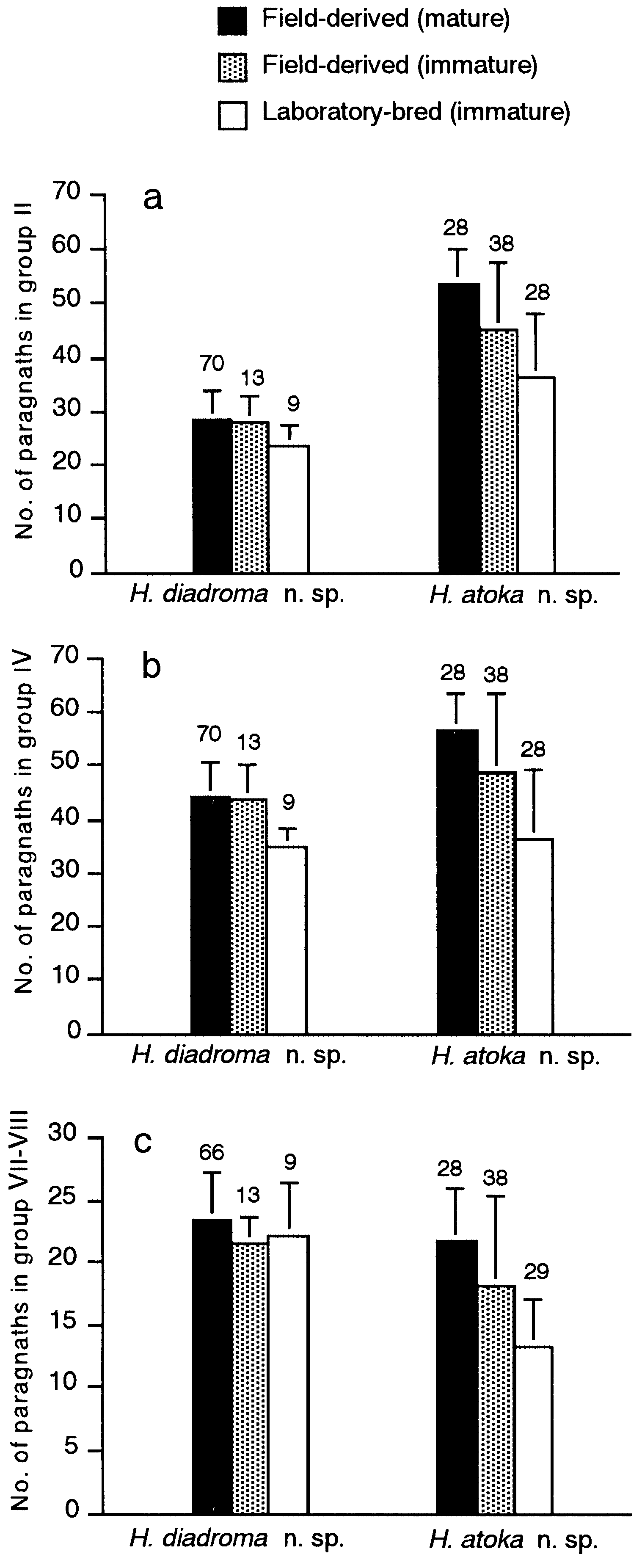
Proboscis with pair of dark brown jaws, each with 6– 9 teeth ( Fig. 16b,c View Figure 16 ). Black paragnaths, usually with pointed tip, present on both maxillary and oral rings; paragnath numbers in holotype (range for all 188 mature specimens in parentheses) as follows. Group I: 2 (1–8); II: 16 on right and 15 on left sides in two or three arched rows each, total 31 (18–52); III: 37 (22– 61) in transverse band; IV: 24 on both sides in three arched rows each, total 48 (25–67); V: none; VI: 6 on both sides in small clusters, total 12 (3–17); VII- VIII: 26 (11–41) in single transverse row .
Parapodia of first 2 setigers uniramous, all following parapodia biramous ( Fig. 16e- l View Figure 16 ). Uniramous parapodia with reduced notopodia consisting of dorsal cirrus and superior ligule, and with ordinary neuropodia.
Notopodia consisting of dorsal cirrus and three ligules in biramous parapodia, i.e. large superior ligule and upper and lower acicular ligules; all notopodial ligules subtriangular with tapering tip. Upper acicular ligule subequal to lower one in anterior setigers ( Fig. 16g View Figure 16 ), gradually diminishing in size in middle setigers and reduced to a small protrusion in posterior setigers ( Fig. 16h- l View Figure 16 ). Superior ligule thick in anterior setigers, thinner in middle and posterior setigers (from around setiger 15), most expanded in middle setigers. Dorsal cirri slender, tapering, gradually diminishing in size in posterior setigers.
Neuropodia consisting of ventral cirrus and three ligules throughout, i.e. prechaetal acicular ligule, postchaetal ligule and inferior ligule. Prechaetal acicular ligule and postchaetal ligule conical with tapering tip, of similar lengths, completely separate in anterior setigers (up to about first 10 setigers, Fig. 16e- g View Figure 16 ), fused at following setigers; tapering tip of postchaetal ligule diminishing to digitate lobe, present up to around setiger 20, and absent in following ones ( Fig. 16i,l View Figure 16 ); prechaetal acicular ligule with blunt tip. Inferior ligule conical. Ventral cirrus slender with tapering tip. Inferior ligule and ventral cirrus gradually diminishing in size in posterior setigers.
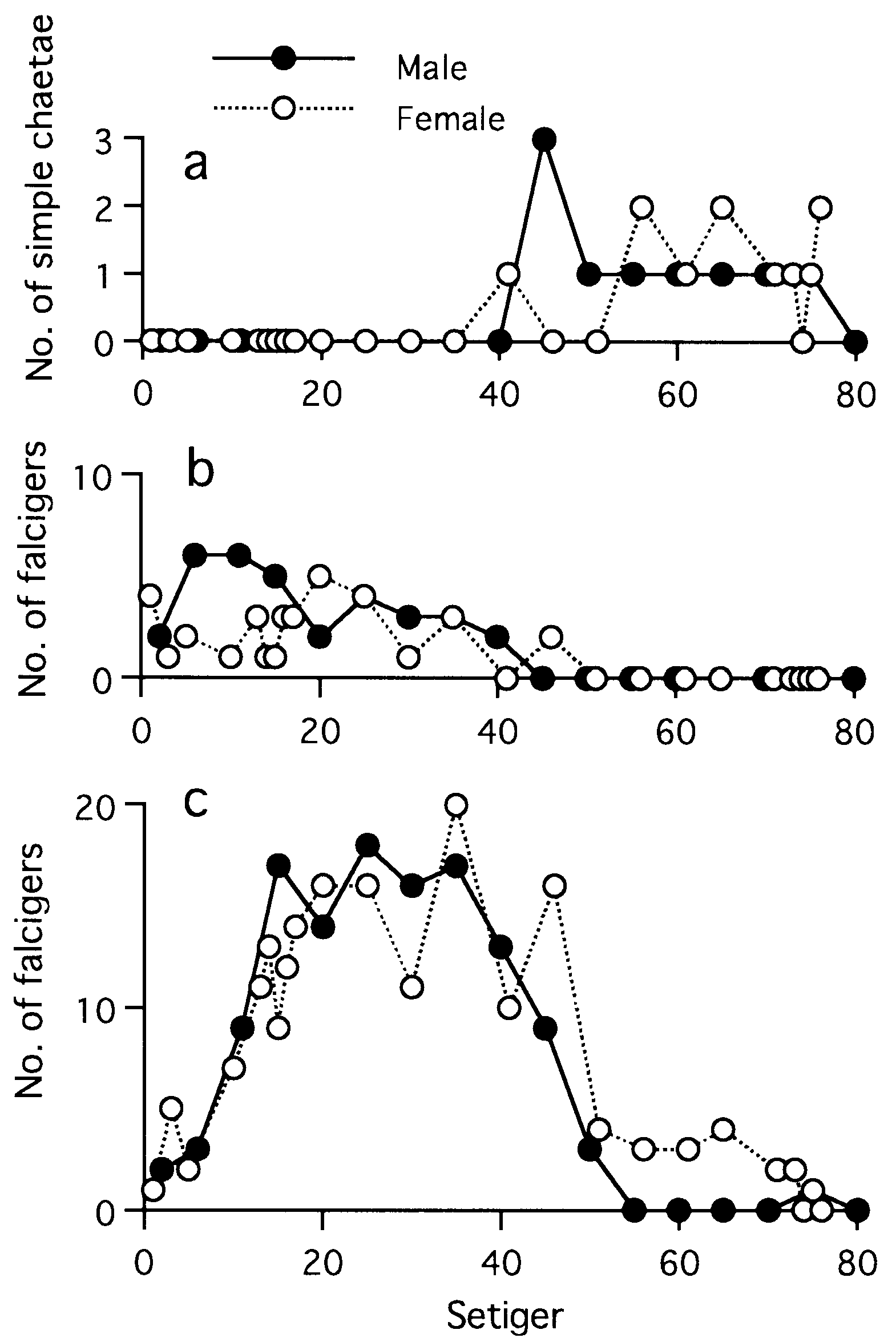
Notochaetae all homogomph spinigers, neurochaetae homogomph and heterogomph spinigers, heterogomph falcigers and simple chaetae, except addition of epitoke-specific chaetae to all notopodial and neuropodial fascicles in mature adults (see below) ( Table 3).
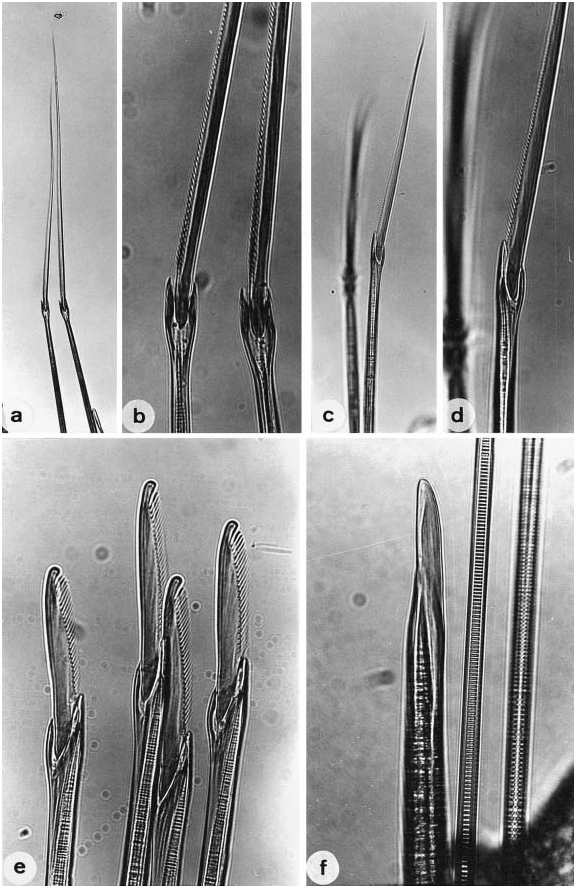
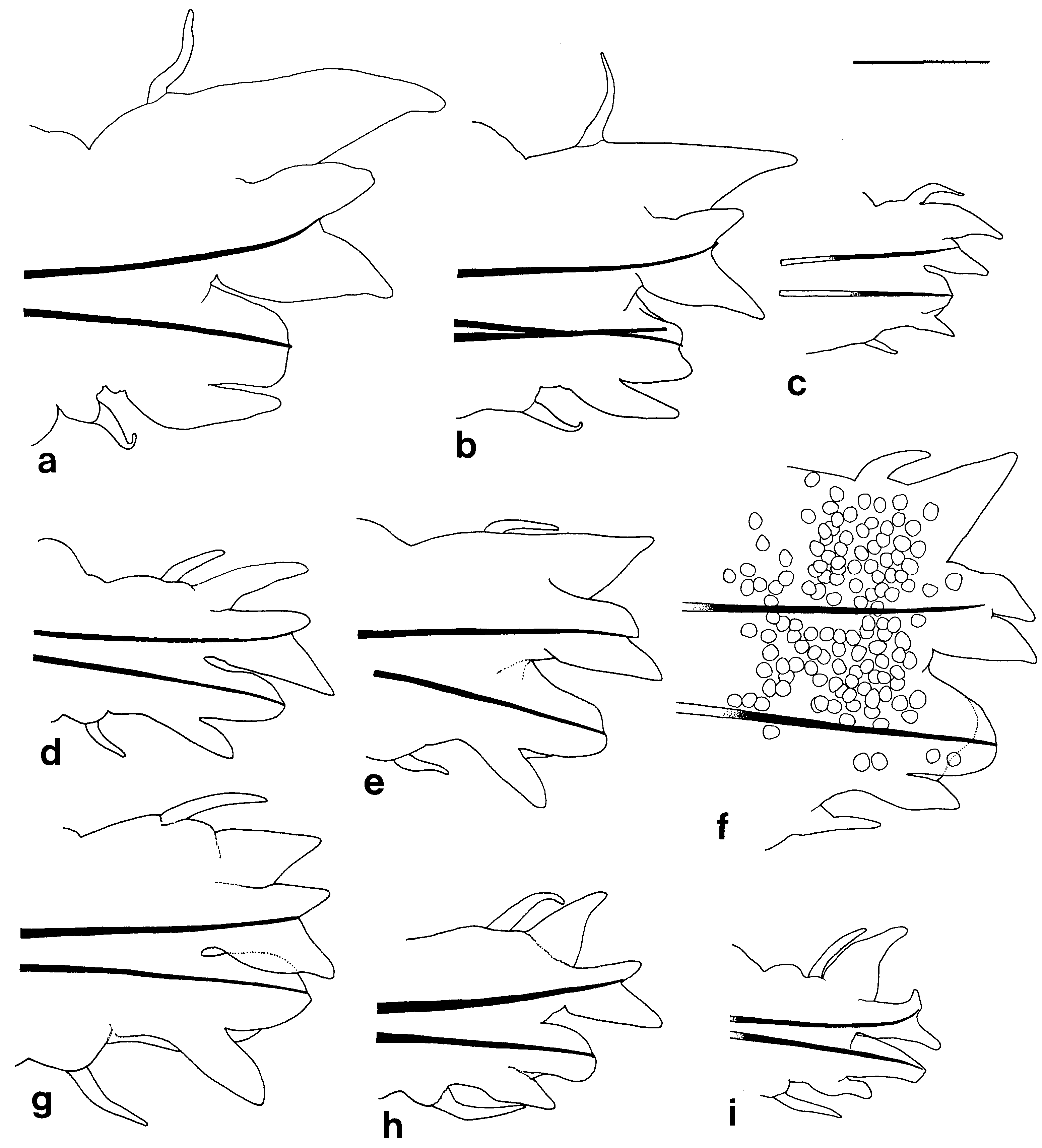
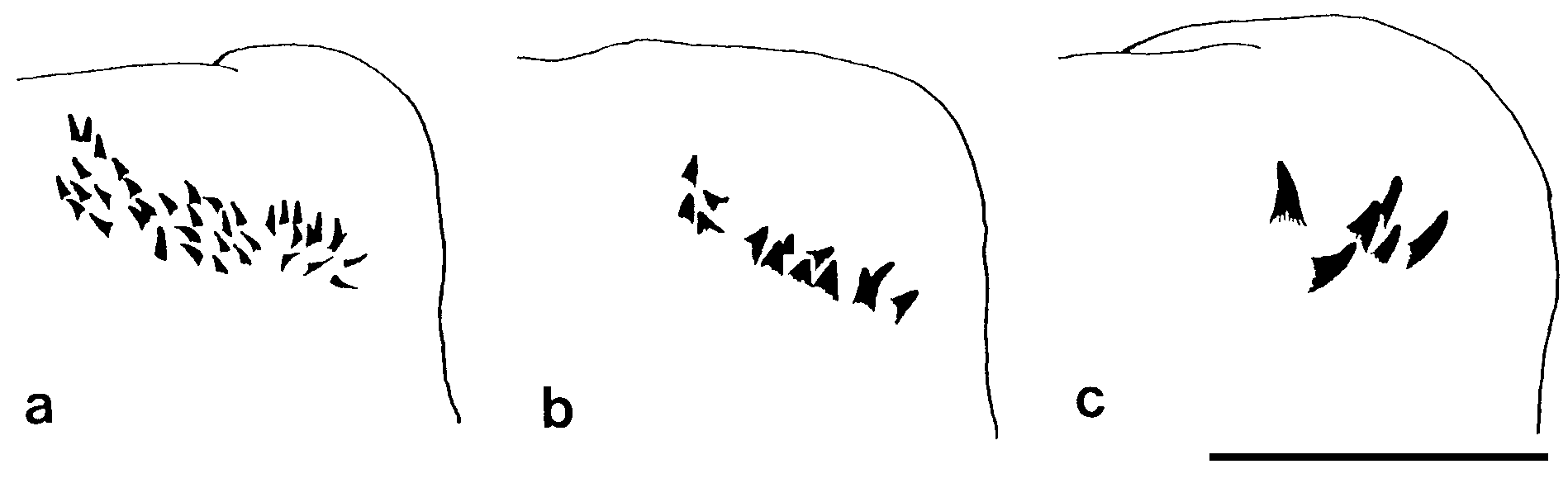
Notopodial homogomph spiniger with slender serrated blade ( Fig. 17a,b View Figure 17 ), 10–50 in number in most setigers, and less than 10 in anteriormost and posteriormost setigers ( Fig. 18a View Figure 18 ).
Neurochaetae of supra-acicular fascicle consisting of homogomph spinigers (up to 20, Fig. 18b View Figure 18 ) and heterogomph falcigers (up to 10, Fig. 19b View Figure 19 ) in anterior and middle setigers (anterior 40–50 setigers in adults). Neuropodial homogomph spinigers similar to notopodial ones in shape and size. Heterogomph falcigers with serrated blade; 1–3 thick simple chaetae with tapering tip present instead of heterogomph falcigers in posterior setigers ( Figs 17f View Figure 17 , 19a View Figure 19 ).
Neurochaetae of infra-acicular fascicle consisting of homogomph spinigers (up to 15, Fig. 18c View Figure 18 ) at upper position, heterogomph spinigers (up to 5, Figs 17c,d View Figure 17 , 18d View Figure 18 ) in middle portion, and heterogomph falcigers (up to 40, Figs 17e View Figure 17 , 19c View Figure 19 ) at lower position.
Aciculae black except colourless proximal part; single acicula present in each ramus (occasionally 2 aciculae present in each ramus).
Pygidium with anus on dorsal side, with pair of cylindrical slender anal cirri ( Fig. 16d View Figure 16 ).
Epitokous metamorphosis at sexually mature stage: body wall becoming thin and transparent; ripe eggs 1 Significance of difference between females and males was tested by Mann–Whitney U -test; ns, not significant (P> 0.05), *significant (P <0.05), **significant (P <0.01).
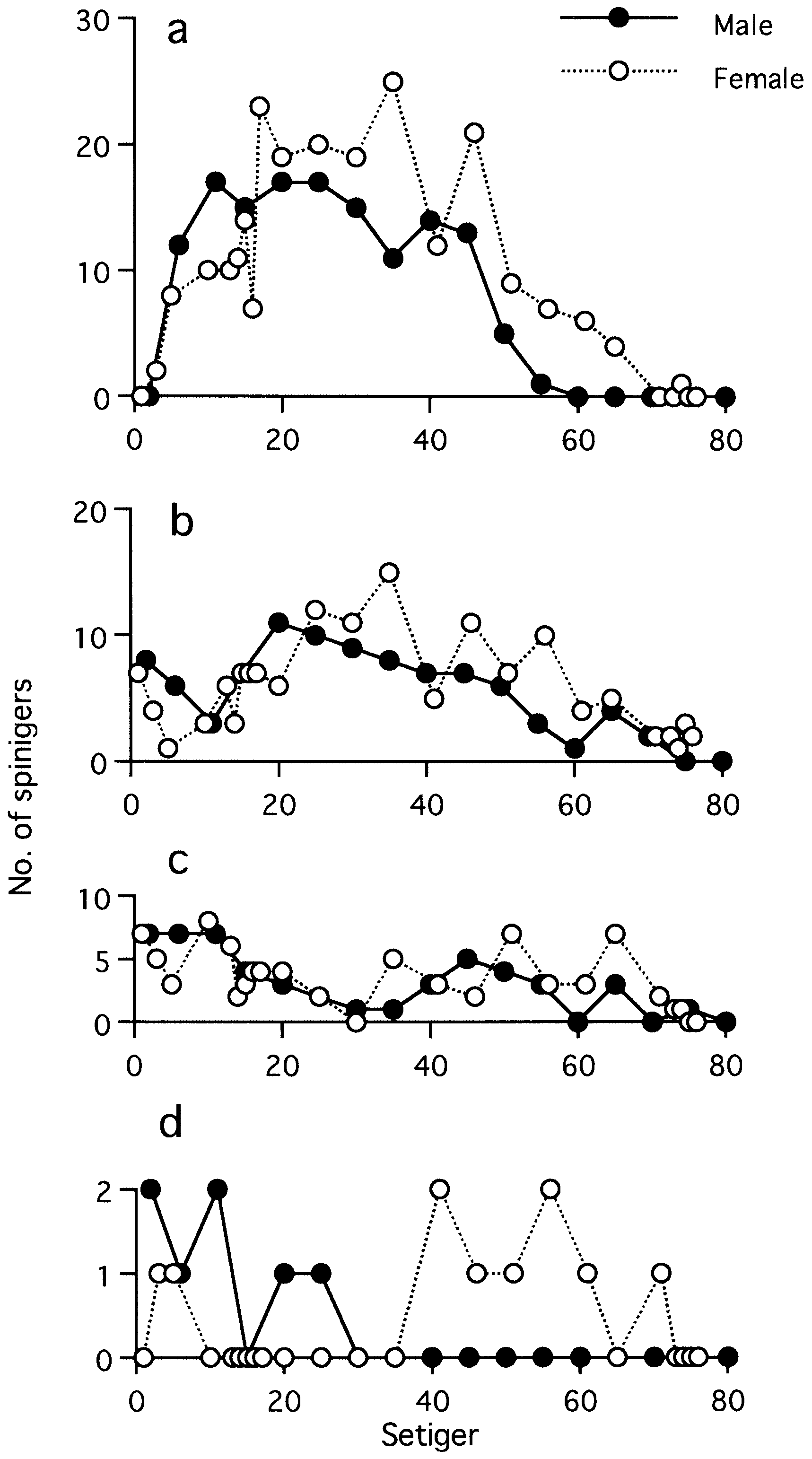
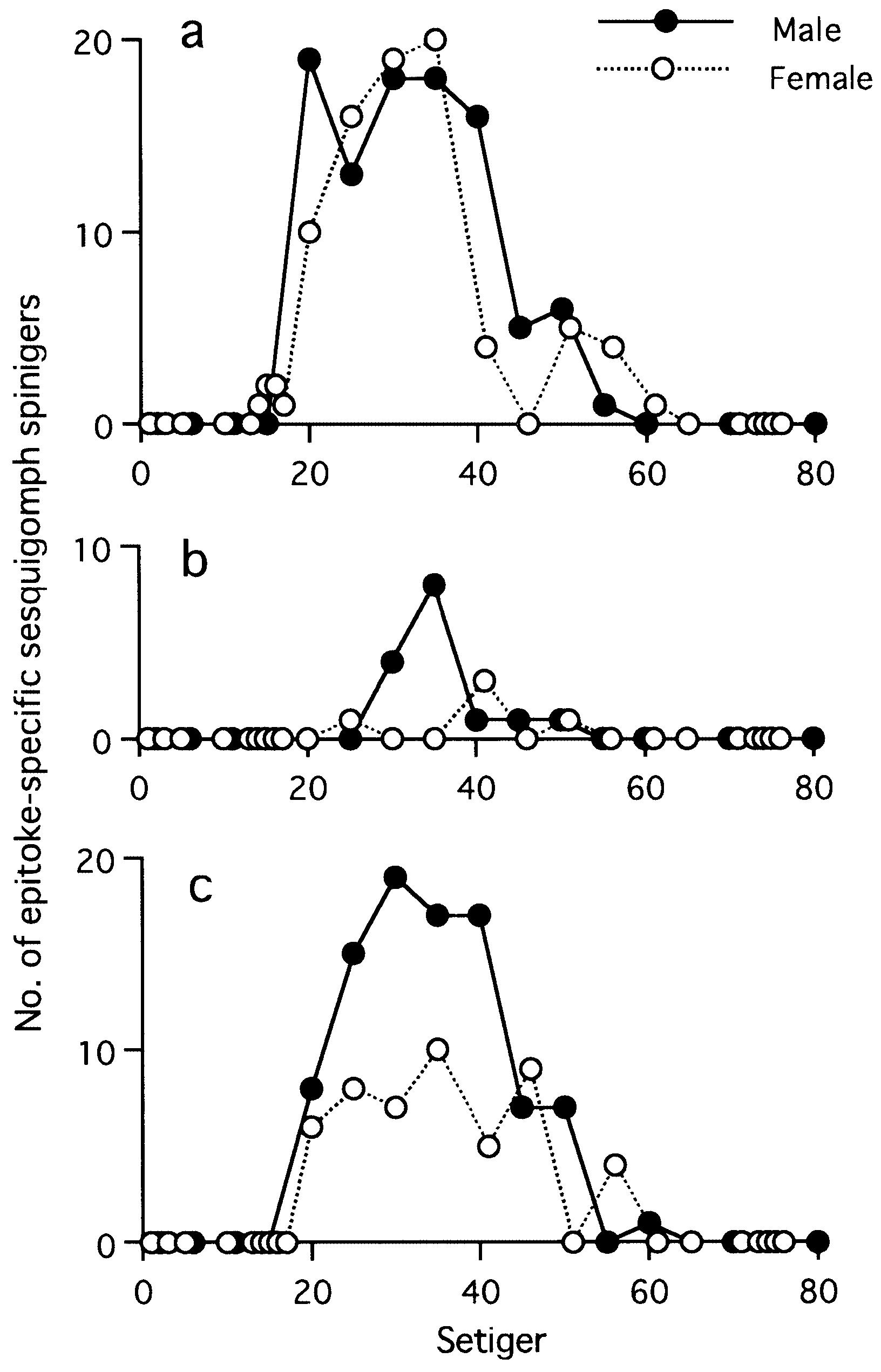
(fully mature oocytes) green colour in females, sperm whitish in males ( Sato & Tsuchiya, 1987; Sato, 1999). Eyes slightly enlarged in both males and females. Parapodial ligules (especially upper and lower acicular ligules of notopodia) enlarged slightly in both males and females; dorsal and ventral cirri also elongated in males ( Fig. 20 View Figure 20 ). Delicate transparent epitokespecific sesquigomph (intermediate between homogomph and heterogomph) spinigers with less serrated blade added posterior to all fascicles of atokous chaetae in notopodia (up to 60) and in neuropodia (up to 60) in middle setigers in both males and females ( Figs 21 View Figure 21 , 22 View Figure 22 ; Table 3). Number of epitoke-specific spinigers in neuropodial infra-acicular fascicle more abundant in males than in females.
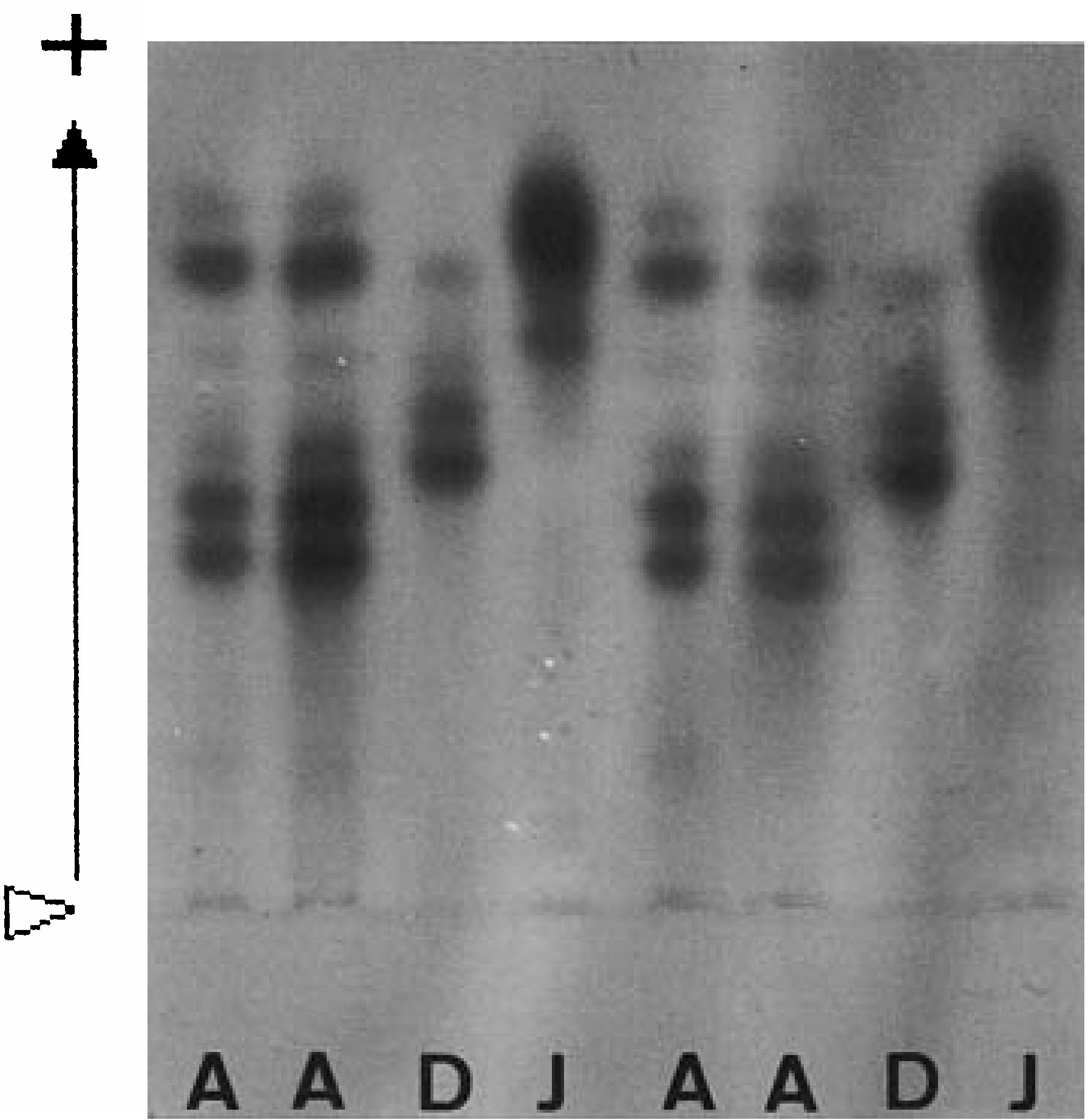
Allozyme pattern of LDH
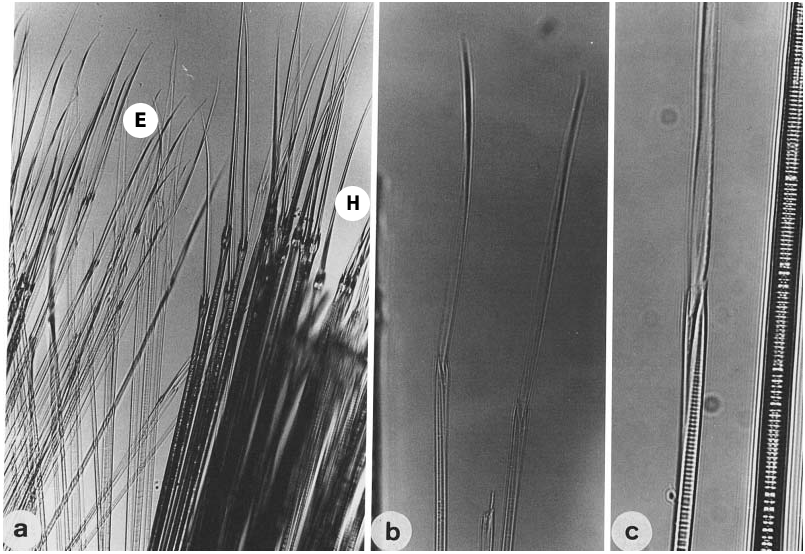
Total of 309 specimens from 12 localities was examined electrophoretically. Three conspicuous bands of anodal migration were observed in most specimens, with two bands of lower mobility closer together ( Fig. 10 View Figure 10 ). Two loci relating to the LDH pattern seemed to be almost monomorphic (the frequency of the dominant allele exceeded 0.9 for each locus) ( Sato & Masuda, 1997).
Allometry
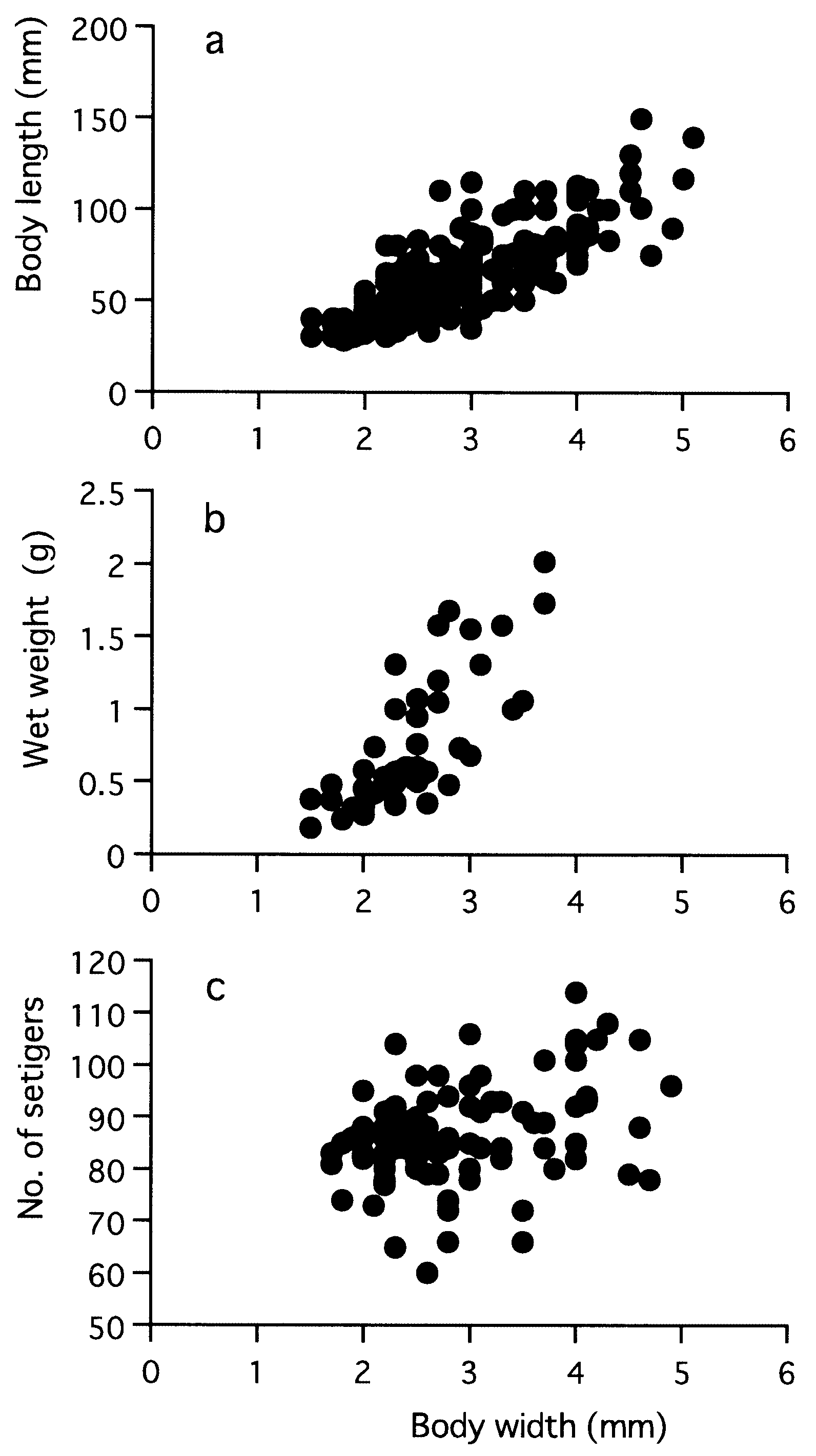
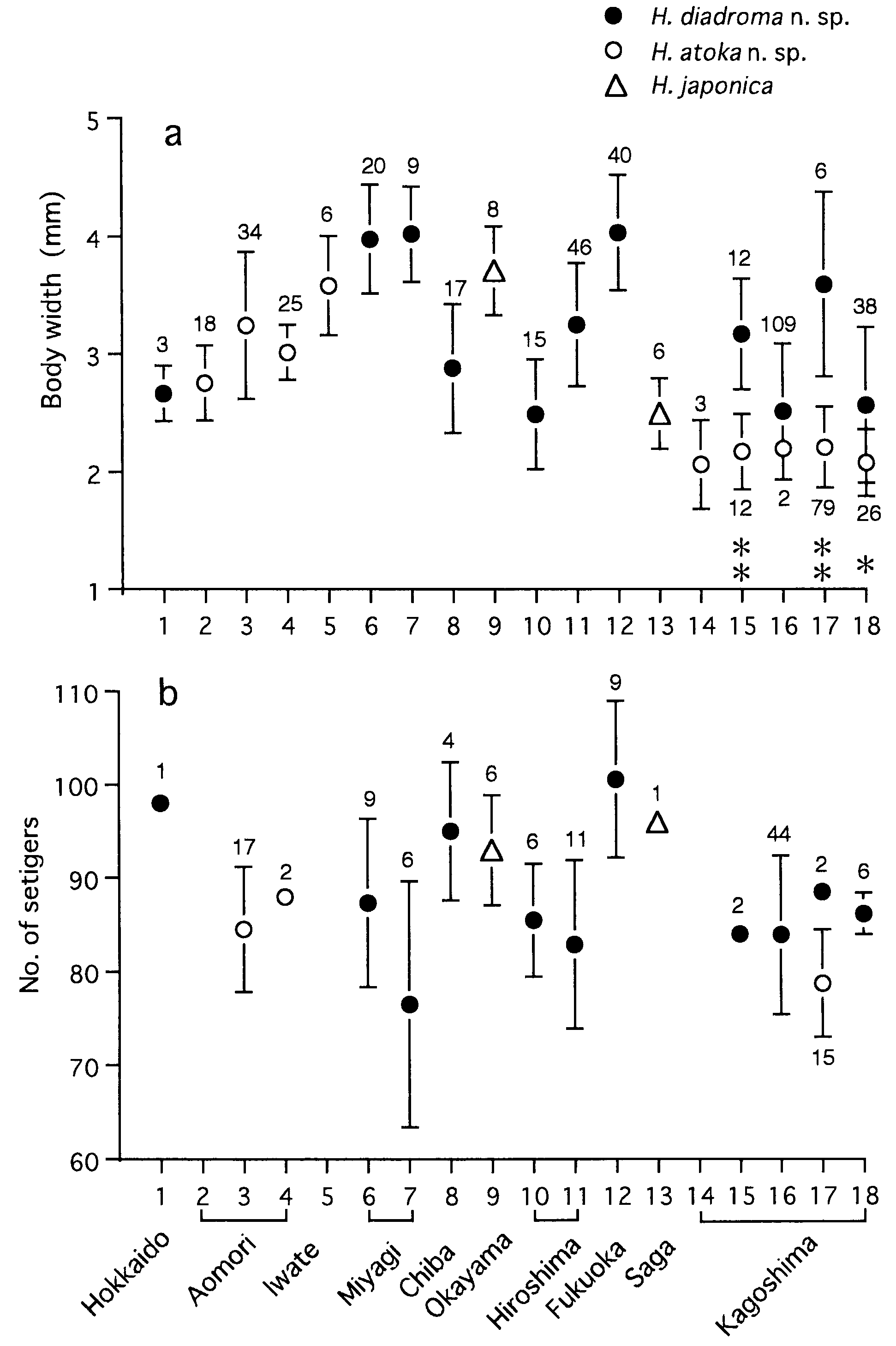
Body length ( BL mm), wet weight before shedding gametes ( WW g) and setiger number (SN) in mature adults correlated with body width ( BW mm) according to the following regression formulae ( Fig. 23 View Figure 23 ): BL = 24.9 BW - 8.1 (r 2 = 0.65, P <0.0001, n = 193), WW = 0.1 BW 2.1 (r 2 = 0.61, P <0.0001, n = 46), SN = 4.1 BW + 75.0 (r 2 = 0.12, P = 0.0006, n = 96). Maximum sizes were 150 mm in BL, 5.1 mm in BW, and 114 in SN for mature adults, and 160 mm in BL, 5.3 mm in BW and 118 in SN for immature specimens which were reared in laboratory for 2 years after artificial fertilization.
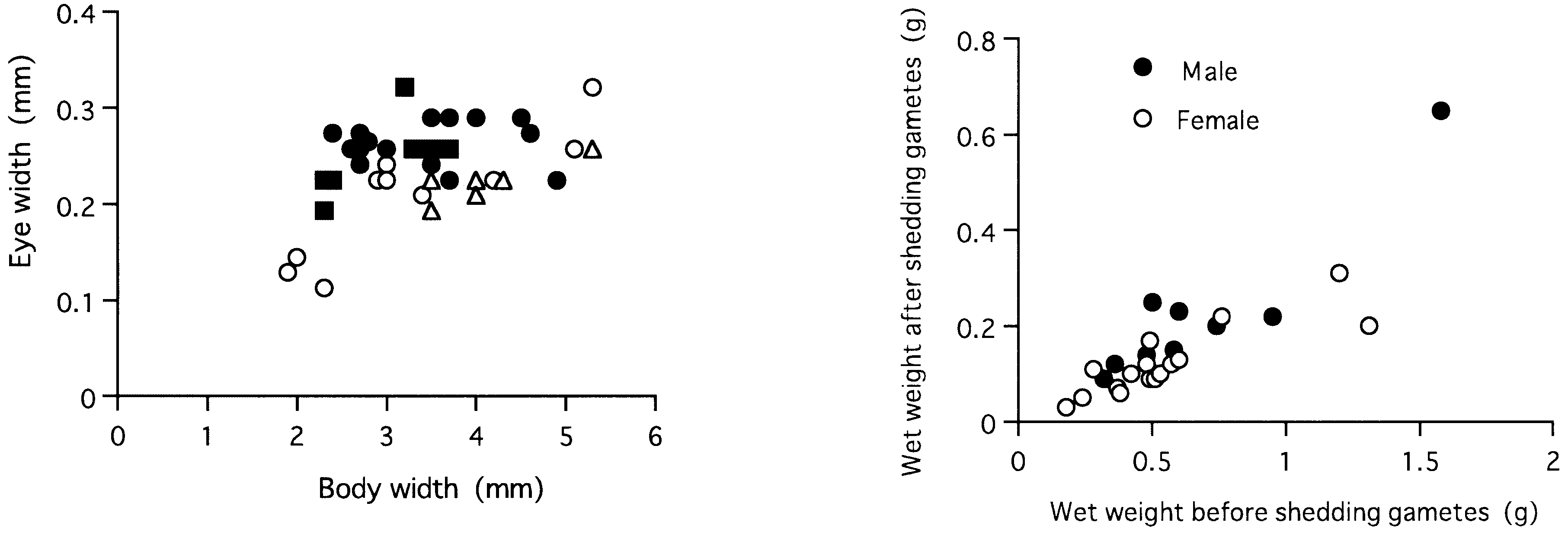
Eye width ( EW mm) was correlated with BW according to the following regression formula in immature specimens: EW = 0.04 BW + 0.09 (r 2 = 0.57, P = 0.0001, n = 18) ( Fig. 24 View Figure 24 ). EW (0.19–0.32 mm) of mature adults (n = 24) was not significantly correlated with BW (r = 0.26, P = 0.2), but significantly larger than EW (0.11–0.26 mm) of immature specimens (n = 13) with corresponding BW of 2.3–4.9 mm (Mann–Whitney U - test, P = 0.0006).
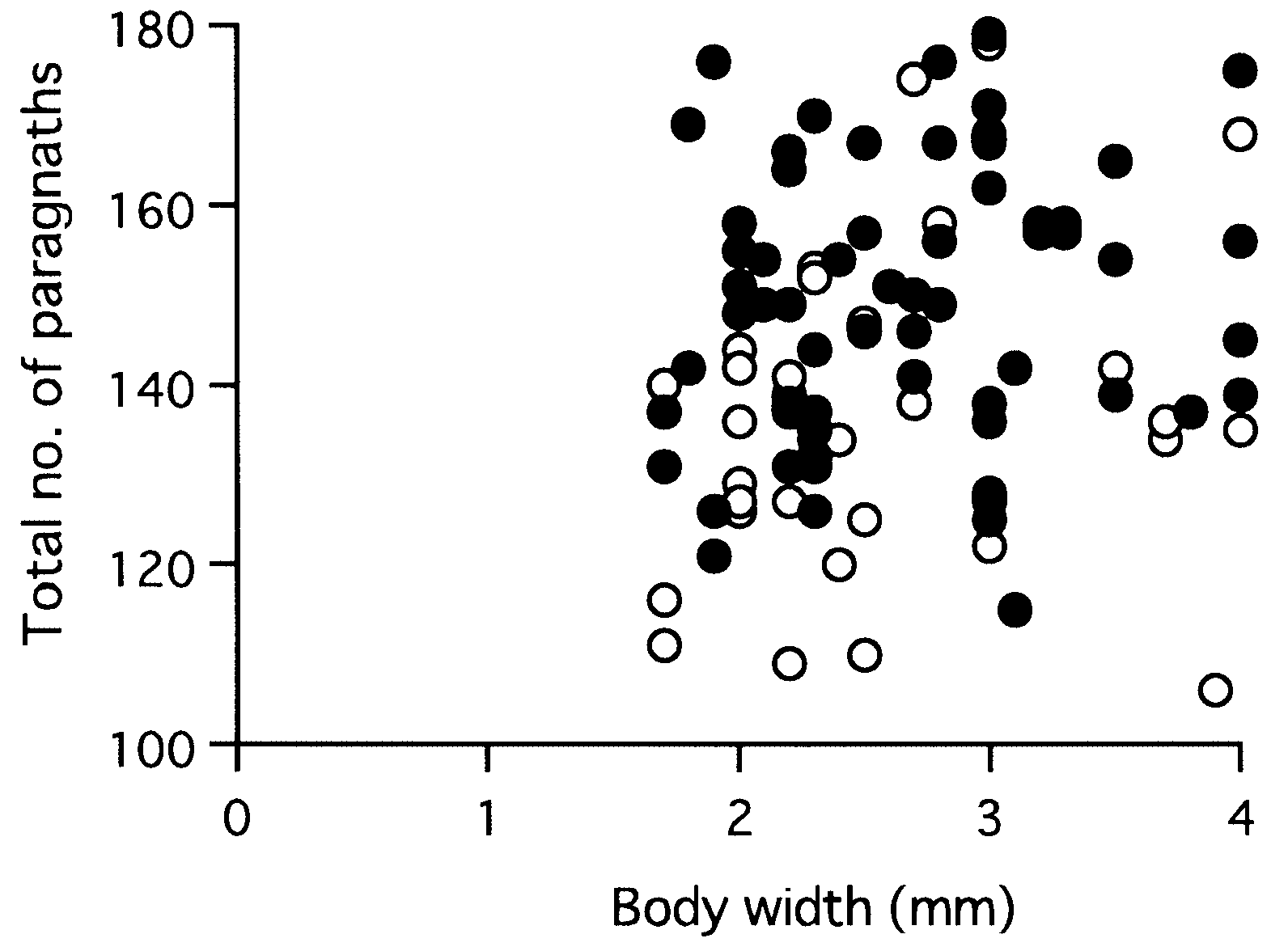
Total paragnath number was not significantly correlated with body size in mature adults collected from a single locality, i.e. Omoigawa River (r = 0.1, P = 0.3, n = 64), and Nagatagawa River (r = 0.2, P = 0.3, n = 35) ( Fig. 25 View Figure 25 ).
Variation of paragnath number
Paragnath numbers in mature (10 localities) and immature specimens (5 localities) are summarized in Table 4. Karyotype and sex determination
Diploid chromosome number was 28, including a pair of heteromorphic sex chromosomes ( Sato & Ikeda, 1992). The sex seemed to be determined by a simple system of male heterogamy, i.e. an XX–XY system, where the Y submetacentric chromosome was larger than the X. A sex ratio of almost 1: 1 was observed ( Sato, 1999). The autosomes consisted of 9 metacentric pairs including 3 distinctly larger pairs and 4 submetacentric pairs.
Reproduction
Reproductive swarming occurred during the cold season, winter or early spring, i.e. January to April, except for June in Hokkaido, northern Japan. Many epitokous males and females simultaneously swam up to the surface just after high-tide at night during the spring tides, and were transported downstream by the ebb tide ( Kagawa, 1955; Sato & Tsuchiya, 1987; Sato, 1999). Eggs or sperm were shed into seawater around the river mouth, where fertilization occurred. After spawning, the adults died. The wet weight of the adult body after spawning reduced to 15–50% of that before spawning (average ± SD: 32.9 ± 0.09% in 9 males, 22.7 ± 0.07% in 16 females) ( Fig. 26). Fecundity (entire number of eggs produced by a female) ranged from over 10 000 to 1 million eggs ( Sato, 1999).
Development
The full-grown oocytes were 130–170 Mm in diameter ( Sato & Tsuchiya, 1991; Sato, 1999). The ooplasm was relatively transparent and contained 20–40 oil drops around a germinal vesicle. The spermatozoa had a cone-shaped acrosome at the tip of the head ( Sato, 1999). The sperm head was about 3 Mm wide, about 4 Mm long, and angular at the base of acrosome. The gamete ultrastructure and early events of fertilization are described in Sato & Osanai (1986) and Sato (1999). In early development, a larval planktonic phase was present during the trochophore, metatrochophore and nectochaeta stages ( Kagawa, 1955; Sato & Tsuchiya, 1991). Salinity approaching full-strength seawater (more than 20‰) was essential for early development. The nectochaeta larvae of the 6-setiger stage, which seemed to gain a tolerance to lower salinity, settled into brackish waters of the adult habitat, moving upstream on rising tides about 1 month after fertilization ( Kagawa, 1955).
Habitat and life history
Adults and juveniles showed euryhaline distributions in estuaries; they lived within burrows mainly in sandy or muddy tidal flats, commonly coexisting with H. atoka sp. nov. along the Japanese coast (excepting that of the Ariake Sea, Kyushu) and coexisting with H. japonica in the Omutagawa River where it flowed into the Ariake Sea. The life-cycle is migratory and diadromous, planktonic (embryos and larvae) in the sea and benthic (juveniles and adults) in brackish waters ( Sato, 1999). This seemed to result in a frequent gene flow from river to river via the sea, and consequently in the lower level of genetic differentiation among geographically separated populations ( Sato & Masuda, 1997).
The life-span of 1 year was suggested by a monthly population survey in China ( Qiu & Wu, 1993).
Geographical distribution
The coasts of Japan and China ( Fig. 27 View Figure 27 ).
Etymology
The specific name refers to the diadromous life history of this species.
Remarks
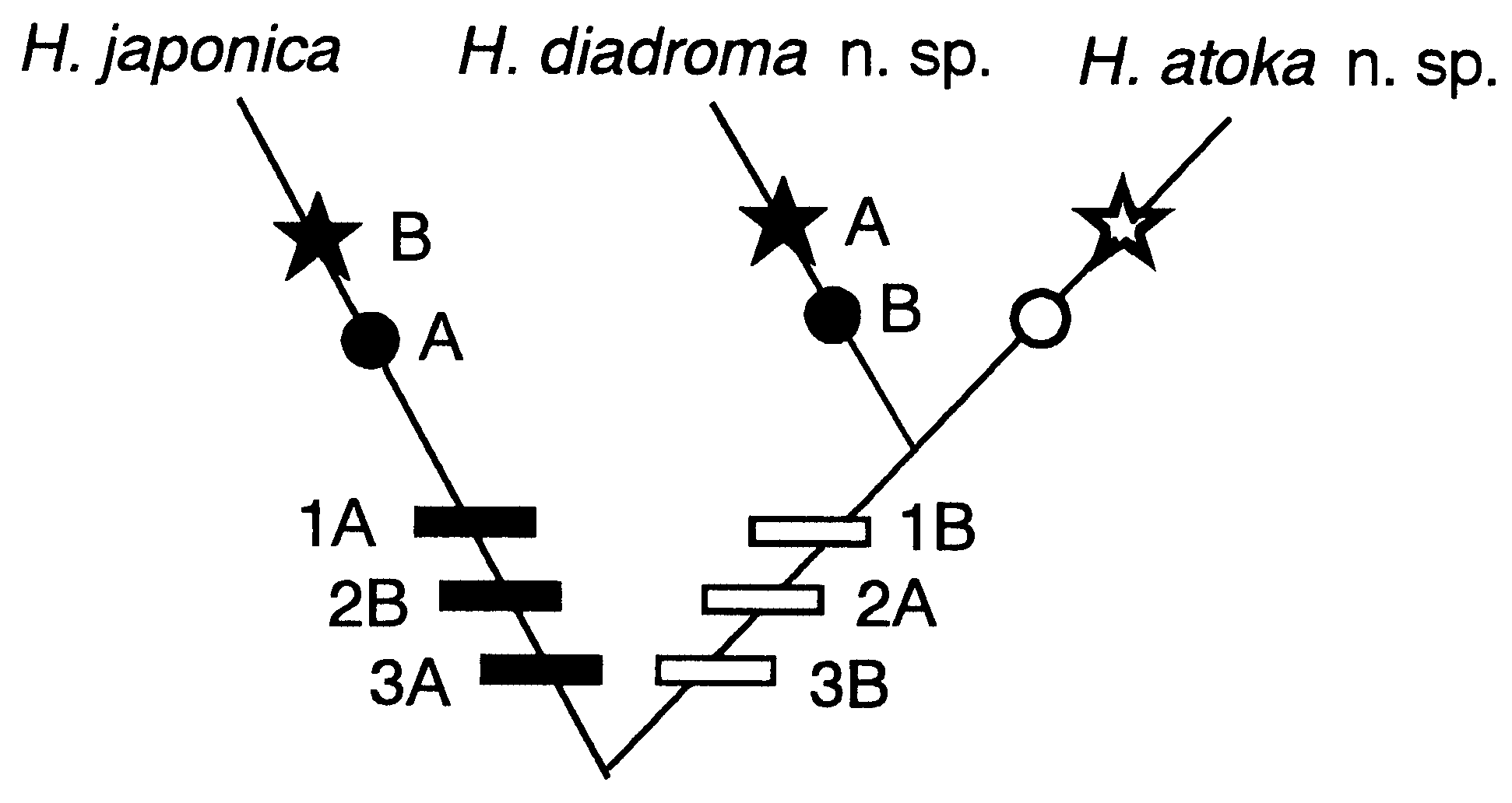
Though Hediste diadroma sp. nov. is very similar to H. atoka sp. nov., they are distinguishable at the mature stage due to the occurrence of epitokous metamorphosis, with the addition of epitoke-specific spinigers and a smaller egg size in H. diadroma sp. nov. They are morphologically indistinguishable at the immature stage. The difference in paragnath numbers in the maxillary ring of the proboscis is significant between the two species of sympatric populations, but cannot strictly distinguish them (see below). However, they are clearly distinguishable by complete allele substitution at several allozyme loci, which is detectable by electrophoretic analysis ( Sato & Masuda, 1997). The electrophoretic pattern of LDH is available as a diagnostic character for identification of immature worms ( Fig. 10 View Figure 10 ).
Nereis japonica sensu Kagawa (1955) and Okada (1960), and Neanthes japonica sensu Sun et al. (1980) View in CoL and Qiu & Wu (1993), seem to be identical to H. diadroma View in CoL sp. nov. because of the occurrence of reproductive swarming and a mature-egg diameter of less than 180 Mm.
| V |
Royal British Columbia Museum - Herbarium |
| VI |
Mykotektet, National Veterinary Institute |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Hediste diadroma
| Sato, Masanori & Nakashima, Akiyuki 2003 |
Hediste japonica: Sato, 2001: 66–86
| Sato M 2001: 86 |
Neanthes japonica:
| Qiu J & Wu B 1993: 360 |
| Sato M & Osanai K 1986: 263 |
| Sun R & Wu B & Yang D 1980: 110 |
Nereis japonica
| Okada K 1960: 63 |
| Kagawa Y 1955: 11 |