Pogonosternum Jeekel, 1965
|
publication ID |
https://doi.org/ 10.5852/ejt.2017.259 |
|
publication LSID |
lsid:zoobank.org:pub:DCD1D671-B95C-4E10-8BC5-2352F25C0D1E |
|
DOI |
https://doi.org/10.5281/zenodo.3796550 |
|
persistent identifier |
https://treatment.plazi.org/id/42783846-E75E-935C-9A17-9FFAFAA274D8 |
|
treatment provided by |
Carolina |
|
scientific name |
Pogonosternum Jeekel, 1965 |
| status |
|
Genus Pogonosternum Jeekel, 1965 View in CoL
Fig. 1 View Fig
Type species: Strongylosoma nigrovirgatum Carl, 1902 View in CoL , by original designation.
Diagnosis
A small to moderate-sized antichiropodine genus (1.5–2.8 cm) with 1–2 broad, yellowish or yellowishwhite longitudinal stripes on dorsum contrasting with more or less dark brown ground; with 20 body rings, each smooth, waist distinct between prozonite and metazonite, pore formula 5, 7, 9, 10, 12, 13, 15–19; paranota poorly developed on anterior rings, absent on posterior rings; small bean-shaped area present behind each antennal socket; male with femoral process (= adenostyle) on legpair 1 and tarsal and tibial brushes from legpair 1 to legpairs 7–12 (variable); ventrolateral hook-like process on anal valves.
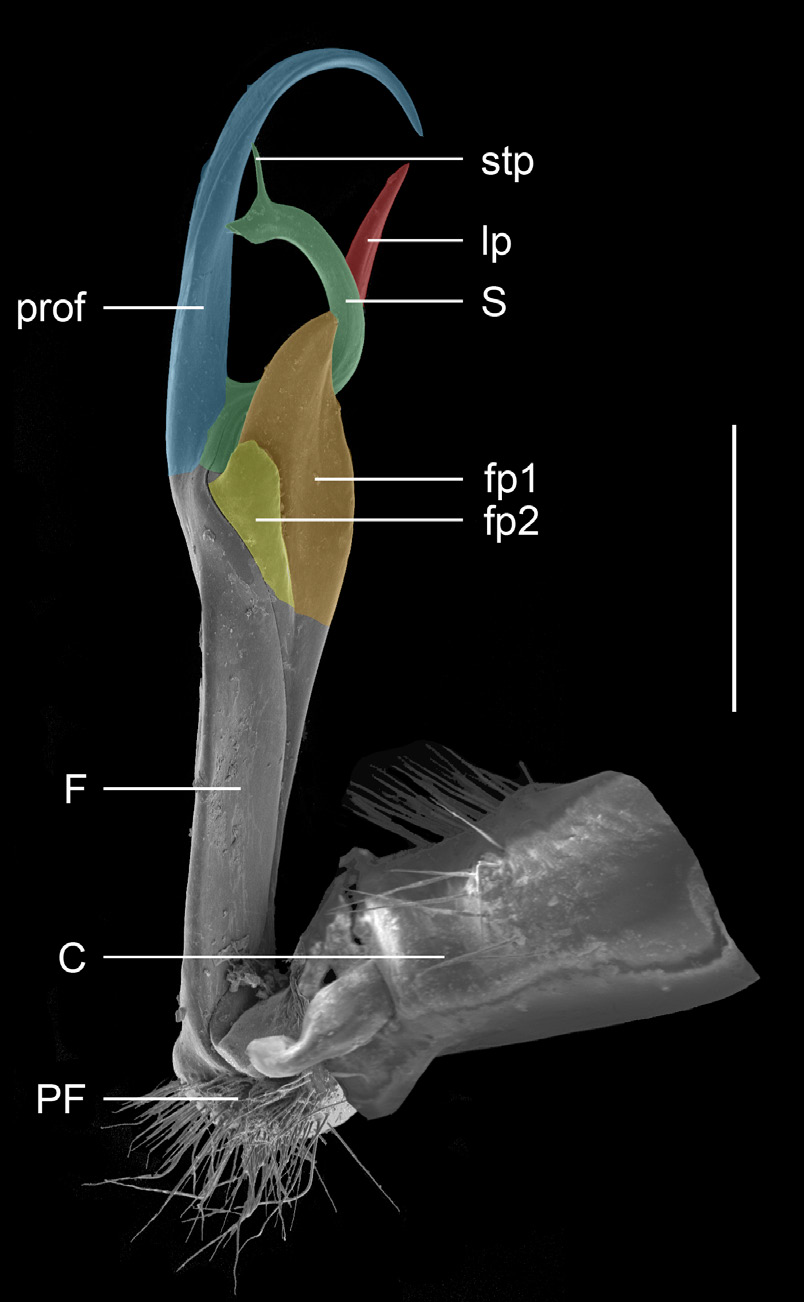
The genus is best defined by the gonopod structure ( Fig. 1 View Fig ). All Pogonosternum species have an anterior laminate femoral process 1 (fp1) with a second adjacent smaller femoral process 2 (fp2). A large prolongation of the femorite (prof) bears close to its base a lateral femoral sub-process (lp), slightly distal to the anteriorly arising solenomere (S). The solenomere is long, laminate and nearly semicircular with a single slender solenomere tip process (stp) directed apically.
A similarly curving solenomere is present in the antichiropodinae genera Antichiropus Attems, 1911 , Helicopodosoma Verhoeff, 1924 and Notodesmus Chamberlin, 1920 , but, apart from Antichiropus , a prolongation of the femorite (prof) is absent or only poorly developed in these genera. Antichiropus species differ in having a longer, nearly circular solenomere with a process on its inner surface one third of the distance to the apex and often additional solenomere processes.
In the field, Pogonosternum species are easily distinguished from other paradoxosomatid species in southeastern Australia by their distinctive colour pattern and lack of midbody paranota.
Description
The members of the genus Pogonosternum are very homogeneous in their morphology, although some non-gonopod characters can be used in combination with gonopod features to distinguish species: body length, colouration, distribution of male leg brushes, coxal processes on second legpair of females and form of anterior spiracles.
The following general description applies to all species if not otherwise mentioned in the text or descriptions of species.
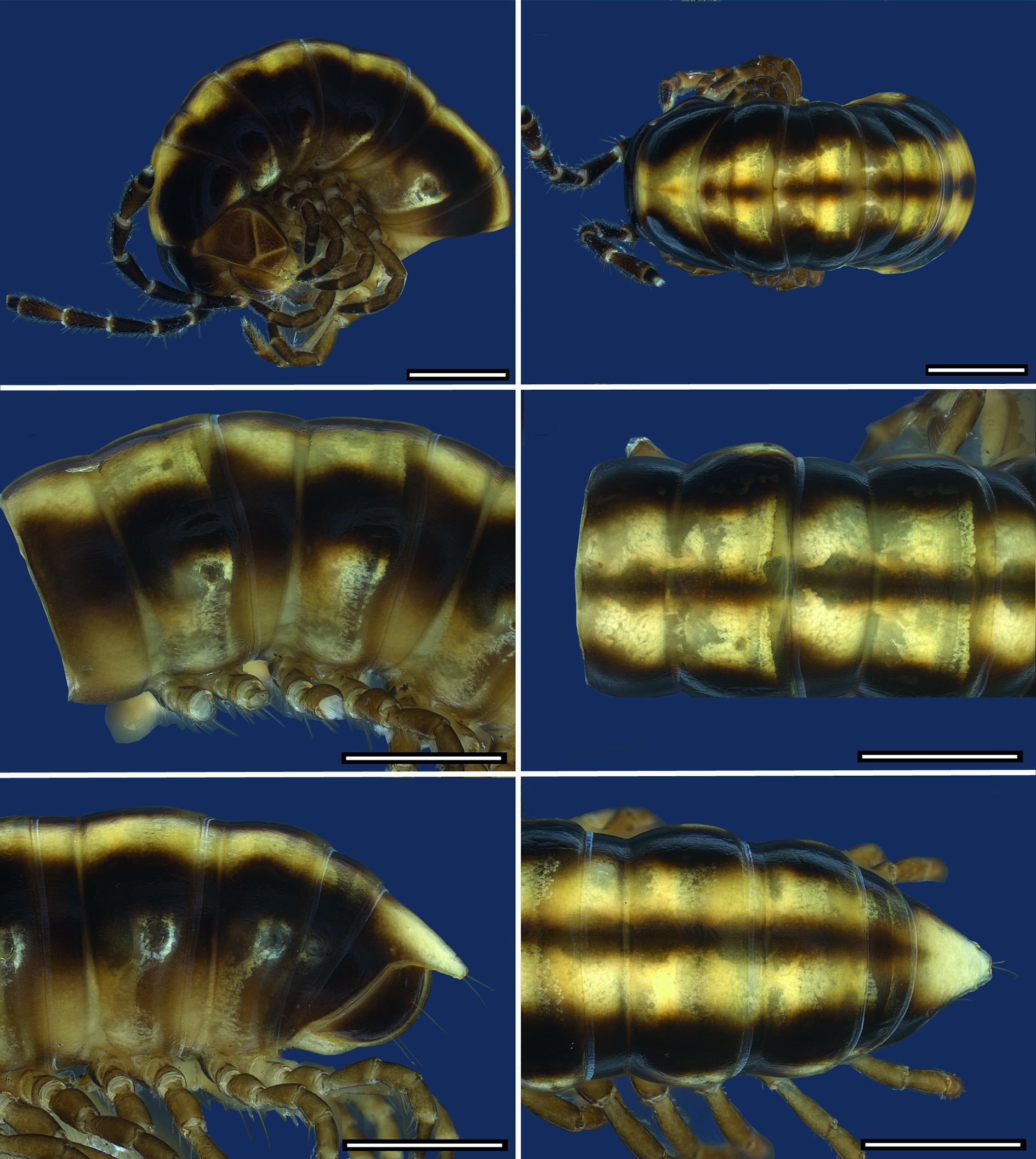
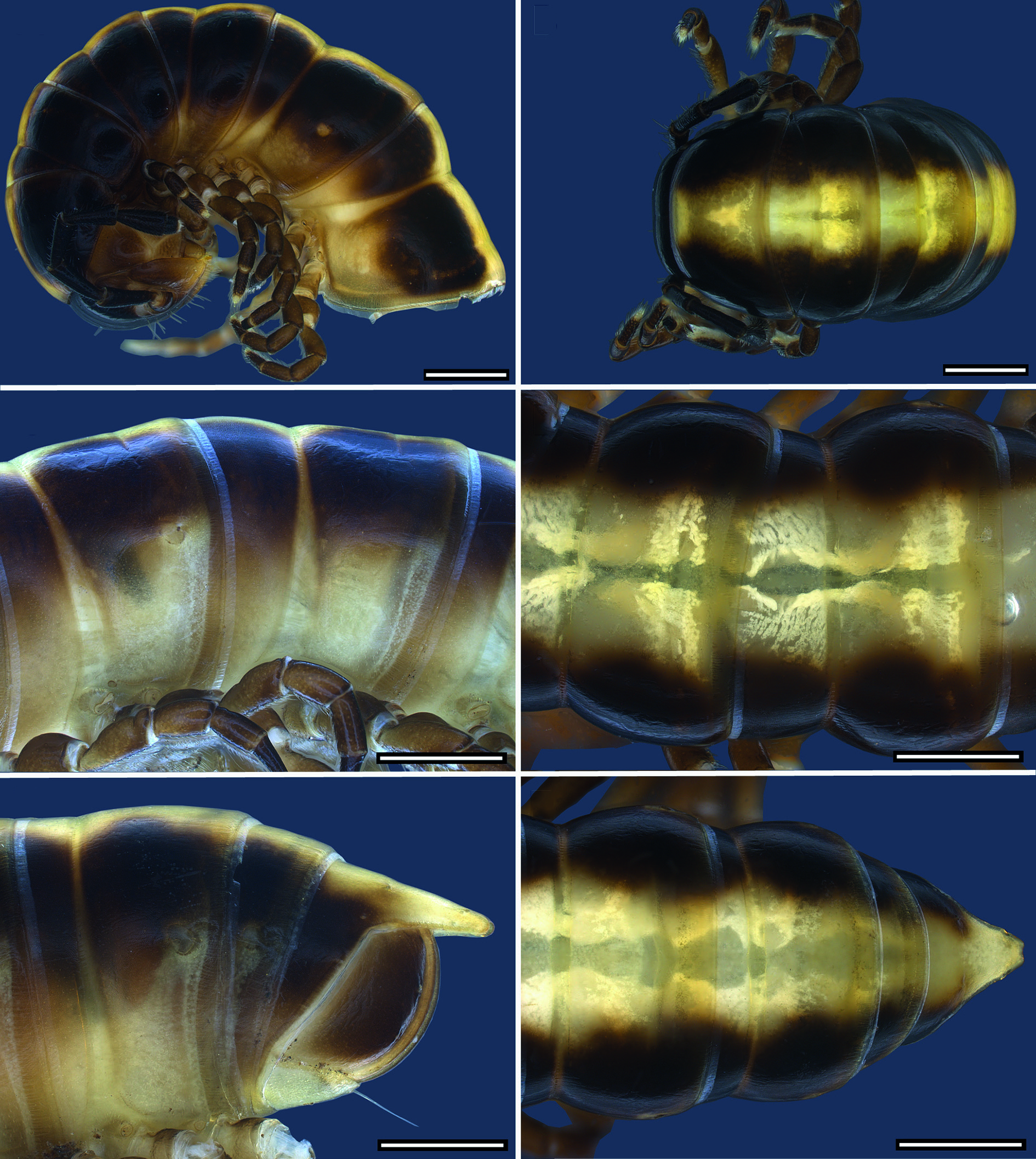
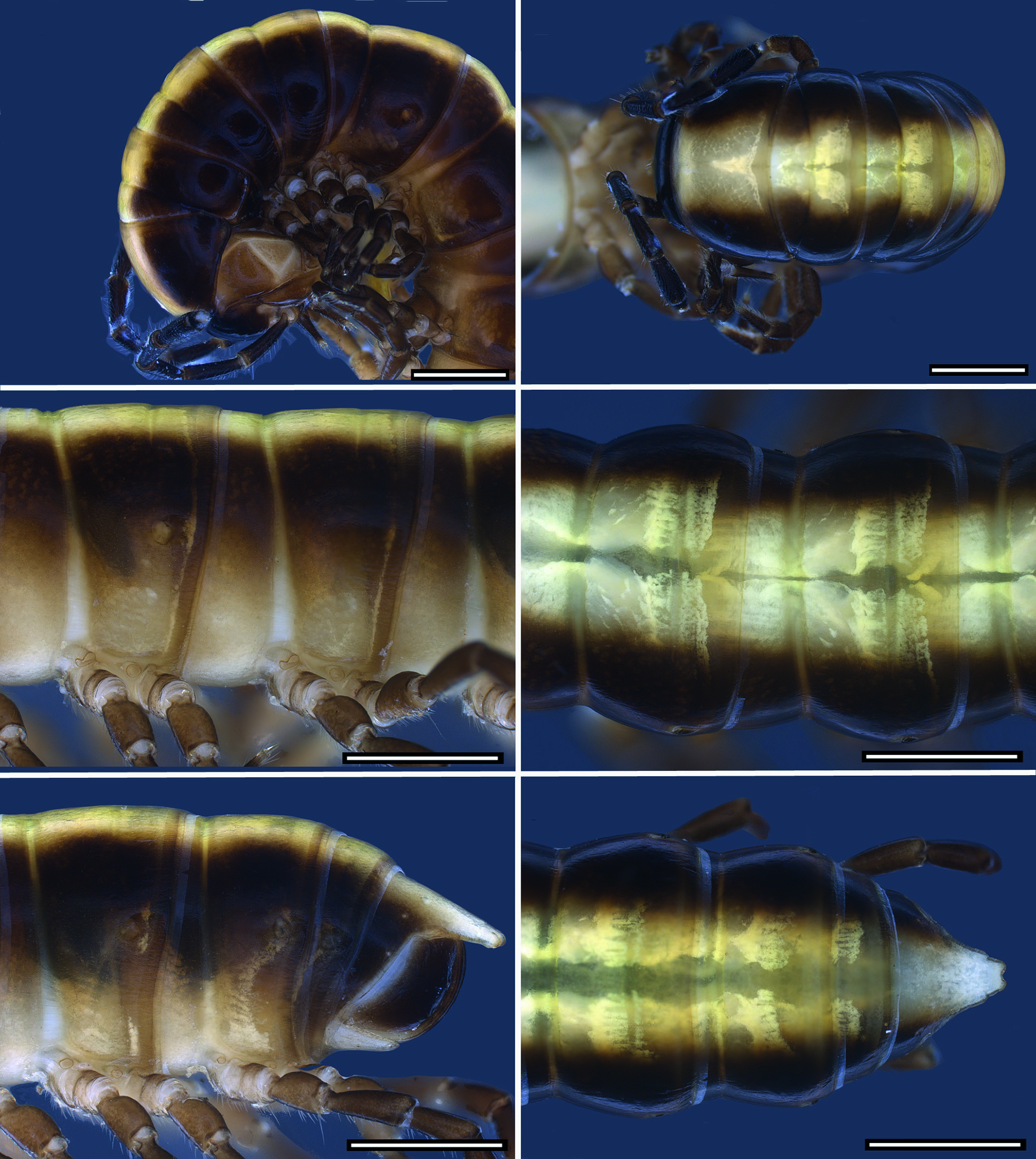
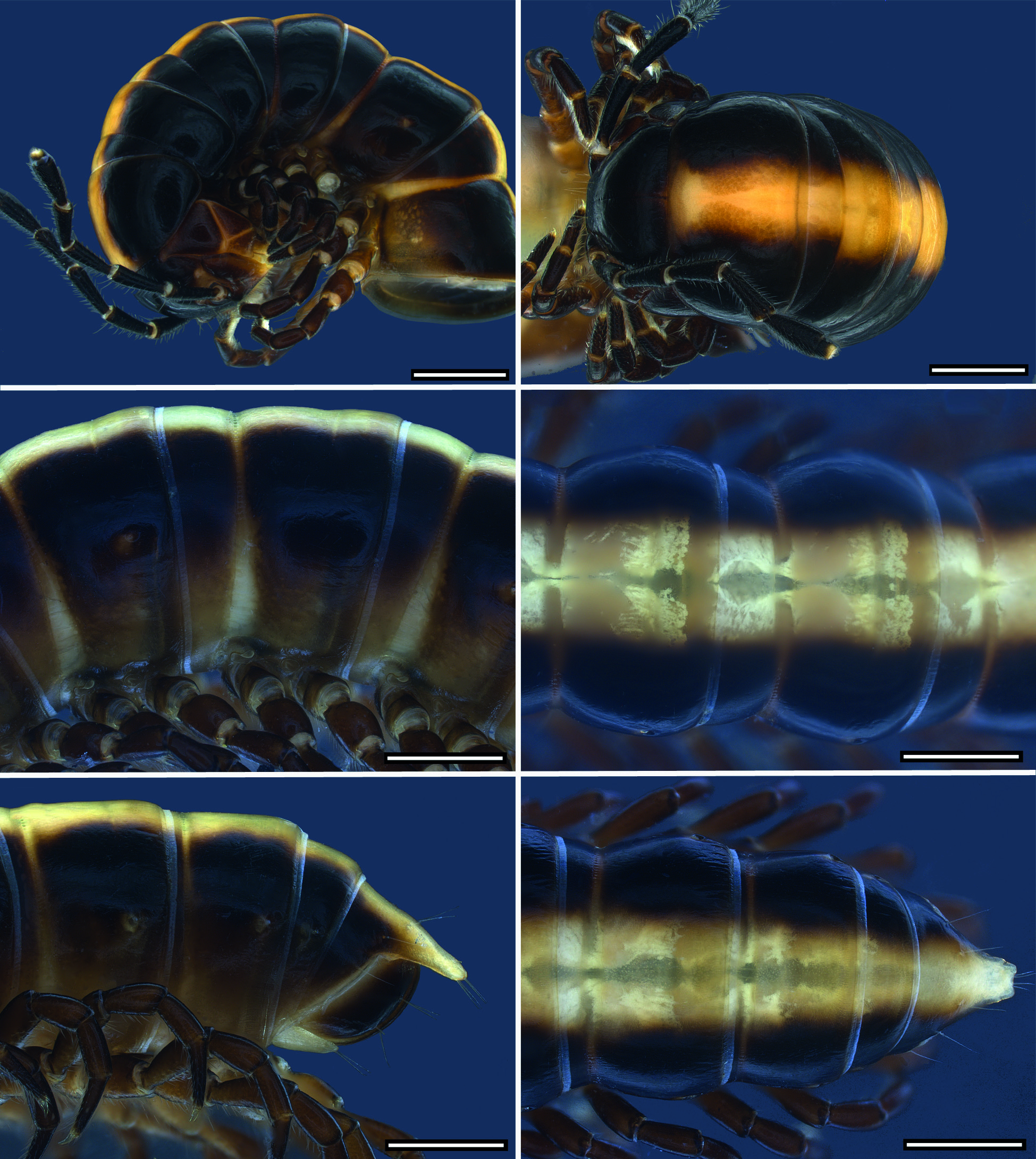
COLOURATION. Colour in fresh material ( Figs 8 View Fig , 12 View Fig , 15 View Fig , 18 View Fig , 22 View Fig , 26 View Fig ): head blackish brown, lateral side, labrum and behind antennal sockets chestnut brown. Antennae blackish brown; distal margins of antennomeres, basal antennomere and base of setae lighter. Legs chestnut brown, tarsus darker. Collum blackish brown with median brownish-yellowish goblet-shaped stripe narrowing in anterior 1/3 of length from anterior margin. Dorsum with one or two contrasting light yellowish brown, broad, longitudinal stripes reaching from collum to epiproct. Flanks and area around ozopores more or less lighter. Anal ring blackish brown with light yellowish brown median stripe, epiproct entirely light brown to pale. Colour stripe sometimes absent from anal ring and only epiproct lighter. Margin of anal ring and anal valves lighter. Hypoproct pale.
G
ONOPOD MORPHOLOGY. Parts of the telopodite are named following Car et al. (2013), with differing terms used by Jeekel (1965, 1982a) given in brackets ( Fig. 1 View Fig ). Coxite = C; femorite = F; femoral process = fp1 (femoral process, process f), fp2 (lobe at transition between femur and postfemur, process a); lateral process = lp (postfemoral process, process c); prefemorite = PF; prolongation of femorite = prof (tibiotarsus, process b); solenomere = S (process k); solenomere tip process = stp.
Femorite (F) moderately long, moderately slender or slightly enlarged distally. Femoral process 1 (fp1) arising from anterior apical part of translucent fringe of femorite, in most species developed as moderate to large subtriangular process. Femoral process 2 (fp2) arising mesad-posteriad to femoral process 1 (fp1) and, except in P. adrianae , often much smaller than fp1 or greatly reduced, as in P. laetificum . Long prolongation of femorite (prof) arising posteriorly.Above base of prof a slender lateral process (lp), except in P. montanum Decker , sp. nov. where it is on mesal side and arises distal to the solenomere. Semicircular solenomere (S) arising anteromesally above lp, with a single slender solenomere tip process (stp).
The solenomere with its stp is the only non-
varying feature of the telopodite in the known species of Pogonosternum . While intraspecific morphological variability of gonopods in P. adrianae and P. montanum Decker , sp. nov. is low, various local forms and intermediate states of gonopod characters have been found in P. nigrovirgatum , P. laetificum and P. jeekeli Decker , sp. nov. As shown by Decker (2016 a), the distribution of these variations does not correlate clearly with geographical distribution or phylogenetics.
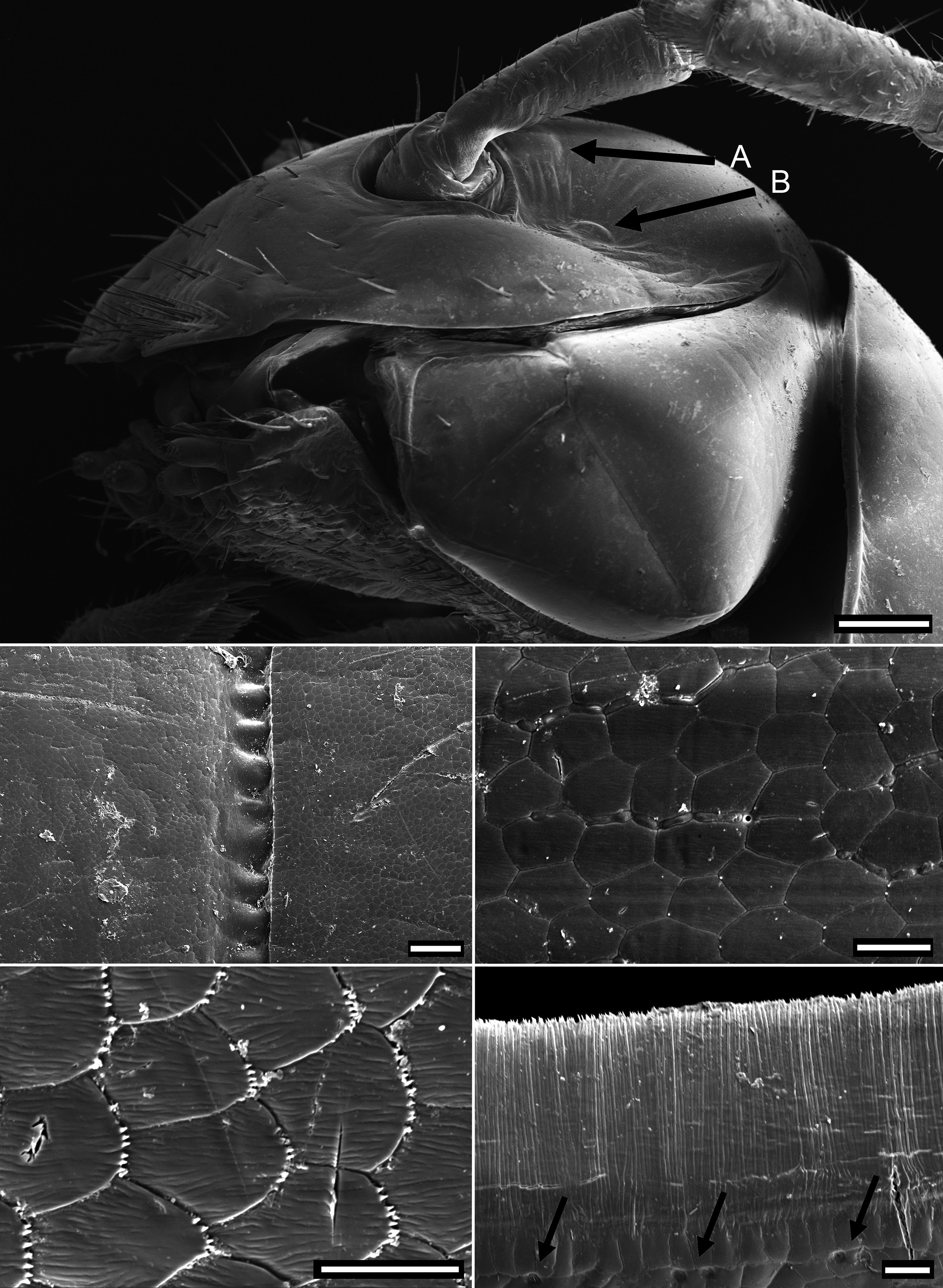
HEAD. Slightly broader than collum. Frons and vertex sparsely setose, clypeus moderately setose, more dense on labrum. Vertigial sulcus well-defined, starting slightly above level of antennal sockets. Postantennal grove moderately wide and deep, the wall in front not conspicuously prominent. Antenna reaching dorsally when stretched to ring 3. Antennomeres slender, nonclavate, antennomeres 2–6 with equal lengths. Antennomere pubescence moderately dense, denser and longer distally. A slightly swollen and lighter circular area, called the bean-shaped area by Jeekel (1982 a), behind antennal sockets and a slightly swollen and lighter circular area in a depression within the postantennal groove ( Fig. 2A View Fig ). The two structures may represent sense organs such as the Tömösváry organ or intracerebral photoreceptors (= accessory lateral eyes), which have not yet been studied in Polydesmida (see Müller & Sombke 2015).
BODY RINGS. Adults with 20 body rings, each smooth. In width, head <collum> ring 2> 3> 4 = 5 <6–16; thereafter body gradually tapering. Collum in dorsal view with anterior margin nearly straight and posterior margin scarcely emarginated; laterally rounded with distinct emargination and a short anterolateral shallow dent. Diplosegments with pronounced
waist, suture moderately deep and strongly corrugated ( Fig. 2B View Fig ). Slightly irregular longitudinal striation sometimes present ventrolaterally. Metatergite with smooth and transverse sulcus from 5 th to 17 th ring, hardly traceable in last two rings. No setae present on metatergites. Paranota only present on rings 2 and 3, sometimes also on ring 4, as ridge with dorsal furrow caudally curving upwards, on subsequent rings only indicated by irregular striation. In females, pleurites of ring 2 with small lobiform toothed lappet directed caudally. Opening of ozopores on lateral side of metazonite at about two-thirds of metazonite length, each lying inside a shallow round pit. Area around ozopores only very slightly swollen; pore formula 5, 7, 9, 10, 12, 13, 15–19. Prozonite and metazonite surface with smooth cellular structure at higher magnification. Micro-scales and pores (= micro-scutes, Akkari & Enghoff 2011) present on metazonites, especially on metatergites and towards posterior margin ( Fig. 2C View Fig ), ventrally cells with dentation ( Fig. 2D View Fig ). Limbus long, lamellar with microdentate fringe arising from a row of subrectangular cells. Single cells of second cell row anterior to limbus anterolaterally with two micropores, between these cells, 3–6 cells without micro-pores ( Fig. 2E View Fig ).
Sternites a little wider than long, transverse depressions deeper than longitudinal, moderate and long setation, directed mostly posteroventrally. Rounded-trapezoidal lamella between anterior legpair on ring 5 ( Fig. 3 View Fig ). No significant variation of shape of lamella was observed within or between species. Small to prominent cones near base of anterior and midbody legs pointing ventrad are only present within local populations of P. nigrovirgatum .
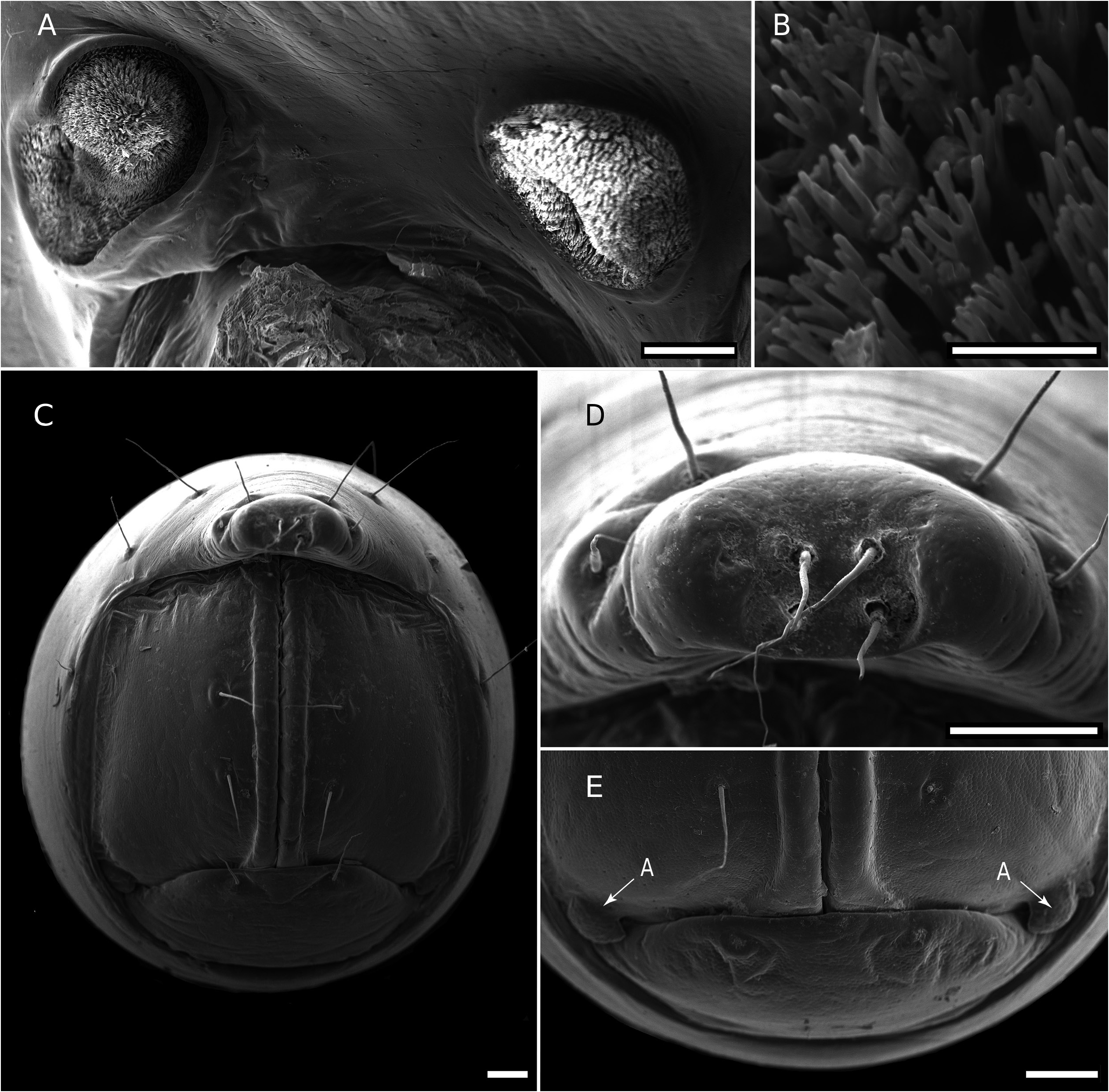
SPIRACLES. Well-separated on diplosegments ( Fig. 4A View Fig ). Anterior spiracle located anterodorsally of anterior leg coxa, posterior spiracle located relatively more anteriorly above posterior leg. Spiracular rim a more or less raised wall. Spiracular opening filled with spiracular filter composed of numerous distally bifurcated lamellae ( Fig. 4B View Fig ); filter can be U-folded or twisted, emergent above rim or not. Anterior spiracles ovoid, rim raised and often with an anterodorsal extension. Posterior spiracle ovoid to subtriangular, rim low without conspicuous extensions.
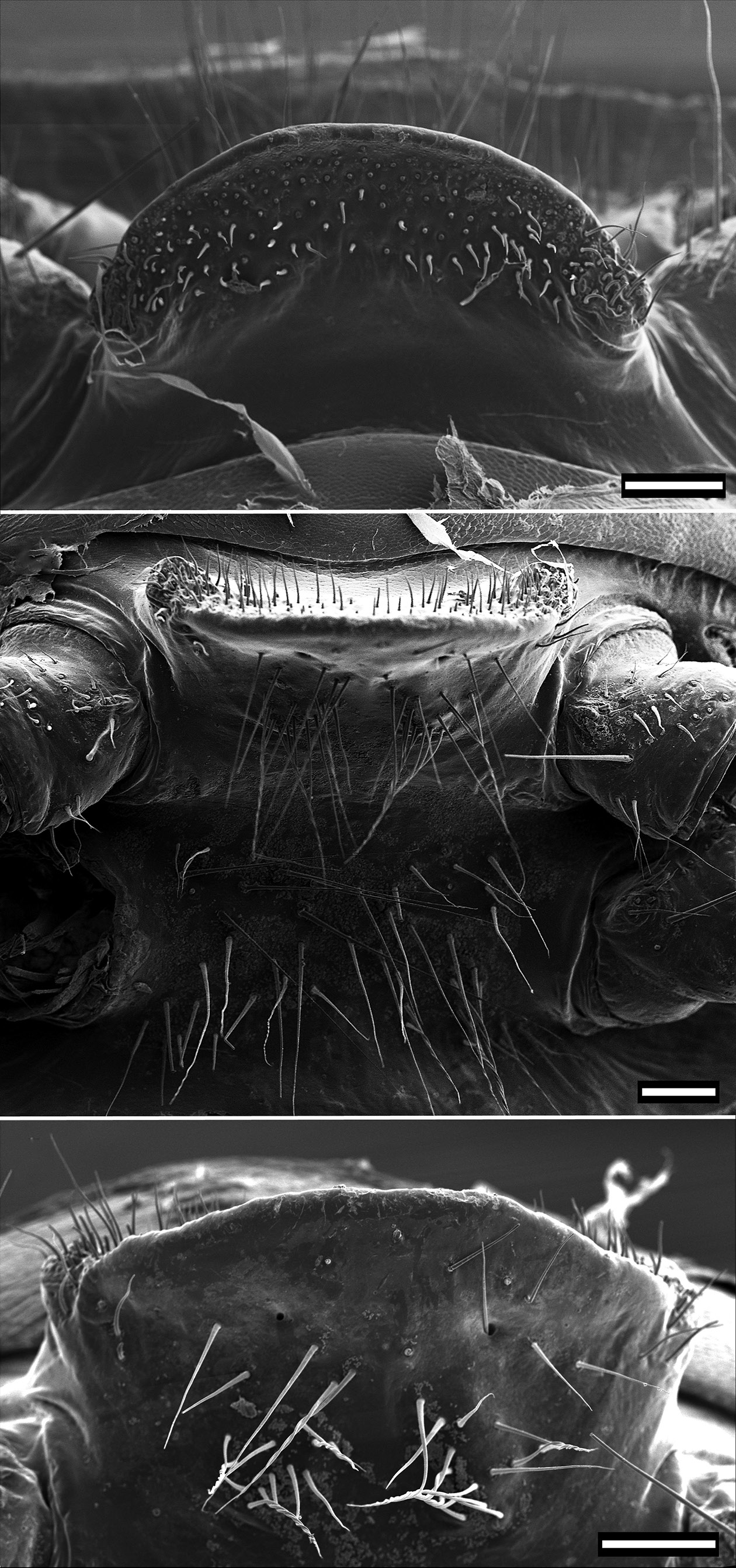
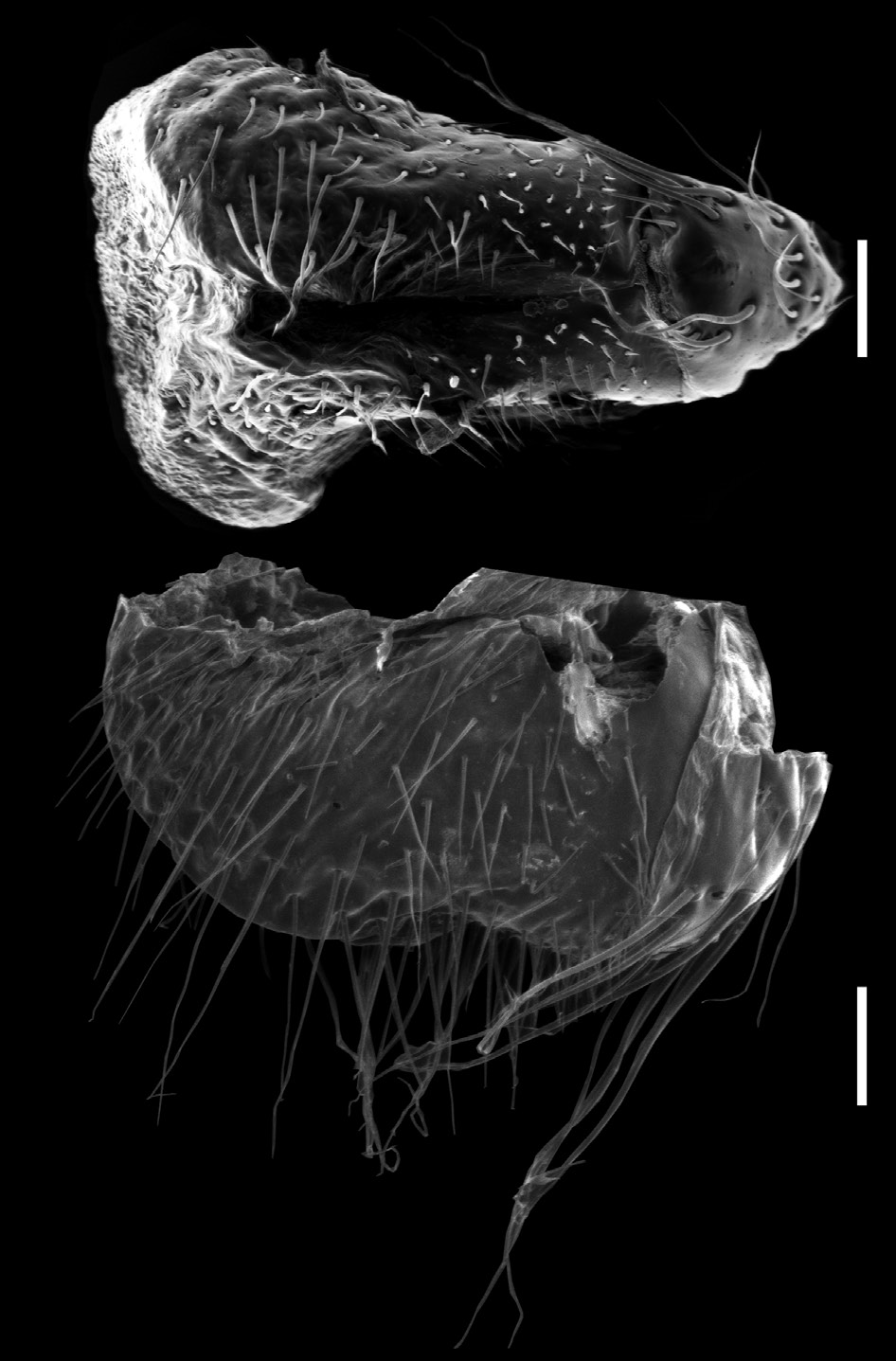
TELSON. With two dorsolateral setae and one lateral seta in one line on each side with slightly produced papillae ( Fig. 4C View Fig ). Epiproct with tip truncate, slightly curving downwards, lateral and dorsal setal papillae moderately produced. Spinnerets located in smooth depression, arranged in trapezoid with dorsal setae slightly closer together than ventral. A more or less distinct rounded notch located lateral to ventral row of spinnerets. Individual spinneret seta with short single-walled sleeve ( Fig. 4D View Fig ). Anal valves each with two long setae and raised margin, with broad uncinate process on lateroventral portion of anal valves. Hypoproct subtrapezoidal with two long setae and slight depression on laterodorsal portion ( Fig. 4E View Fig ).
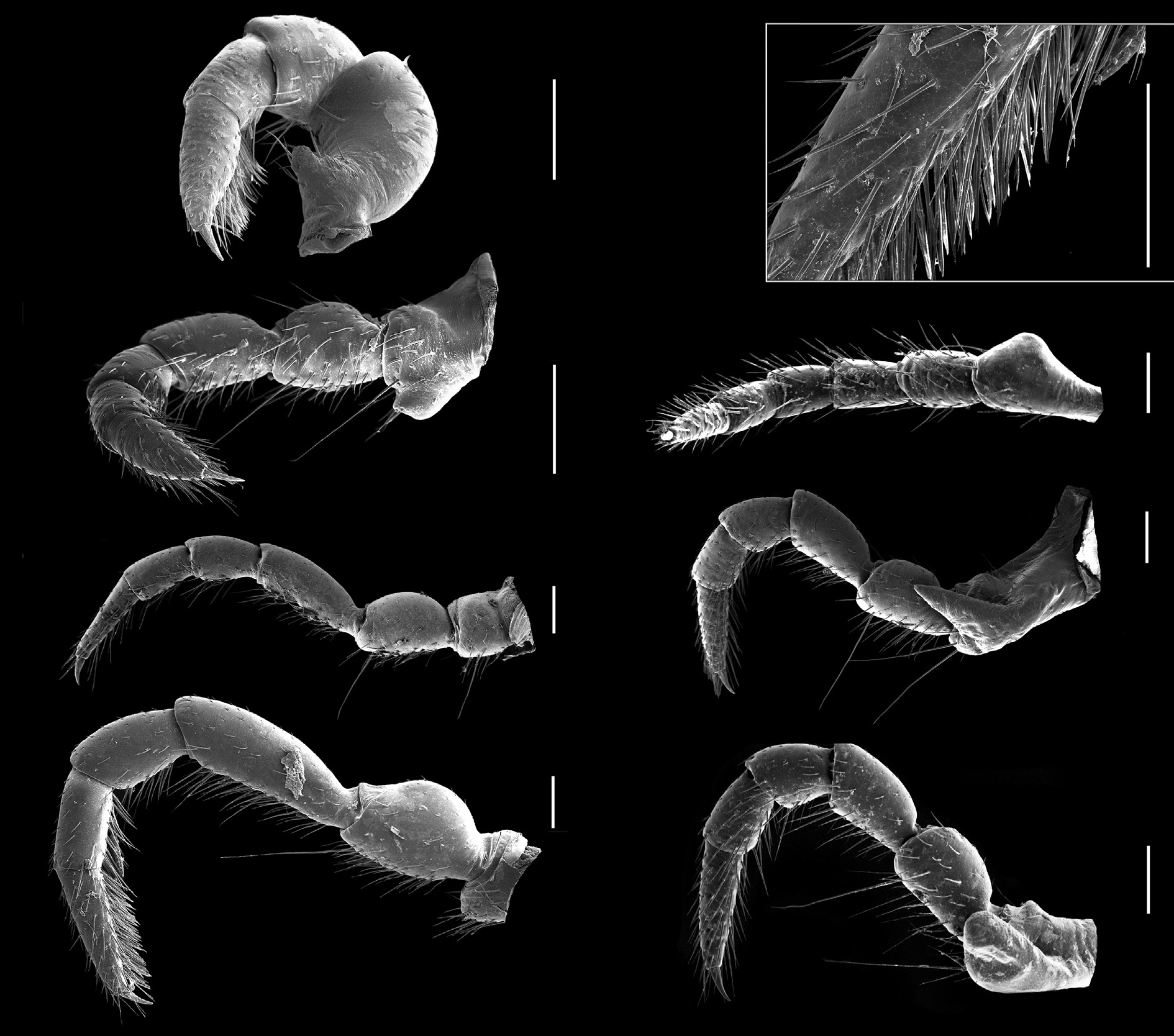
LEGS. Of moderate length extending laterally and easily visible from above.All species of Pogonosternum have similarly shaped legs. Male legpair 1 with well-developed ventral adenostyle on femur ( Fig. 5A View Fig ). Coxa of legpair 2 with posteroventrally directed conical process bearing laterally a set of setae and the gonopore opening at tip of process ( Fig. 5B View Fig ). Dense setal pads or brushes on tarsus and tibia of males present from legpair 1 to legpair 7–12 ( Fig. 5 View Fig D–E), thereafter gradually thinning out or abruptly absent. Posterior-most walking legs without dense brushes ( Fig. 5C View Fig ). In female, legs are slightly shorter than in male. Coxa of legpair 2 with prominent swelling on posterior side ( Fig. 5F View Fig ) especially, distinct processes in P. montanum sp. nov. and P. adrianae ( Fig. 5 View Fig G–H). VULVA. Subtriangular, widest posteriorly ( Fig. 6 View Fig ). Numerous setae on inner and outer valves, longest ventrally and posteriorly. Operculum with 3–4 long and 2–3 small setae on each side ventrally. Above 9–12 moderate long setae. No significant interspecific differences were observed, and both shorter and longer setae on valves can occur within a species.
Distribution
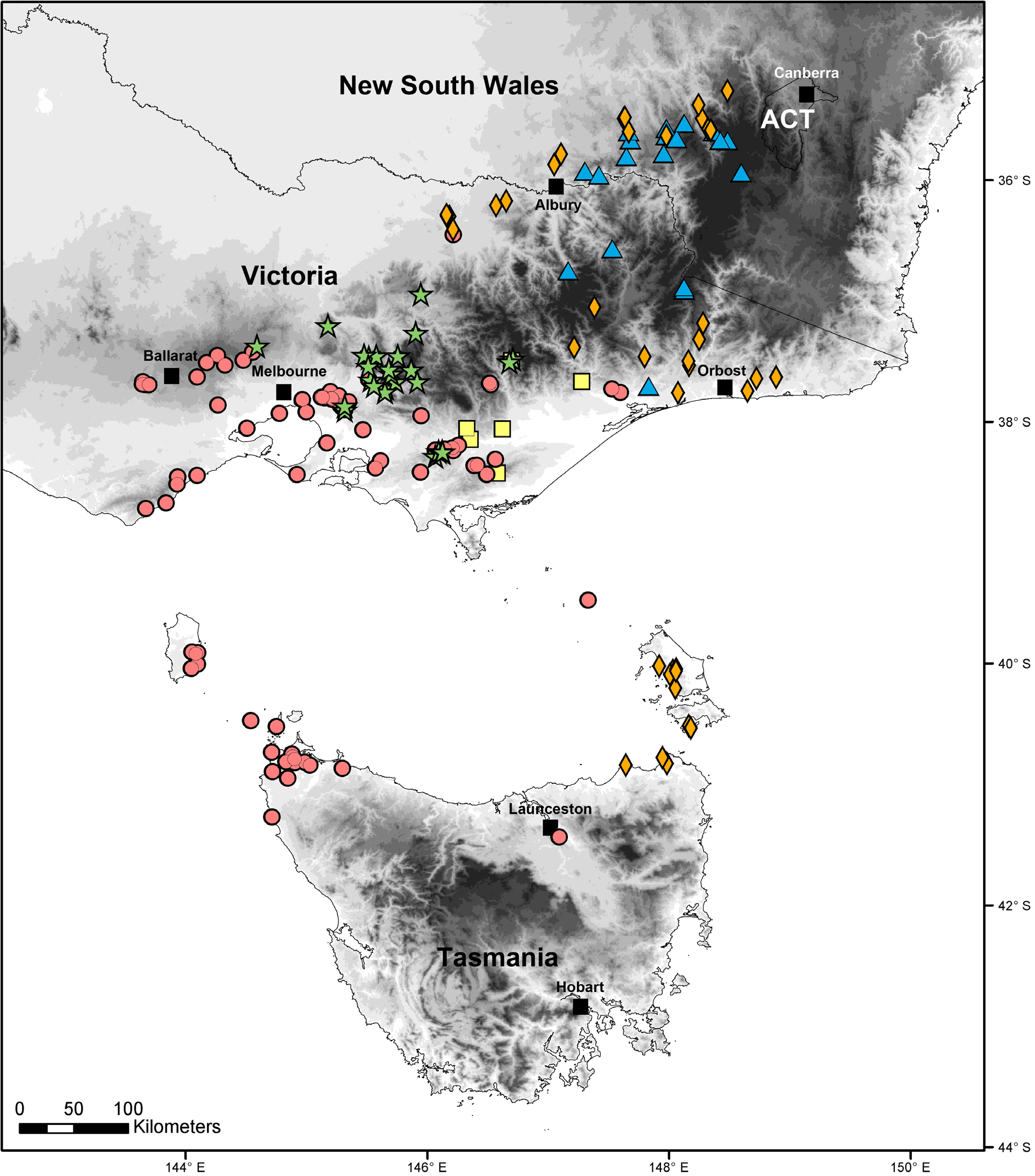
On the Australian mainland, the genus
Pogonosternum has its main distribution area
in eastern, northeastern and central Victoria and
southeastern New South Wales ( Fig. 7 View Fig ).
Pogonosternum has not yet been found in far
Eastern Gippsland or west of the Otway Ranges
and the Ballarat area. Like the paradoxosomatid
species Dicranogonus pix Jeekel, 1982 and Notodesmus scotius Chamberlin, 1920 ( Mesibov 2014) , Pogonosternum nigrovirgatum and P. jeekeli sp. nov. have ranges extending from Victoria through the Bass Strait Islands to the Tasmanian mainland. None of the known Pogonosternum species can be regarded as a short-range endemic.
Notes on ecology and biology
Adults and juveniles (mostly subadults) of Pogonosternum are present on the surface in the cold or cool and rainy winter months, with a mating and activity peak between July and September. The genus can be found from near sea level ( P. nigrovirgatum , P. jeekeli sp. nov.) to 1110 m a.s.l. ( P. montanum sp. nov. in Kosciuszko National Park) and mostly inhabits forests, but P. jeekeli Decker , sp. nov. can also be found in coastal heathland in northeastern Tasmania ( Mesibov & Churchill 2003), whereas P. jeekeli sp. nov. and P. montanum sp. nov. also occur in pine plantations ( Car 2010). Pogonosternum species were preferably recorded from moist, but not wet, leaf litter. In some cases, specimens were found under loose bark on the forest floor and in a few cases, adult males were observed walking during the daytime on gravel roads.
Relationships
According to Jeekel (1982a) the Western Australian genus Antichiropus Attems, 1911 or the Queensland genus Aulacoporus Verhoeff, 1924 is the nearest relative. Humphreys & Shear (1993) mentioned Pogonosternum as related to Antichiropus and Stygiochiropus Humphreys & Shear 1993 . Jeekel (2006) suggested Howeosoma Jeekel, 2006 from Lord Howe Island as the nearest relative of Pogonosternum .
However, the gonopods of Pogonosternum are unique within Antichiropodini , and its relationships within Antichiropodini are unclear due to our lack of understanding of the homology of the different processes of the gonopod. Jeekel (1987: 11) even questioned the division of Australian Australiosomatinae into two tribes: “Although these two groups are maintained here, it must be emphasized that this division probably gives an oversimplified picture of the actual systematic relationship between the genera involved. With the recent discovery of more taxa, it becomes more and more obvious that the classification of the Australian Paradoxosomatidae is more complicated than formerly understood and needs a critical revision”. Future integrative phylogenetic studies covering the described and the many undescribed genera will increase our knowledge of relationships and the importance of gonopod characters.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |