Schizomyia botellus Dorchin and Freidberg
|
publication ID |
https://doi.org/10.5281/zenodo.203443 |
|
DOI |
https://doi.org/10.5281/zenodo.6194107 |
|
persistent identifier |
https://treatment.plazi.org/id/415887DF-2652-4238-FF14-FF4AFBA0F8AA |
|
treatment provided by |
Plazi |
|
scientific name |
Schizomyia botellus Dorchin and Freidberg |
| status |
sp. nov. |
Schizomyia botellus Dorchin and Freidberg View in CoL , new species
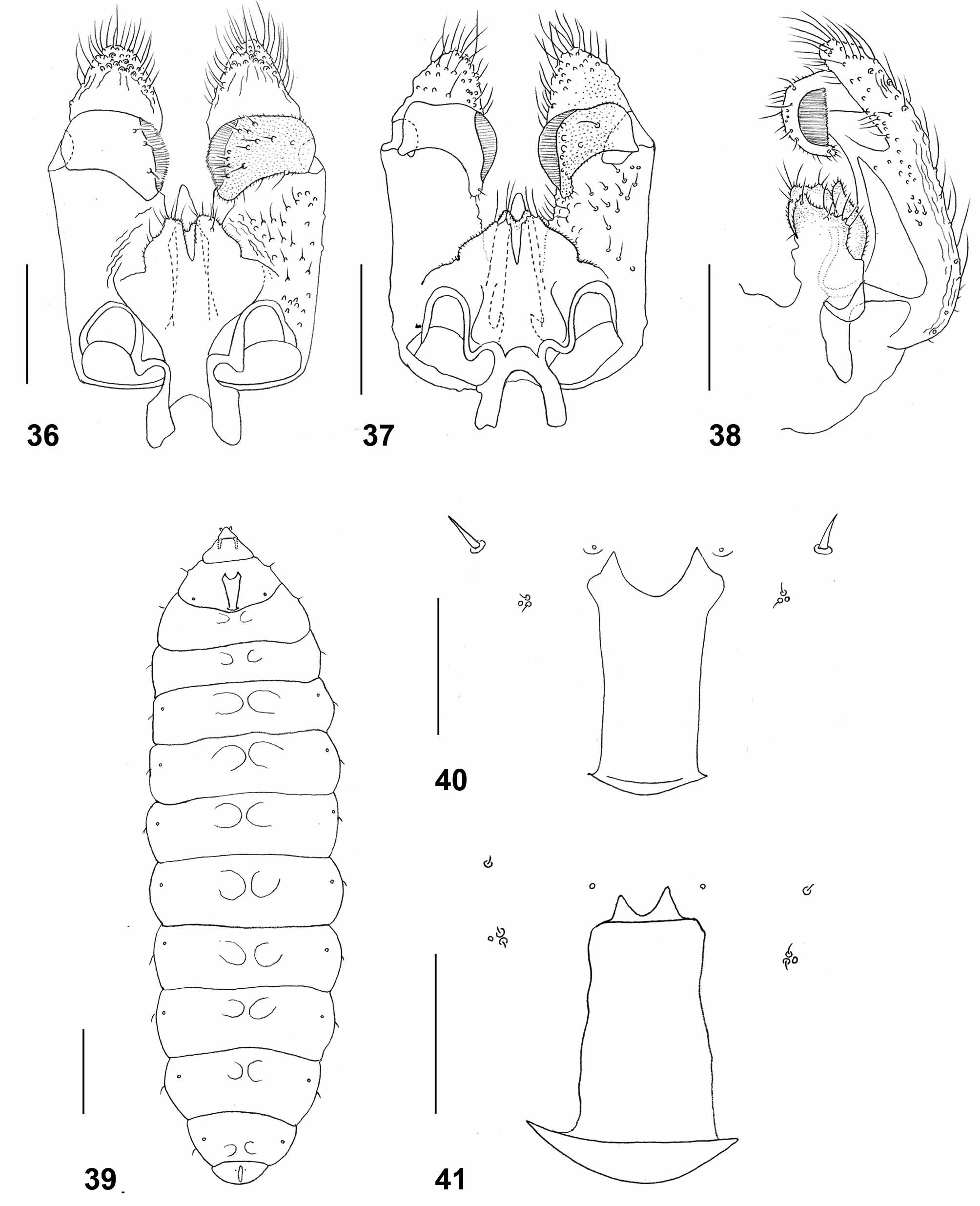
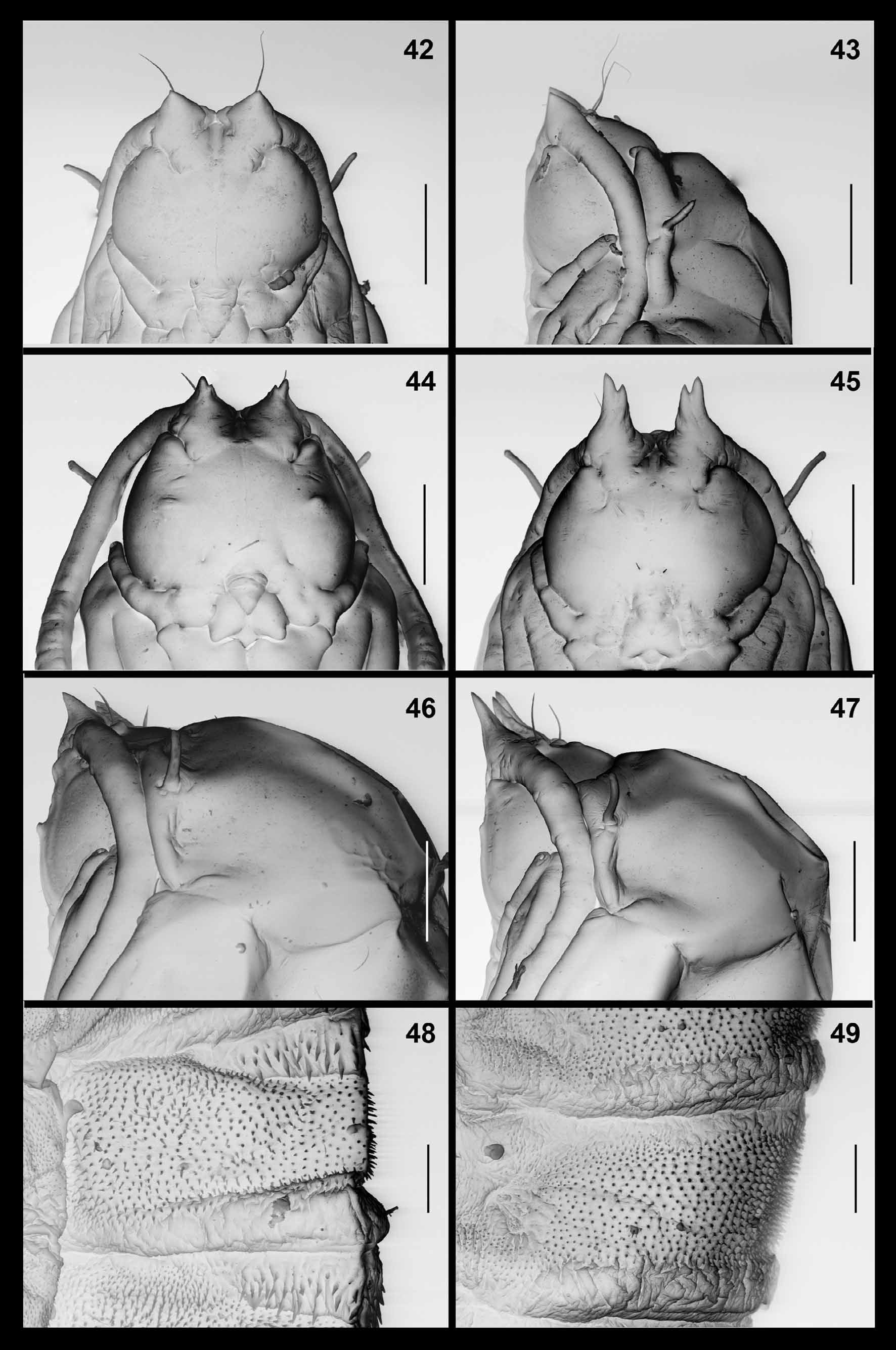
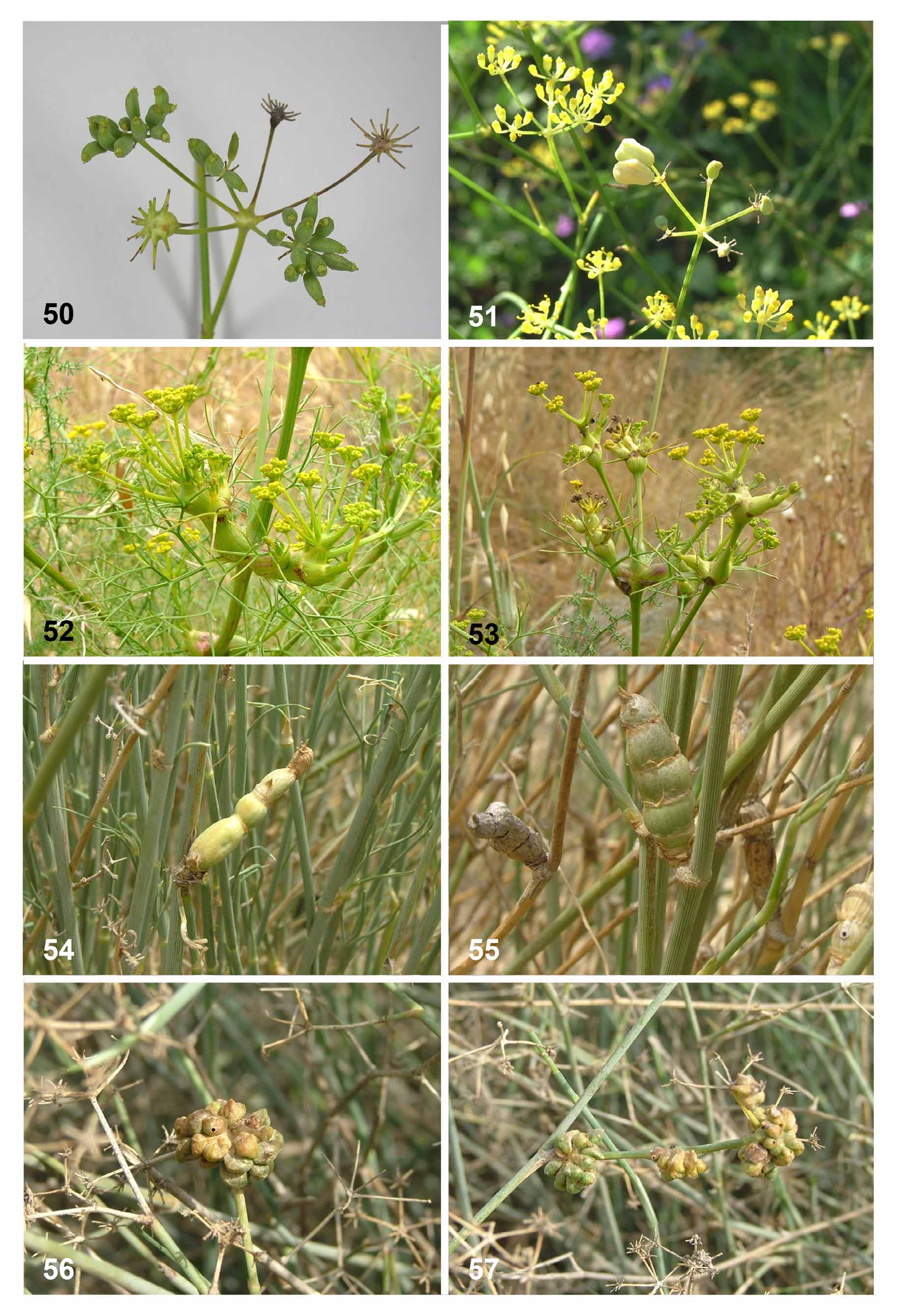
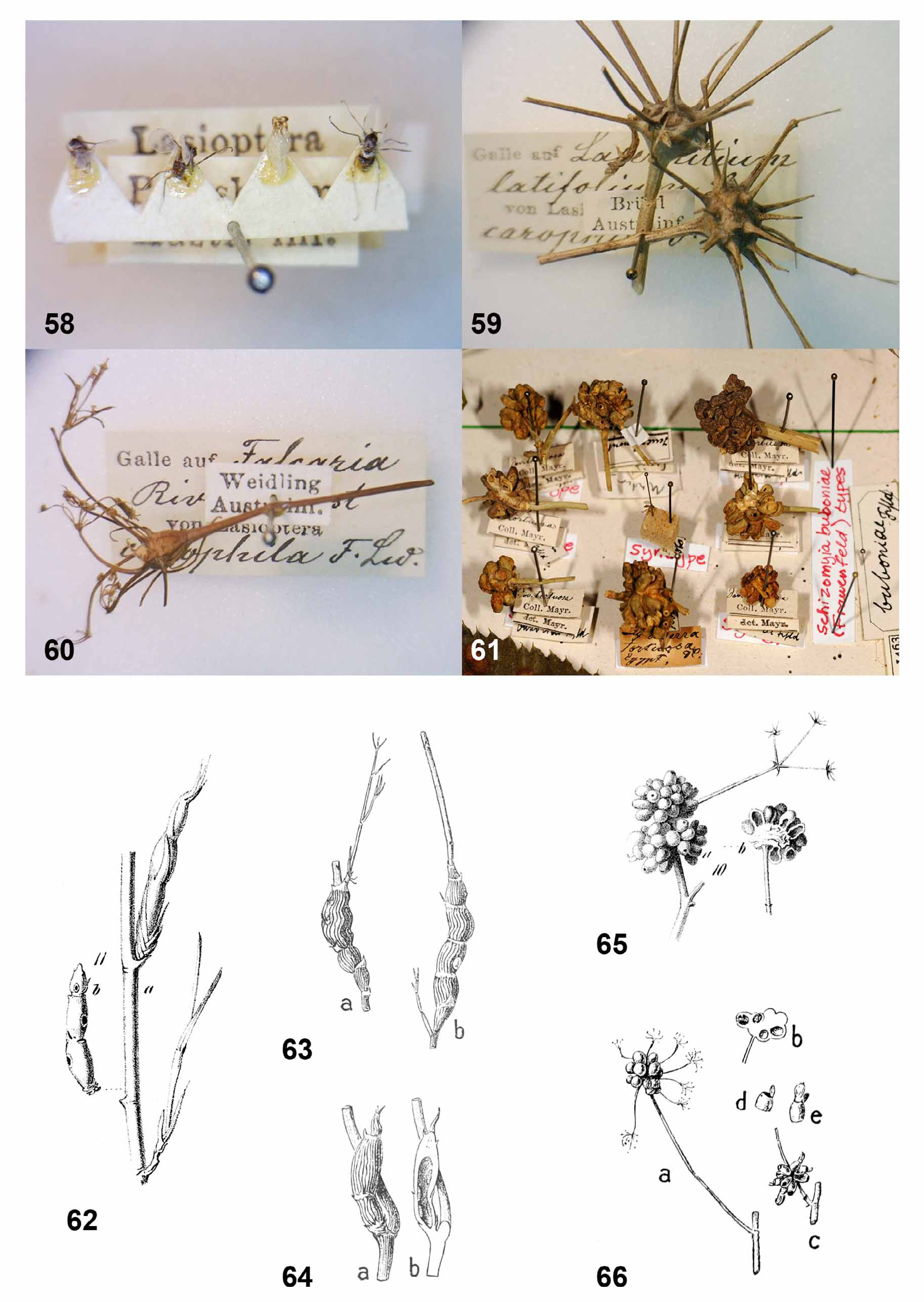
( Figs. 30–36, 39–40 View FIGURES 30 – 35 View FIGURES 36 – 41 , 44, 46, 48 View FIGURES 42 – 49 , 54–55 View FIGURES 50 – 57 , 62–64 View FIGURES 58 – 66 )
Adult: Head ( Fig. 30 View FIGURES 30 – 35 ): Eye facets circular to hexagonal. Gap between eyes on vertex 0-1 facet wide. Palpus 4-segmented, segments successively longer; first segment round, about as long as wide; consecutive segments much longer than wide. Labellum slightly tapered, with few long setae. Antenna: 12 flagellomeres in both sexes, covered by microtrichia. Female flagellomeres 1–9 cylindrical; flagellomeres 1–2 partially fused; flagellomeres 5 and on successively shorter; flagellomere 10 as long as wide; flagellomere 11 rounded, slightly wider than long; flagellomere 12 rudimentary, partially fused with preceding; flagellomeres 1–10 each with two whorls of anastomosing circumfila, strong setae posterior to basal whorl and between whorls ( Fig. 31 View FIGURES 30 – 35 ); segments 11–12 with only one whorl of circumfila. Male flagellomeres cylindrical; flagellomeres 1–2 longer than succeeding, flagellomeres 3–11 about the same length; flagellomere 12 slightly shorter than preceding, rounded apically; all flagellomeres covered by spacious interconnected whorls of winding circumfila and bear relatively few strong setae mostly at flagellomere bases ( Fig. 32 View FIGURES 30 – 35 ).
Thorax: General color gray, covered by sparse, white hair. Wing: hyaline; length 2.26–2.70 mm in female (n=15), 2.04–2.52 mm in male (n=8); R1 joins C at about mid-length of wing, R5 joins C posterior to wing apex, M present as very weak fold, Cu forked. Haltere stalk orange, knob white. Legs: general color orange, covered by short black hairs dorsally, white hairs ventrally; coxae with long, sparse, white hairs; tarsal claws evenly curved, untoothed; empodia longer than bend in claws ( Fig. 33 View FIGURES 30 – 35 ).
Female abdomen ( Fig. 34 View FIGURES 30 – 35 ): General color brownish-orange; sclerites black; entire abdomen covered by sparse white hairs. Tergites 1–5 rectangular, less sclerotized anteriorly than elsewhere, with posterior and median rows of strong setae, and a pair of tiny sensory setae anteriorly; tergite 6 similar but more setose; tergite 7 longer than preceding, distal half covered by strong setae, proximal half with several shorter setae and pair of tiny sensory setae; tergite 8 shorter than preceding, with short, tapered projection posterioly, bare except for pair of anterior sensory setae. Sternites 2–6 each comprising two sclerites, anterior rectangular, with several strong setae, posterior narrow band-like, with row of strong setae; anterior sensory setae not visible. Sternite 7 at least three times as long as preceding, posterior part shovel-like, strongly sclerotized; posterior two thirds covered by long setae. Base of ovipositor posterior to sternite 7 forms setose invagination. Ovipositor curled inside abdomen, distal part needle-like, slightly sclerotized ventrally, with several short setae on and towards apex ( Fig. 35 View FIGURES 30 – 35 ).
Male abdomen: General color and hairiness as in female. Tergite 1 with narrow band-like sclerotized area medially, posterior row of long setae, and few long setae elsewhere; tergites 2–7 rectangular, with posterior row of long setae, several long setae mesally, and evenly scattered scales; tergite 8 hardly differentiated from surrounding membrane, with small amorphous sclerotized patch and no long setae. All tergites with pair of small, anterior sensory setae. Sternites 2–6 rectangular, with posterior row of long setae separated from anterior area of sternite by weakly sclerotized band; anterior area of sternite with several strong setae. Sternite 7 entire, without weakly sclerotized band and with more numerous, evenly scattered strong setae. Sternite 8 hardly differentiated from surrounding membrane, with small but strongly setose sclerotized patch. Anterior sensory setae not vsible. Terminalia ( Fig. 36 View FIGURES 36 – 41 ): Gonocoxal apodeme comprises two strongly sclerotized, wide lobes connected by concave bridge. Gonocoxite angular proximally, with long apical lobe extending far beyond gonostylus, ridged medially; most surface proximal to insertion of gonostylus covered by short, strong setae; apical lobe rounded apically, ridged and densely covered by long, strong setae especially around apex. Gonostylus stout, rectangular except for slight apical projection pointed anteriorly, evenly covered by microtrichia on both dorsal and ventral surfaces; dorsally with several strong setae mostly on distal half, ventrally with row of strong setae along apical denticles and group of strong setae along posterior margin; unfused denticles extend almost to tip of projection. Aedeagus tapered, notably longer than hypoproct and cerci. Hypoproct narrow rectangular, slightly widened just before notched apex; setulose, with few longer setae apically. Cerci separated by deep triangular notch, considerably wider than hypoproct, setulose throughout, jagged and strongly setose around apex.
Larva (third instar) ( Fig. 39 View FIGURES 36 – 41 ): Orange. length 2.6–3.2mm (n=6). Integument covered entirely by pointed spicules. Antennae about twice as long as wide. Cephalic apodemes about as long as head capsule. Spatula ( Fig. 40 View FIGURES 36 – 41 ) with wide shaft and two triangular anterior lobes widely separated. On each side of spatula one asetose sternal papilla and one group of three lateral papillae, two with short setae, one asetose. Pleural and dorsal papillae with strong setae. Thoracic segments 2–3 and all abdominal segments each with two small mesoventral bulges. Terminal segment with two tiny, barely perceptible papillae.
Pupa ( Figs. 44, 46, 48 View FIGURES 42 – 49 ): Antennal bases developed into prominent horns, each divided into two tips close to apex; arched along dorsal contour. Cephalic seta about as long as antennal horn, originating from elevated base. Frons on each side with pair of papillae at mid-posterior area, one with long seta, the other asetose and group of three tiny lateral papillae, two setose, one asetose. Mid facial area with conspicuous blunt bulge on each side. Prothoracic spiracle long and blunt; trachea ends at apex. Dorsal and lateral parts of abdominal segments ( Fig. 48 View FIGURES 42 – 49 ) each evenly covered by short, pointed spinules except for short crinkled posterior area and anterior area of striking, complex spines.
Holotype: 3, Israel, Mishor Paran, 17.iii.1995, N. Dorchin, reared from Deverra triradiata gall.
Paratypes: 2Ƥ, 23, same data as holotype, TAUI: 7Ƥ, 9 exuviae (on two microscope slides), Nahal Lavan 24.iii.2000, N. Dorchin (4Ƥ, exuviae TAUI, 3Ƥ ZFMK); 1Ƥ, 23, 6 larvae (on one microscope slide), Israel, Nahal Yamin, 8.iii.2010, A. Freidberg, TAUI; 13, 1Ƥ, Sede Boqer, 18.iii.2010, N. Dorchin and E. Morgulis, TAUI. All material from Deverra triradiata .
Distribution. Tunisia (Matmata), Egypt (Sinai, Wadi El Raha), Israel (Negev desert: Nahal Lavan, Mishor Paran, Nahal Paran, Nahal Yamin, Sede Boqer, Zomet Mash’abim, Nahal Raham).
Etymology. The species name is Latin for “small sausage”, a noun in apposition, with reference to the conspicuous jointed galls.
Biology. Galls of this species were recorded from Deverra scoparia (in Tunisia) and Deverra triradiata (in Egypt and Israel). The species is univoltine, with galls developing from late winter (February) to spring (April). Pupation takes place inside the gall and adults emerge from early March to mid April. Other life-history aspects are unknown, but eggs are presumably laid on the plant and first-instar larvae probably spend the summer in diapause until they become active the next winter. The galls ( Figs. 54–55 View FIGURES 50 – 57 , 62–64 View FIGURES 58 – 66 ) develop from axillary buds into elongate, jointed structures, 2–8 cm long, depending on the number and size of joints. They are light green, often with fine longitudinal striation, and are very conspicuous on the background of the virtually leafless stems. After adult emergence, the gall turns yellow and then gray when completely dry. Such old, dry galls remain on the plants for more than a year so that old and new galls are often found in spring side by side ( Fig. 55 View FIGURES 50 – 57 ). The gall is hollow ( Fig. 64 View FIGURES 58 – 66 b) and the spaces within joints are often connected to each other. Larvae feed gregariously inside the gall, several in each joint. A large gall may contain more than 20 larvae. Before pupation, the larvae prepare holes in the gall’s walls, which remain sealed by a thin layer of plant tissue through which the pupae will break out. The larvae then spin thin, transparent sheaths of silk that divide the space inside the gall into separate compartments in which they pupate with or without spinning a cocoon. The gall does not contain visible mycelia. Larvae can be heavily attacked by external hymenopteran parasitoids, each of which feeds on several larvae and may kill all the larvae in a large gall. Endoparasitoids were also observed.
Remarks. Frauenfeld (1859) found the galls of this species while on the Novara expedition, during which he also collected and described the closely related Schizomyia buboniae . He stated that he was unable to determine the identity of the host plant or rear the insects because the plant was too young, but his accurate description of fully developed galls and the mention of emergence holes suggest that the galls were already empty. This species has therefore been known so far only from its distinctive galls, which were beautifully illustrated by Frauenfeld (1859) ( Fig. 62 View FIGURES 58 – 66 ) and later by Houard (1912) ( Figs. 63-64 View FIGURES 58 – 66 ). The latter reported Deverra tortuosa as the host of Frauenfeld’s galls, but it is clear from Frauenfeld’s original drawing ( Fig. 62 View FIGURES 58 – 66 ) that the host was in fact D. triradiata (the two plant species differ strikingly). Houard (1912) found galls of variable sizes in Matmata ( Tunisia) and, like Frauenfeld, failed to rear the adults although he mentioned that the larvae pupated inside the galls. For some reason he found larvae only in smaller galls ( Fig. 64 View FIGURES 58 – 66 ), whereas larger galls ( Fig. 63 View FIGURES 58 – 66 ) were occupied by coccids, which no doubt invaded the galls once the original inhabitants left. Although galls of all sizes are very similar in structure and despite illustrating them in detail, Houard (1912) did not recognize that they were all caused by the same insect and erroneously attributed the large galls to the coccids.
Schizomyia botellus and S. buboniae are the only Schizomyia species that are known to pupate inside their galls, which explains the well developed structures on the pupal head and dorsum, and possibly also the reduced number of terminal papillae in the larvae. Schizomyia botellus is larger than S. buboniae but adults of the two species are otherwise very similar morphologically. In contrast, the immature stages are easily distinguishable. The larval spatula in S. buboniae has smaller teeth that are not as widely and deeply separated as those of S. botellus , and the pupae of the two species differ strikingly in their head and abdominal structures; the pupa of S. botellus has two conspicuous facial protuberances that are absent in S. buboniae , significantly shorter and not as broadly separated antennal horns, and a dorsal field of complex spines on each abdominal segment that is absent in S. buboniae . The relation of these two species to other described species of Schizomyia is unknown.
| ZFMK |
Zoologisches Forschungsmuseum Alexander Koenig |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |