Agrionympha Meyrick, 1921
|
publication ID |
https://doi.org/ 10.5281/zenodo.203553 |
|
DOI |
https://doi.org/10.5281/zenodo.6189649 |
|
persistent identifier |
https://treatment.plazi.org/id/3E6D87FC-FFDA-FFC9-FF69-A0EEAE1BFCC9 |
|
treatment provided by |
Plazi |
|
scientific name |
Agrionympha Meyrick, 1921 |
| status |
|
Agrionympha Meyrick, 1921 View in CoL
Type species A. pseliacma Meyrick, 1921 .
Description. Head. Vertex between ocelli and compound eyes densely microtrichiated. Anterior tentorial arm at base with a high dorsolateral crest, the invagination line of which is demarcated as a curved sulcus extending upwards/outwards from the tentorial pit; dorsal tentorial arm small but distinct, situated some distance behind/ above the inner end of the crest. Antennal scape ( Fig. 3 View FIGURE 3 F) elongate and swollen (length ~1.5x first flagellomere) with constriction near midlength; pedicel swollen, width equal to scape, equidimensional (length 1.0x first flagellomere). Flagellum in male of 40–45 flagellomeres, scale-covered on 2–3 basal flagellomeres. Female antennae shorter, 26–33 flagellomeres with 3–10 scaled. Fully developed ascoids ( Fig. 4 View FIGURE 4 A) with about 15 radiating branches, equally spaced around the base. Fenced sensilla coeloconica not conspicuous, probably generally absent. Mandibles and armature of labral epipharynx of ‘well-developed micropterigid type’ repeatedly described, in most detail for Micropterix ( Hannemann, 1956; Chauvin & Faucheux, 1981). Labial palp 2-segmented.
Legs. Fore tibial epiphysis well developed.
Wing structure ( Fig. 3 View FIGURE 3 ). Most species have the + rounded-lanceolate wings typical of Micropterigidae ; a very narrow and pointed wing shape in A. capensis (males in particular) is extreme in the family. Forewing (FW) venation with Sc and R forked; Rs3 and 4 ‘sessile’ (‘free’) in all but A. capensis ( Fig. 3 View FIGURE 3 B) where these veins are ‘stalked’; Rs4 to apex or termen/dorsum. In the four examined A. capensis males (and in one of the [four] examined more broad-winged females of that species) the anterior M branch anastomoses with the stem of Rs3&4, and the free terminal M1 hence is stalked on the latter. A M3-CuA cross-vein may or may not be present. It can be variably developed within a single species (also within the same sex), and so can a Sc-R cross-vein as well as the crossvein/vein which connect the base of CuA to the bases of M and CuP, respectively. In the hindwing (HW) venation the intriguing ‘double’ configuration of Sc first described from micropterigids by Philpott (1923) is sometimes very prominent ( Fig. 3 View FIGURE 3 E), and before the apex the two, now diverging, Sc branches may be united by an apparent cross-vein ( Figs 3 View FIGURE 3 C, D). Exceptionally, however, in one male specimen [of three of which slide mounts were examined] of A. capensis the anterior Sc branch becomes obliterated just beyond the bifurcation. Discrete stem of vein R at most present in the form of short ‘spur’ (referred to as ‘recurrent’ R vein by Philpott, 1923) immediately before its anastomosis with Sc2. 1–3 frenulum bristles. Following veins overall similar to FW counterparts. The putative base of A1 is indistinguishably fused with CuP, and a free distal part of this vein may or may not be developed; when present it is markedly crossvein-like, oblique and soon anastomosing with A2.
FW pattern ( Fig. 2 View FIGURE 2 ). Very conservative and reminiscent of many European and Australian species in the family. Somewhat uniform throughout the genus, but with clear specific differences in details. Ground colour dark brown to black, with purplish-coppery iridescence and brilliant silvery-white maculation which, when most complete (e.g. Fig. 2 View FIGURE 2 B), consist of four components: a ‘claval mark’ (along the claval furrow, i.e., the concavity within which CuP is lying), a median band, a postmedian band, and a subapical band (or spot).
Scale colour on body. Head variable on vertex, frons and antennae (see descriptions) but with a black lateral streak extending from the base of the antenna to the area below the compound eyes in all species; vertex with long tufts of piliform scales. Dorsum of thorax with flat black or brown-black scales, usually with metallic iridescence (see descriptions), lateral walls with silvery white scales, concolorous with fore and midfemora. Tegulae either black (in 3 species) or whitish (in 5 species) clothed in a mix of elongate flat scales and long piliform scales. Legs variable (see descriptions); but fore and mid femora glistening white with grey distal joints; hindtibiae uniformly dark brown-black, but fore and midtibiae and tarsi with variable pale bands. Abdomen black.
Pregenital abdomen. Dorsum I with or without a trace of a narrow transverse sclerite, unscaled. Sternum V gland ( Figs. 4 View FIGURE 4 B, D) orifice on a relatively large (about 0.4 mm diam.), modestly elevated, near-circular protuberance; markedly microtrichiated on discal area around the orifice, with 8–12 long fluted piliform scales concentrated around its posterior border.
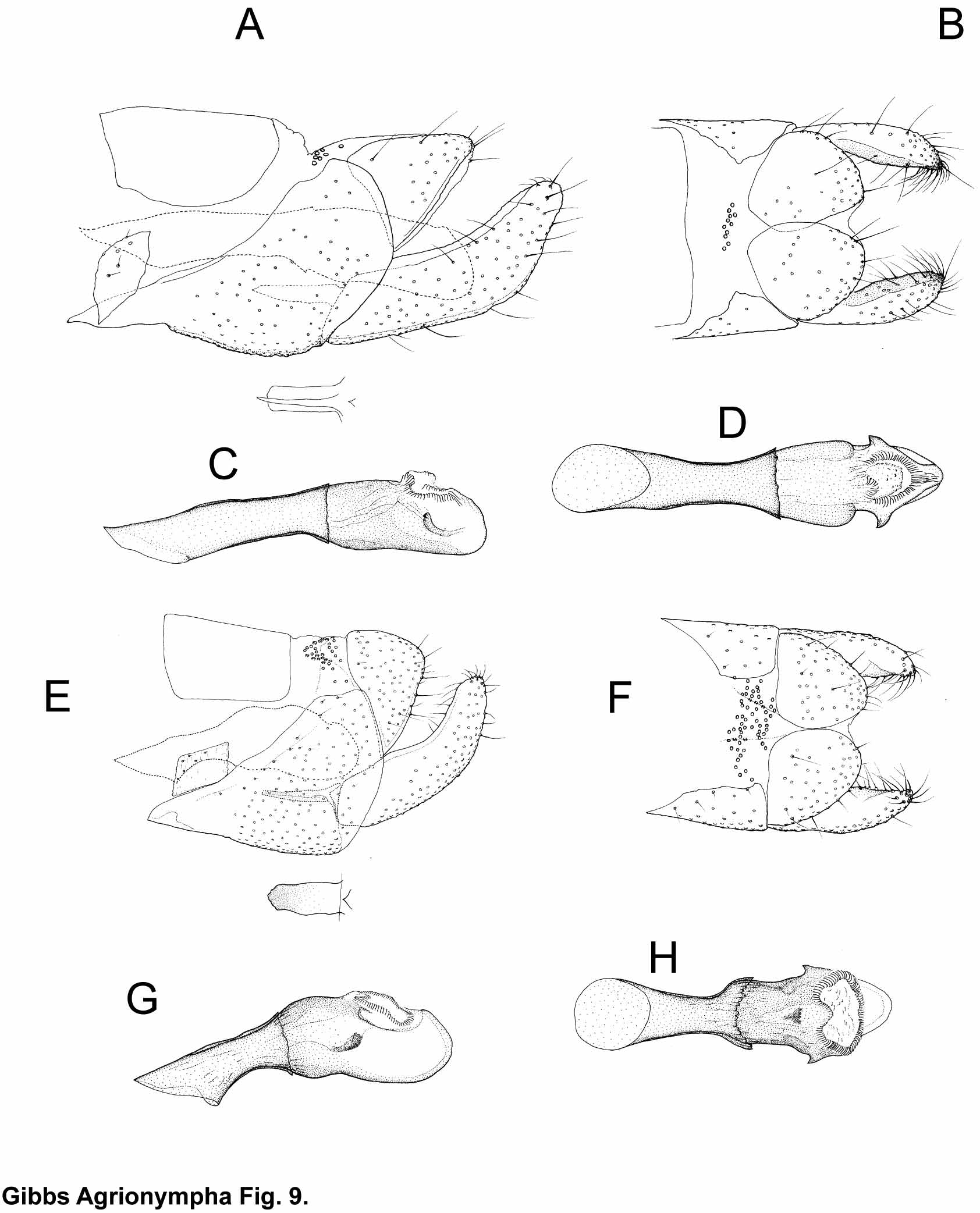
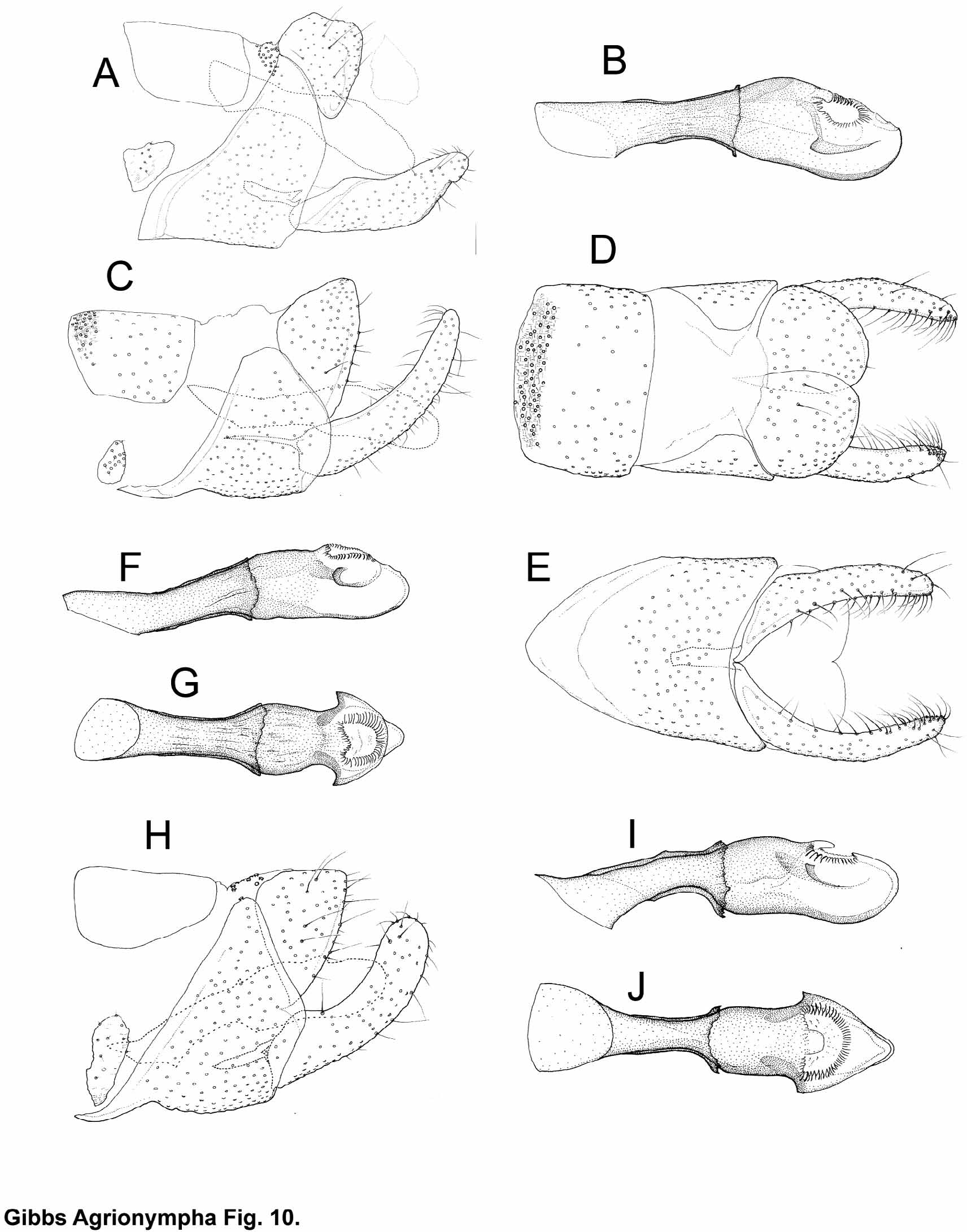
Male genitalia ( Figs. 7–10 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 ). Sternum VIII remnants present as a pair of small, but distinct, isolated and variably shaped sclerotisations bearing some minute setae. Segment IX sclerite (= ‘vinculum’) forming a dorsally open ring, its thickened anteroventral margin extended forward, telescoping into sternum of segment VII; anterodorsal margins straight or very slightly concave and neither pigmented nor markedly thickened; posterior margin strongly convex, overlapping base of valve. A cluster of specialised sensilla (circular elevations surrounding a small central hair-like process, Fig. 4 View FIGURE 4 C) is present on dorsum IX in all species except A. capensis and A. sagittella ; in the latter what may be serial homologues occur on an anterior zone of tergum VIII ( Fig. 4 View FIGURE 4 C). Valvae simple, slender, bent upwards, weakly tapering toward a rounded apex; ratio of length:width at mid-length 3.6–5.7; lacking any medial projections or processes but with an area of stiff retro-bristles near the apex. Median plate well developed, sometimes with anterior part more or less strongly darkened. Tergum X plate completely divided in mid-line, posteriorly produced into a pair of rounded sclerotised ‘uncus’ lobes. Lateroventral walls of anal cone with a pair of irregular sclerotised plates immediately below the tergal plates and largely concealed by the latter, bearing numerous large microtrichia but no setae; membranous distal part of anal cone small, inconspicuous. Phallus short, 1.3–1.7 of ventromedial length of segment IX sclerotisation. Distal/posterior part of phallus tube equal to or shorter (1.0–0.6x) than anterior part, enclosed in sclerotised phallocrypt, subdivided into ‘head’, ‘neck’ and ‘body’ regions, the latter with heavily-sclerotised walls produced into a pair of small ‘arrowhead’-like lateral lappets level with the proximal margin of the gonopore; ‘neck’ more or less constricted in dorsal view but not apparent in lateral view; slightly swollen ventral bulb projecting beyond gonopore, rather thickwalled but unsclerotised (mesocuticular), not appearing as a separate branch; gonopore generally heart-shaped or circular, orientated dorsally or tilted slightly towards apex, bordered by lightly sclerotised radial folds. Just inside the gonopore the cuticular ‘floor’ of the ejaculatory duct may be markedly thickened and textured, and may be produced into a small sclerotised apodemal formation ( Fig. 5 View FIGURE 5 B); retractor muscle fibres inserting on this posterior duct portion, such as have been identified in a Malagasy micropterigid genus, were not observable in histological sections. Posterior phallocrypt with scaly texture, raised free edges of the ‘micro-scales’ with fine comb-like margins. Valve musculature originating on (here incomplete) segment IX ring comprising both the unusual (in micropterigids) ventral ( Fig. 5 View FIGURE 5 A) and the more widespread, but also not ubiquitous, dorsal set; dorsal muscle inserting on membranous body wall just proximad to/above valve base rather than on the latter itself.
Female genitalia ( Fig. 7 View FIGURE 7 F). Relatively uniform among the species. Segment VIII tergite and sternite independent and unmodified; segments IX and X capable of extreme elongation but normally IX is telescoped inside segment VIII; segment IX sclerite a discontinuous ring, with a gap along mid-dorsal line, the gap being wider anteriorly than posteriorly, the sclerite slightly higher than long. Melanised ‘terminal plates’ (lateral sclerites of segment X) vary from equidimensional to longer than high (about 1.6x), sometimes appearing emarginated along anterior margin. In Micropterigidae , the spermatheca arises from the genital chamber inside a thickened and highly malleable, collar-like papilla, often with complexly folded latero-ventral walls which can be distorted according to the reproductive status of the individual. It is situated adjacent to the entrance to corpus bursae which, in Agrionympha , is not constricted at this point. The spermatheca itself ( Fig. 7 View FIGURE 7 G) is subdivided into three parts; a thickwalled, heavily staining basal duct, a more voluminous thin-walled utriculus, and a terminal lagena. In Agrionympha , the duct is narrow, slightly sinuous (both papilla and basal duct stain heavily with chlorazol black), its distal end abruptly bulbous; the utriculus of three sections but relatively short overall (no longer than 8th tergite), broadly connected to duct bulb through a thin-walled bladder-like proximal section, a short generalised middle section, leading through a constricted, often convoluted junction to its most distinctive feature—a large distal spherical bulb 2– 3 x diameter of middle section of utriculus but often collapsed so as to appear crescentic; a short lagena extends from the distal utricular bulb. Corpus bursae small, simple, without spines, signa, or other sclerotisations in the wall.
Immature stages ( Fig. 6 View FIGURE 6 ). Early stage (probably second instar) larvae have been found (by GWG) at Mariepskop, Mpumalanga Province (formerly Transvaal), and Kogel Bay, Western Cape, almost certainly representing two species. Both are of the ‘sabatincoid’ morphotype (Gibbs, 2010) characterised by the possession of eight pairs of abdominal spiracles; the trunk broadly hexagonal in cross-section; and a cryptic pigment pattern (pigmentation disappearing after several years of storage in 70% ethanol) integument with a honeycomb of raised ridges. The head can be totally recessed into the prothorax as in all micropterigid larvae. The cranial ecdysial line is distinct, a cluster of 5 stemmata occurs on each side within a melanised ‘cage’, and a medial melanisation is present on the hypostomal bridge. The thoracic legs are short, three-segmented, lack a distinct melanised coxa and the femur carries an inflatable membraneous sac on its inner side near the base (see Gibbs, 2010). Abdominal prolegs are lacking. Cranial chaetotaxy was not examined. Macro-setae of the trunk could be scored with accuracy from both taxa but confirming the presence or absence of short micro-setae was not always possible. The macro-setae are relatively short, thickened, with clubbed apices, D1 arising from low integumental flanges on the conspicuously honey-combed integument. These larvae of Agrionympha are notable for the reduction of D2 setae on abdominal segments to minute spherical knobs. Prothorax with 9 pairs of setae, directed forward, six aligned around the margin of the head recess fold: dorsal and lateral setae as long as any on the trunk; five pairs on dorsum, with two forming a longitudinal row either side of mid-dorsal line (D1 & D2, with D2 on the anterior head recess margin), and three forming a discrete oblique row along a dorso-lateral ridge (anterior-most are XD1, XD2, posterior L1), the two remaining L setae are aligned vertically at the level of the spiracle (L2 & L3) close to the margin of the head recess; a single short SV seta is situated dorsad to the tibiotarsus insertion and a small thinner seta is located on the margin of the head recess at the level of the leg base. No V1 seta could be found associated with the coxae on these larvae. Mesothorax with five pairs of setae, aligned around the middle of the segment: the D setae longer than L setae, a much smaller spherical SV seta; D1 and D 2 in vertical line dorsally; L1 and L2 just above the lateral flange of the trunk at level of spiracles, more or less vertically aligned with the more ventral of the pair slightly forward; the single SV seta dorsad of the leg. Four pairs of setae on the metathorax are similarly arranged except that the D2 seta is absent. Abdominal segments 1–8 each with only three pairs of setae aligned around the middle of the segments: two D setae, the minute spherical D2 seta is repeated along all trunk segments to A8; a single L setae, about half the length of D1 on the lateral flange slightly below and posterior to the spiracle. Two pairs of clubbed setae occur on A 9 in the dorsal region, the more ventral one slightly forward and shorter; A10 with a single pair of minute globular setae.
Remarks on morphology. The subcosto-radial region of the micropterigid hindwing has long attracted particular attention, and a configuration observed in some specimens of A. jansella and A. sagittella proved to be of particular note. Here the two branches of the ‘doubled’ Sc are connected by a short, oblique apparent cross-vein. Had it not been for the presence of the R ‘spur’ (distinct in the A. jansella specimen illustrated in Fig. 3 View FIGURE 3 D, only just indicated in the A. sagittella specimen illustrated in Fig. 3 View FIGURE 3 C) diverging from the posterior Sc branch immediately distad from the cross-vein, it would in this case have appeared straightforward to interpret the posterior Sc branch as vein R. Similarly, it is the presence of the ‘spur’ in conspecific/congeneric moths which prompts us to reject the interpretation of the unusually discrete Sc2, itself devoid of any R-’spur’, of the A. sagittella specimen illustrated in Fig. 3 View FIGURE 3 E as vein R. Moreover: where a vestigial cross-vein between Sc and the R+Rs-stem is identifiable in the basal part of the wing, it joins vein Sc distad from the level where the doubling of the latter has become evident; this evidently speaks against the said cross-vein actually being the basal part of R, and the apparent posterior component of the doubled Sc being the more distal part of R.
Huang et al. (2010) suggested that the hindwing Sc is unforked in all non-agathiphagid Lepidoptera (a few scattered exoporians excepted), and that what has been interpreted as a Sc 2 in micropterigids is consistently a Sc-R crossvein. The cuticle thickening which Kristensen & Nielsen (1982) interpreted as a true Sc-R cross-vein in a specimen of Sabatinca calliarcha Meyrick, 1912 may indeed likely be a teratological formation as maintained by Huang et al., but we do not consider these authors’ evidence (flat/slightly concave lower surface, absence of erect setae) for the apically-posteriorly-curving branch from Sc 1 in this taxon being a cross-vein rather than Sc2 to be compelling. Because the posterior component of the ‘double’ Sc may be discrete as far basad as it occasionally is (afore-mentioned A. sagittella specimen in Fig 3 View FIGURE 3 E), we consider it most unlikely that it can be a Sc-R cross-vein, even though in less extreme cases ( A. jansella specimen in Fig. 3 View FIGURE 3 D) its diverging apical part does have some resemblance (also position-wise) with the fore wing Sc-R cross-vein in certain micropterigids, as noted by Huang et al. Seemingly unquestionable forking (even twice in one case) of Sc in some members of the family was documented by Hashimoto (2006, Fig. 6 View FIGURE 6 ); the 'doubling' of the hindwing Sc is in any case an intriguing phenomenon which deserves closer scrutiny.
It remains uncertain whether the oblique vein between the apical Sc branches here referred to as an ‘apparent cross-vein’ (i.e., a Sc1-Sc2 cross-vein), or represents an anterior branch (R1) of a forked vein R, the posterior branch (R2) of which has, then, coalesced with Sc2. The position of the vestige (‘spur’) of the R-stem is so close to the lower end of this ‘apparent cross-vein’ that both possibilities seem open.
The anterior portion of the micropterigid phallus is tightly surrounded by the sclerotised phallocrypt (and hence appearing double-walled), and the posterior portion beyond the sclerotised phallocrypt have been referred to as ‘phallobase’ and ‘aedeagus’, respectively ( Kristensen, 1984). However, the two together are apparently homologous with the phallobase (or phallotheca) ascribed to the lepidopteran ground plan, while a true aedeagus is undeveloped in micropterigids as in most non-agathiphagid Lepidoptera ( Kristensen 2003) .
Diagnosis. Agrionympha is unique within the family by the combined presence of a forked FW vein R (plesiomorphy shared with all S. Hemisphere genera except Austromartyria Gibbs, 2010 and an undescribed Malagasy genus), absence of a discrete full-length hindwing vein R and of a R/Rs fork (apomorphy shared with some SW Pacific genera), and complete longitudinal division of bilobed male tergum X plate (apomorphy elsewhere ocurring only in the S. American Hypomartyria and one Australian Tasmantrix species). The cluster of hair-sensilla on the membranous IX region may be a generic groundplan autapomorphy, and its absence in A. capensis and A. sagittella hence secondary; the dorsum IX location is suggestive of homology with the dorsum IX sensilla in Hypomartyria which, however, are more close-set, have more prominent marginal rims, and are devoid of the central hair-like process. Larvae with D2 setae of trunk segments A1–A8 minute and spherical, as in Austromartyria .
Affinities and intrageneric relationships. Findings emerging from preliminary analyses of 16S and 28S rRNA (Gibbs et al. 2004, Gibbs 2010) place Agrionympha in a (weakly supported) clade, referred to loosely as the ‘southern sabatincoid’ lineage, which also includes undescribed genera from South Africa, Madagascar and Costa Rica as well as Austromartyria (N. Qld, Australia) and Hypomartyria ( Chile) . The structure of the sternum V gland protuberance with its marginal piliform scales, the presence of both dorsal and ventral valve muscles from the segment IX ring, and the morphology (including chaetotaxy) of the early-instar larva is compatible with this placement. So too is the structure of the spermatheca which closely resembles Austromartyria from Australia and Squamicornia from Ecuador. However, in all these cases the resemblances are due to traits which occur in other micropterigids as well and which may be plesiomorphic within the family. Comprehensive sets of morphological and molecular data on all Micropterigid genera are currently being developed, and combined analyses of these will likely provide a more robust generic phylogeny in due course. A preliminary morphological analysis of the known larvae of Micropterigidae (GWG unpublished) emphasised the clear distinction between two morphotypes: 1) ‘ Micropterix- type’ known from genera in Europe, Australia and New Zealand (see Gibbs 2010 for further detail), and 2) the ‘sabatincoid’- type. Within the latter group larval characters prove useful for distinguishing between the northern hemisphere members (e.g. Epimartyria, Neomicropterix , Paramartyria, Issikiomartyria, Kurokopteryx ) found in North America, Japan, Taiwan, Vietnam, and the remaining genera which occur around the southern hemisphere ( Agrionympha , Austromartyria , Sabatinca , Hypomartyria in Africa, Australia, New Zealand, New Caledonia and Chile.
We consider the morphological characters so far studied and found useful for species discrimination to be an insufficient basis for making suggestions about intrageneric phylogeny. Suffice it here to note that A. capensis and A. sagittella both in their way stand particularly out from the rest: both lack the dorsum IX sensillum clusters in males, the former is moreover distinctive by the extreme wing shape and the Rs3&Rs4 stalk (from which, probably at least consistently in the male, M1 issues as well) and the latter by the presence of dorsum VIII sensilla. We are unable to make well-substantiated decisions about character polarity in these cases, and both species may well prove to be quite subordinate within the genus.
Species discrimination in Agrionympha is best achieved from the forewing maculation and confirmed with male genitalic details. Female morphological details contribute little to taxonomic precision, as in most micropterigid genera, but are presented here for comparative purposes.
Bionomics. Agrionympha species occur in moist forest or fynbos shrubland from sea level to 1700 m, wherever there is sufficient seepage and a shade canopy to promote the growth of liverworts at some time of the year and provide the sheltered humidity necessary for adult moths. The day-flying adults are generally found amongst low vegetation (usually ferns) on cool roadside banks or alongside streams. In the hot arid climate of parts of southern Africa, these flight conditions are only fulfilled for a very limited part of the day, leading to an interesting variation of diurnal activity times in which these moisture-sensitive moths appear to be active only for an hour or so after dawn, prior to the evaporation of overnight dew. This behaviour has been observed at Toorberg in the Karoo and similarly at Storms River, Eastern Cape, where A. capensis can be taken at night (females are attracted to light) or amongst the low fynbos shrubland before the heat of the day builds up.
Adults have been taken between mid-October and March but each species appears to have a relatively restricted flight period at any one locality.
The known larvae, putatively representing two species, are liverwort-feeders.
Distribution ( Fig. 11 View FIGURE 11 ). The presently-known species are restricted to the eastern escarpment ranges and coastal fringe of Republic of South Africa from 24º 34´S in Mpumalanga (formerly Transvaal) to 34º 16´S, Western Cape at Cape Town, with outliers in the Karoo near Graaff Reinet (32º 11´S) and Worcester (33º 38´S).
Associated taxa of plants and animals indicate that Agrionympha is a component of the well-known “Cape Biota”. It is not found in the lowland Afrotropical rainforests of southern Africa.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |