Parabrachyodus hyopotamoides (Lydekker, 1883)
|
publication ID |
https://doi.org/10.1093/zoolinnean/zlac111 |
|
DOI |
https://doi.org/10.5281/zenodo.7927136 |
|
persistent identifier |
https://treatment.plazi.org/id/3E5A87FC-FFBB-9D1C-4841-F956FD39B711 |
|
treatment provided by |
Plazi |
|
scientific name |
Parabrachyodus hyopotamoides |
| status |
|
SYSTEMATICS OF PARABRACHYODUS HYOPOTAMOIDES
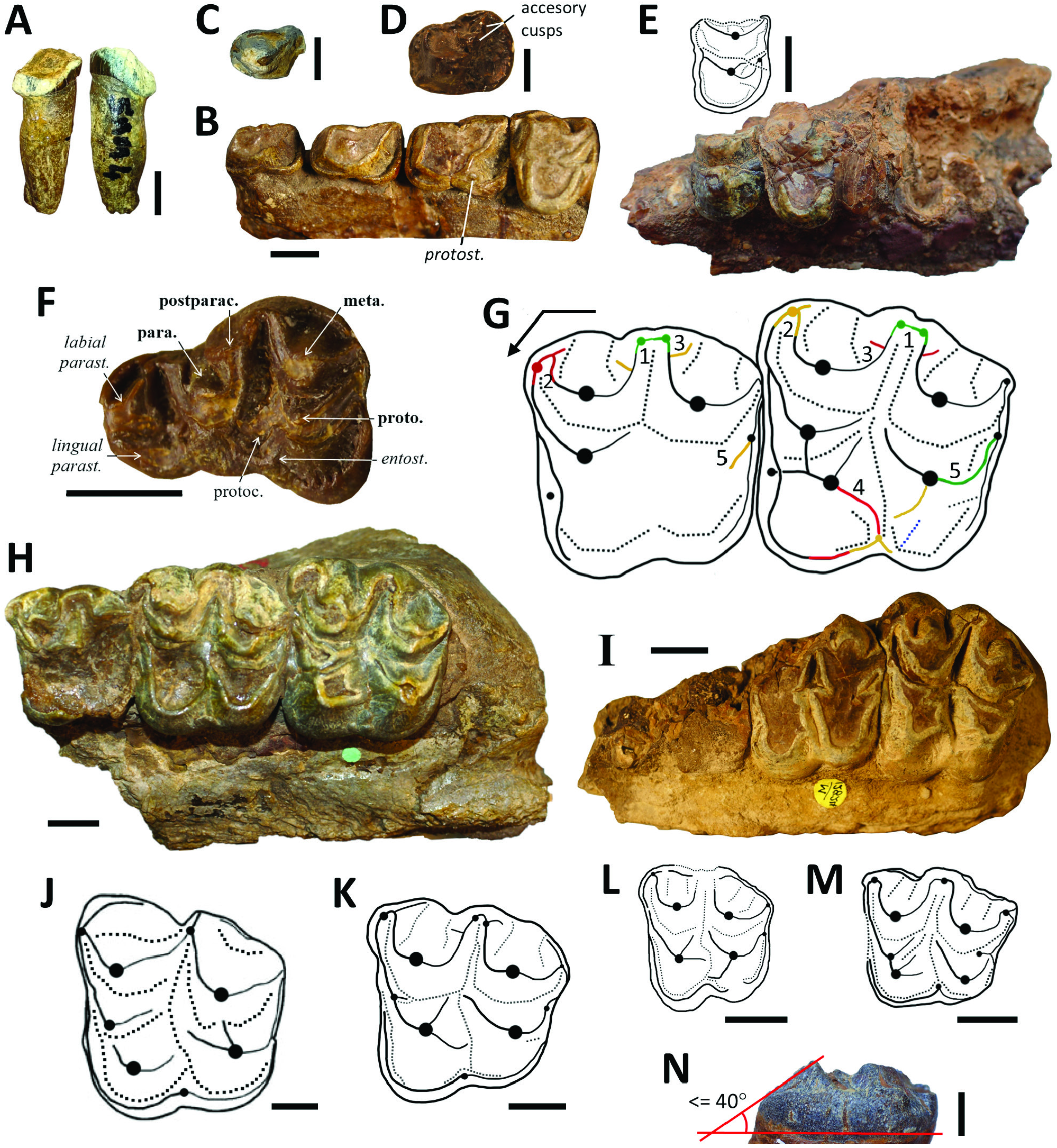
Based on unstable dental characters, early authors like Lydekker, Pilgrim and Forster-Cooper created up to ten species of Brachyodus ( Br. africanus , Br. gandoiensis , Br. giganteus , Br. hyopotamoides , Br. indicus , Br. manchharensis , Br. obtusus , Br. orientalis , Br. pilgrimi and Br. platydens ). Our analyses of character variance (namely the multiplication of original crests and styles around the protocone of upper jugal teeth) led us to synonymize these taxa with Par. hyopotamoides . For example, Brachyodus platydens ( Fig. 5I View Figure 5 ; Forster-Cooper, 1924), described by the flattest upper molars of the historical collections, cannot be maintained because its definition is based on a degree of wear. The square shape of the upper molars of Br. gandoiensis is the only distinctive feature on which this species was founded ( Forster-Cooper, 1924: 27–28), but we have shown that the shape of the upper molars in our sample highly depends on characters submitted to intraspecific variability. Moreover, the mesostyle is not loop-shaped but pinched in the type species of Brachyodus (e.g. Fig. 5J View Figure 5 ). The absence of biometric discriminations between upper teeth invalidates Brachyodus orientalis defined essentially from upper molars smaller than other specimens ( Fig. 5H View Figure 5 ; Forster-Cooper, 1924: 32) but with morphology equivalent to all the synonyms of Par. hyopotamoides . On the basis of the morphology of the paraconule, upper molars from the Bugti Hills attributed to Brachyodus africanus belong to S. palaeindicus (e.g. GSI B463; Pilgrim, 1912: pl. 22, fig. 1) and to the larger Par. hyopotamoides (e.g. GSI B462; Pilgrim, 1912: pl. 22, fig. 2). Pilgrim (1912: 50) considered the M/3 displaying postentostylids as pertaining to Brachyodus giganteus , the M/3 lacking the postentostylid belonging to Br. hyopotamoides . However, as we have shown, this character is commonly subjected to intraspecific variability in close relatives of Par. hyopotamoides . The single-cuspidate P4/ were grouped in Parabrachyodus obtusus (= Br. obtusus ) by Forster-Cooper ((1915, 1924: 33) based on this criterion, interpreted as a dental anomaly by Viret (1961) among those that can appear in isolated series of anthracotheres ( Ducrocq et al., 1995). Considering the frequency of this character state in the studied series of anthracotheres, it is included in the common variability of Par. hyopotamoides , as it is for the P4/ of some hippopotamids ( Boisserie, 2005: fig. 5D).
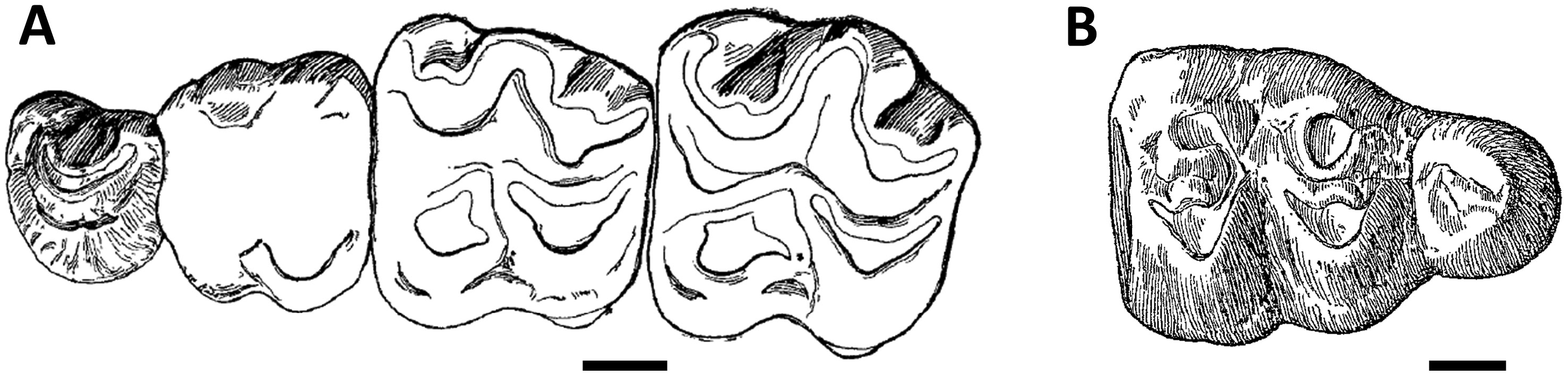
Even after attempts to resolve the systematics of Par. hyopotamoides ( Viret, 1961; Pickford, 1987), the diagnostic characters of this species remained partially misunderstood, with the strength of the convex labial ribs on paracone and metacone or the loop-like styles on upper molars being considered here as plesiomorphic characters shared with all bothriodontines from the Bugti Hills. Our biometric analysis shows that a smaller mesial than distal width of the lower molars was erroneously considered by Dineur (1981) and Pickford (1987) as a distinctive character between Parabrachyodus and Brachyodus . Conversely, the M/3 of Parabrachyodus is not defined by a trigonid larger than the talonid, as suggested by Forster-Cooper (1913), despite the proportions of the first M/3 allocated to the genus ( Fig. 1B View Figure 1 ). Furthermore, drawings of early authors show that the presence of the ectoprotocrista linked to a protostyle (a diagnostic character of Par. hyopotamoides ) was not considered as a relevant feature ( Fig. 1A View Figure 1 ). It is noteworthy that Pilgrim (1912: 55) was the first to mention some character states of Parabrachyodus (= Brachyodus ‘ giganteus ’) such as the ‘rudimentary additional cusp’ on P3/ underscoring the anthracotheriine affinity of this tooth, without interpreting them as diagnostic.
The sample of Parabrachyodus is comprehensive in the Bugti Hills, with at least 103 specimens (mostly molars) when including the remains newly described here. All these remains belong to the single species of the genus, Par. hyopotamoides . Pickford (1987) stressed the difficulties of discriminating the lower molars of Par. hyopotamoides from those of Telmatodon and Hemimeryx , presumably because the lower molars display a greater number of polymorphic traits and are less represented in number of specimens than the upper molars. Also, the convergent morphology of the hypoconulids of Anthracotherium and Parabrachyodus probably brought confusion in distinguishing these genera (e.g. Welcomme & Ginsburg, 1997: 1002), further inspiring the assignment of Parabrachyodus in the Anthracotheriinae instead of the Bothriodontinae ( Pickford, 1987: fig. 4), as such following Lydekker (1883) who considered this anthracothere as a representative of Anthracotherium . However, the upper molars are more distinctive. Gonotelma major is defined by a worn upper molar with a paraconule ( Forster-Cooper, 1924: pl. 5, fig. 1) and it is here considered as a junior synonym of Par. hyopotamoides . We concur with Pickford (1987) in considering Gonotelma a monospecific genus with small tetracuspidate molars, first interpreted as pentacuspidate ( Pilgrim, 1908, 1912).
AGE AND GEOGRAPHICAL DISTRIBUTION OF PARABRACHYODUS
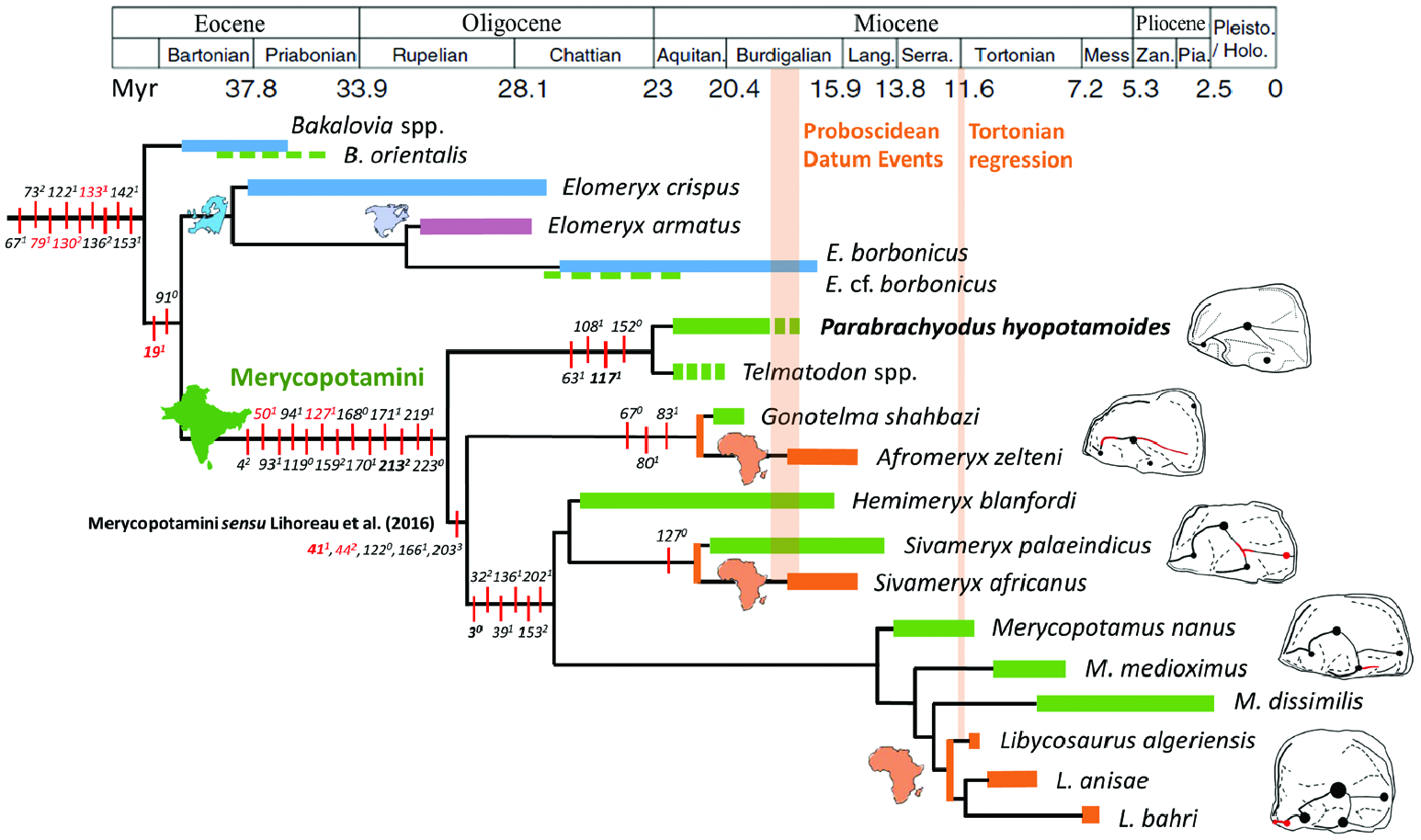
In addition to the series of Parabrachyodus hyopotamoides from SAM 4 ( c. 21 Mya), the isolated M3/ UM-SAM5-001 is the first reported occurrence of a fossil from SAM 5, which extends the age formally given to Par. hyopotamoides to approximately 19 Mya ( Roddaz et al., 2011; Antoine et al., 2013). We thus confirm the presence of the species in the Vihowa Formation (e.g. Antoine et al., 2013; Nanda et al., 2017). Parabrachyodus hyopotamoides is also recorded with confidence in the Zinda Pir Dome, Sulaiman Province of Pakistan, about 200 km north of the Bugti Hills, through three isolated specimens in localities Z114, Z154 and Safed Nala ( Lindsay et al., 2005). The ‘Interpretation B’ of Lindsay et al. (2005: fig. 6B) for the correlation of the Zinda Pir localities to the Geomagnetic Polarity Time Scale (GPTS) being the most satisfactory ( Antoine et al., 2013: 416), the localities Z114 and Z154 then lie between the magnetochrons C6AA and C6Bn. The corresponding approximate age is 21.95 Mya ( Speijer et al., 2020; J. Barry, pers. comm., 2022), which makes it the oldest known occurrence of Par. hyopotamoides . The species is also documented in the Khari Nadi Formation (Kutch Basin, Gujarat, India), via a palate with molars that have, among other traits, the characteristic morphology of the protocone and a large distostyle ( Bhandari et al., 2010: fig. 7A). It is also accompanied by SiƲameryx palaeindicus in the Kutch fauna. This assemblage could be younger than Samane Nala 4 and 5 according to Bhandari et al. (16.5 +/– 0.5 Mya). However, they precisely considered a last local appearance (LLA) of 16.5 Mya for Par. hyopotamoides to propose the most likely age of the Kutch mammal fauna. It is unclear whether or not Parabrachyodus also occurs in the Level 6 of the Chitarwata Formation ( c. 17.5–18.0 Mya, Roddaz et al., 2011; Antoine et al., 2013: fig. 16.4) and Par. hyopotamoides is certainly not documented in younger levels in the Bugti Hills. Nevertheless, a gap is observed in terms of fossilyielding levels in the concerned overlying sequence, i.e. between the poorly documented Level 6sup (‘Assemblage B’) and the Middle Miocene ‘Assemblage C’ (Level W; Antoine et al., 2013). In any event, the biochronological age of the Kutch fauna is potentially questionable ( contra Bhandari et al., 2010, 2021; Sehgal & Bhandari, 2014; Patnaik & Prasad, 2016). Conversely, it may restrain the use of anthracotheriid species from the Bugti Hills for biochronological purposes ( Antoine et al., 2013: 413).
Russell & Zhai (1987) reported one P4/ and one M/2 from the Benara fauna in Georgia ( Gabounia, 1966: fig. 9d, e) referred to as Parabrachyodus . The upper premolar lacks the two distal ridges and large distostyle characteristic of this genus, and it is more oblong transversely. The lower molar is too small to belong to this taxon; its selenodont morphology with pinched lingual cuspids and the connection between the preprotocristid and the premetacristid are consistent with Elomeryx instead. According to the provided drawing, it could also be a worn, ruminant-like lower tooth. A fragmentary pentacuspidate upper molar from the Dingdanggou fauna ( China) has been interpreted as Parabrachyodus sp. ( Wang & Qiu, 2004), but it has more selenodont ridges than unambiguous representatives of this genus, and the postparacristule joins the base of the paracone rather than the transverse valley. In this context, it is more likely that this tooth belongs to S. palaeindicus than to Par. hyopotamoides . Hence, no occurrence of Parabrachyodus is documented in Oligocene–Miocene deposits of China, and there is no dispersal event either between the north and south sides of the Tibetan Plateau involving this bothriodontine during the Early Oligocene ( contra Li et al., 2016; Wang, 2020; Li et al., 2022). The occurrence of Parabrachyodus sp. in the Irrawaddy area of Myanmar ( Burma) immediately east of the Indian subcontinent ( Bhandari et al., 2010) remains uncertain due to the lack of illustration for the referred specimen. The provided measurements of the concerned M3/ (46.5 × 54.9 mm) fall outside the range of variation defined for Par. hyopotamoides in this study ( Table 2 View Table 2 ).
In the current stage of our knowledge, Par. hyopotamoides seems to be restricted to the western part of the Indian subcontinent and it is not formally known in the Palaeogene, in contrast with what the compilation of Sulaiman Range faunas studied by Raza & Meyer (1984) and Pickford (1987) may have suggested. Despite the ‘gigantic Hyopotamus ’ known ‘from Sind’ ( Lydekker, 1882: 107), Parabrachyodus is not mentioned in the Manchar Formation, with maybe the exception of ‘cf. Brachyodus sp. ’ listed by Raza et al. (1984: table 2) in its lower member. Given that the Sind deposits lie south of the Bugti Hills and north of the Kutch Province ( Bhandari et al., 2010: fig. 1), fossils of Par. hyopotamoides are also likely to occur in the Manchar Formation. Fossil collections without any stratigraphical context in the Bugti Hills where Oligocene sediments have been identified (M12030 and M12033) are suspected to somewhat pre-date the Oligocene–Miocene transition ( Forster-Cooper, 1913; Antoine et al., 2013: fig. 16.4) as those referred to as Hemimeryx blanfordi have been in the same work of Forster-Cooper ( Lihoreau et al., 2016). This occurrence of Hem. blanfordi in the Late Oligocene of the Bugti Hills, together with the basal phylogenetic position of the clade ( Par. hyopotamoides + Telmatodon ), support the potential appearance of Parabrachyodus as early as in the Late Oligocene.
PHYLOGENETIC RELATIONSHIPS BETWEEN BOTHRIODONTINES FROM THE BUGTI HILLS
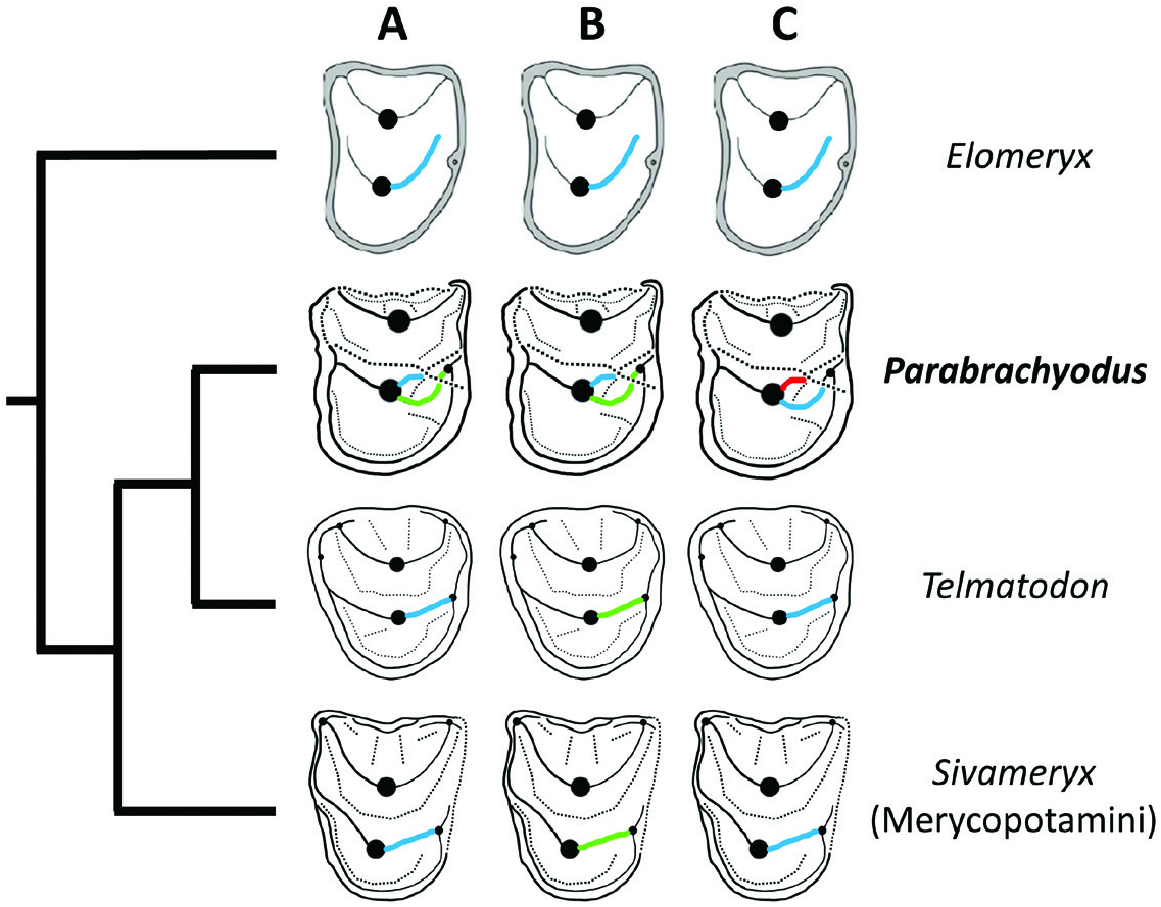
The three genera, Gonotelma , Parabrachyodus and Telmatodon , recorded in the Bugti Hills can be seen as early Merycopotamini sensu Lihoreau et al. (2016) in the present study. We refute the hypothesis that Gonotelma shahbazi is more closely related to SiƲameryx than to Afromeryx zelteni due to the shared retention of the paraconule ( Lihoreau & Ducrocq, 2007). First, the absence of a postparacristule on the M3/ of G. shahbazi shows that there is no paraconule ( Fig. 5L View Figure 5 ). In comparison, the holotype of Telmatodon orientalis ( Forster-Cooper, 1924) has a reduced paraconule with a vestigial cristule ( Fig. 5K View Figure 5 ). Second, the presence of the paraconule is here considered as a derived character that appeared independently in SiƲameryx and Parabrachyodus . The position of Gonotelma as sister-group to Afromeryx instead supports the hypothesis of closer phylogenetic relationships with this small Libyan merycopotamine ( Pickford, 1987, 1991). Shared characters relating to the morphology of the lower molars of these genera explain they are close relatives, while only the similarity of their upper molars had been pointed out by Pickford (1987, 1991). As Telmatodon is closer to Parabrachyodus than to Gonotelma , the hypothesis of a synonymy between Telmatodon and Gonotelma ( Viret, 1961; Kumar & Kad, 2003) is not verified by the present analysis, despite their general similarity. These findings involving the phylogenetic position of Telmatodon and Gonotelma suggest that they do not constitute a clade with Parabrachyodus contrary to what Pickford (1987: fig. 4) had informally proposed. Instead, Parabrachyodus and Telmatodon can be seen as a lineage that appears to be restricted to the Indian subcontinent during the Early Miocene. These conclusions remain uncertain insofar as the scarce fossil material for Telmatodon and Gonotelma (lacking lower premolars, rostral teeth, mandible and skull) has not allowed us to discriminate their diagnostic characters as precisely as for Par. hyopotamoides . In all cases, the phylogenetic proximity of these three associated early merycopotamines, together with their size differences ( Parabrachyodus and Telmatodon being gigantic compared to Gonotelma ), contradict the existence of a body size increase through time in this lineage, as argued by Pickford (2009) for the African merycopotamines Afromeryx and Libycosaurus .
Of the two distal ridges of the protocone of P4/ of Parabrachyodus , the one that joins the distostyle can be interpreted at first as the postectoprotocrista and the other one, shorter and labially situated, as the postprotocrista, considering the convergent condition in Anthracotherium ( Scherler et al., 2018) . In the context of the basal position of Par. hyopotamoides in relation to Merycopotamini such as SiƲameryx palaeindicus (also documented in the Bugti Hills), the hypothesis of homology involved by this terminology implies that a reduction of the postectoprotocrista – accompanied by a lingual displacement of the postprotocrista and its development until it reaches the distostyle – would have led to the occlusal pattern of the P4/ of this tribe ( Fig. 10A View Figure 10 ). In view of the configuration of the distal crest of the P4/ of Elomeryx ( Kostopoulos et al., 2012: fig. 4) resembling those of Merycopotamini , two hypotheses are equally parsimonious. Since Par. hyopotamoides is the first branching species of the clade excluding Elomeryx together with species of Telmatodon , it may be expected that the Parabrachyodus -like postprotocrista has been lost and the postectoprotocrista is retained in all Merycopotamini ( Fig. 10B View Figure 10 ). The postprotocrista of Par. hyopotamoides can be seen as an additional ‘endoprotocrista’ (formed from an enamel fold or a fossa) and the postectoprotocrista as the true postprotocrista that connects to the distostyle, inherited from a common ancestor with Elomeryx ( Fig. 10C View Figure 10 ). Since the single known P4/ of Telmatodon orientalis ( Fig. 10 View Figure 10 ; Forster-Cooper, 1924: pl. 5, fig. 5) has a two-crested protocone, with one distal crest, the second proposal is the most likely in the context of the present topology (the P4/ of Gonotelma being unknown). This autapomorphic scenario ( Fig. 10C View Figure 10 ) is consistent with the overall trend towards the addition of styles and ridges, which have only been reported on the jugal teeth surrounding the P4/ of Par. hyopotamoides , namely the small distolingual style on the P3/ and the protostyle and ectoprotocrista of upper molars.
IMPLICATIONS FOR THE DEFINITION OF MERYCOPOTAMINI
The paraphyly of Merycopotamini sensu Lihoreau et al. (2016) and the weak support of the clade, assuming that G. shahbazi (the sister-species to A. zelteni ) is part of it, highlights the need for a more inclusive diagnosis of the tribe. Two-thirds of the non-ambiguous synapomorphies that made Merycopotamini a clade at the time of its definition were then interpreted as convergences with Elomeryx and Bothriodon ( Lihoreau et al., 2016) . The phylogenetic position of Parabrachyodus with respect to Elomeryx on the one hand and Merycopotamini on the other hand establishes the suspected link between Elomeryx and Merycopotamini ( Lihoreau & Ducrocq, 2007; Böhme et al., 2013, Rincon et al., 2013). Hence, seven out of nine characters defining Merycopotamini sensu Lihoreau et al. (2016) are retrieved as unambiguous synapomorphies in the branching sequence leading to the robust node comprising Par. hyopotamoides ( Fig. 11 View Figure 11 ). For instance, the pinched loop-like hypoconulid on M/3, the parastyle issued from the preparacrista and the absence of ectocristyle on upper molars are actually inherited from a common ancestor with Elomeryx and BakaloƲia . Thus, considering the first diagnosis of Merycopotami ( Lihoreau et al., 2016), Par. hyopotamoides differs in only three out of 13 traits relative to the morphology of the crestids of the protoconid on P/3 and P/4, namely the orientation of the P/3 postprotocristid, the position of the P/4 postprotocristid and the direction of the P/4 preprotocristid. Nevertheless, the occlusal pattern of lower P/4 has proved to be of great interest for distinguishing Merycopotamini from each other ( Lihoreau et al., 2019: fig. 4).
Considering the robust relationship between Elomeryx and Merycopotamini through the inclusion of Par. hyopotamoides , and the primitive condition of its P/4 compared to Merycopotamini sensu Lihoreau et al. (2016) ( Fig. 11 View Figure 11 ), we suggest a redefinition of Merycopotamini encompassing the basal position of Parabrachyodus and its relatives Telmatodon and Gonotelma . Interestingly, the new traits of the larger tribe concern mainly the eruption of tubercles appearing subsequently during the development of the dP3/, recently considered as bearing strong diagnostic characters ( Gomes Rodrigues et al., 2020), the preferential development of the I/1 among the lower incisors, the multiplication of tubercles and crests on the P3/ and that of the number of mandibular foramen; hence different characters from the previous definition. Nonetheless, the critical role of the enamel microstructure in distinguishing this clade ( Alloing-Séguier et al., 2014; Lihoreau et al., 2016) is here reinforced, the substantial development of radial outer enamel being completed by the Schmelzmuster composed of two layers and weakly developed HSB. This diagnosis also clearly distinguishes merycopotamines from Elomeryx through the lack of connection between the protocone and the metaconule, closing the transverse valley on upper molars, except for E. borbonicus (e.g. Geais, 1934; Hellmund, 1991; Lihoreau et al., 2009: fig. 3), and between the premetacristid and the preprotocristid on lower molars. We note a tendency for the number of protocone crests to decrease in the extended tribe, from Parabrachyodus with a quadricrescentic protocone to Afromeryx , Gonotelma , Hemimeryx , SiƲameryx and Telmatodon with three crests, and Libycosaurus and Merycopotamus with only two crests. Finally, this lineage is also characterized by a tendency to complexify the occlusal morphology of the Parabrachyodus -like P/4, as suggested above. A mesial curvature of the preprotocristid and a lingual orientation of the postprotocristid are acquired in Afromeryx , the hypoconid and a partial fusion of the postprotocristid and endoprotocristid in Hemimeryx and SiƲameryx , the distal crest of the entostylid in Merycopotamus , and a multiplication of accessory cuspids mesially to the preprotocristid are independently developed in Hemimeryx and Libycosaurus ( Fig. 11 View Figure 11 ; Lihoreau et al., 2019).
PALAEOBIOGEOGRAPHICAL IMPLICATIONS
The northern distribution of Brachyodus relative to the Himalaya Range during the Early to Middle Miocene ( Ducrocq et al., 2003) indicates that these mountains must have constituted a barrier to dispersal for this genus and vice versa for Parabrachyodus from India, inasmuch as no specimen from China similar to those from the Indian subcontinent is documented. An alternative hypothesis for their non-overlap is a mutual exclusion for ecological reasons. Elomeryx cf. borbonicus is known from two jugal teeth in the Zinda Pir Dome in Z108 locality ( Ducrocq & Lihoreau, 2006), an older locality than those that have yielded the oldest material of Parabrachyodus (Z114 and Z154; Lindsay et al., 2005: fig. 6B). The occurrence of this European species in Pakistan shows that the palaeobiogeography of the Late Oligocene and the Early Miocene allowed interchanges with Europe, as massively illustrated by mammalian assemblages of the Early Miocene in general (e.g. Antoine et al., 2010, 2013). In Burdigalian times (late Early Miocene), the two concomitant dispersal events involving SiƲameryx on the one hand and Gonotelma and Afromeryx on the other (Proboscidean Datum Events, Fig. 11 View Figure 11 ), via the probable connection of the Indus with the Tiger–Euphrate drainage basin, provide evidence that a passageway to Africa was also open from Pakistan (e.g. Barrier et al., 2018; Grossman et al., 2019; Lihoreau et al., 2019). An origin for Merycopotamini , rooted by Par. hyopotamoides , in this north-western province of the Indian subcontinent (i.e. Pakistan), is therefore consistent with this second phase of dispersal in the evolutionary history of this lineage of bothriodontines ( Fig. 11 View Figure 11 ).
Unlike the small merycopotamines, Par. hyopotamoides appears to be endemic to the Indian subcontinent. The absence of dispersal towards Africa is probably linked to its extinction just before the contact between the two continents ( Fig. 11 View Figure 11 ). Parabrachyodus hyopotamoides displaying the thickest enamel among bothriodontines, allowing more resistance to wear that could explain the strong wear gradients of molars rows ( Alloing-Séguier et al., 2014: 691), the flattest occlusal surface among bothriodontines and molars with a selenodonty less marked than in other merycopotamines, we question if a high degree of ecological specialization may explain its extinction.
The basal position of Parabrachyodus in relation to Merycopotamini , its relatively short temporal range and atypical morphology (for a bothriodontine), as well as a fossil record limited to the Indian subcontinent, blurs its palaeobiogeographical history. Furthermore, Asian records of Elomeryx cf. borbonicus are scarce ( Ducrocq & Lihoreau, 2006), and there is a large gap with the record of BakaloƲia orientalis from the Late Eocene ( Böhme et al., 2013), which contributes to the uncertainty of the geographical origin of Parabrachyodus ( Fig. 11 View Figure 11 ). Arretotherium , and especially A. meridionale from Central America, must be included in further phylogenetic analyses, since the phylogenetic position of this genus is unclear, either in an Elomeryx clade ( Kostopoulos et al., 2012) or in a polytomy with E. borbonicus and Merycopotamini ( Lihoreau & Ducrocq, 2007; Böhme et al., 2013; Rincon et al., 2013). It is a critical point for biogeographical purposes regarding the concomitant origination of merycopotamines in Asia.
The identification of Gonotelma in the same stratigraphic level as Parabrachyodus , SiƲameryx , probably Telmatodon ( Antoine et al., 2013) and Hemimeryx ( Lihoreau et al., 2016) , implies that the Bugti Hills faunas simultaneously comprised at least five phylogenetically related bothriodontines. Such diversity is not surprising for the megafaunas of the region, as nine distinct species of rhinocerotids are known to co-occur from Kumbi 4 ( Antoine et al., 2010), the lateral equivalent of SAM 4. The diversity of merycopotamines from the Early Miocene of the Bugti Hills is unique in that no homotaxic assemblages are known elsewhere. The available craniomandibular material for Par. hyopotamoides did neither allow us to define a sexual dimorphism in the studied populations, nor a semi-aquatic lifestyle, as for SiƲameryx ( Rowan et al., 2015) and contrary to what is observed in certain bothriodontines with a proven semi-aquatic lifestyle ( Orliac et al., 2013; Lihoreau et al., 2014). Yet, the predominance of the remains of Par. hyopotamoides over other Bugti anthracotheres ( Pickford, 1987: table 6), and the proportion of unicuspidate P4/ for Par. hyopotamoides , show that these megaherbivores must have lived in sufficiently isolated and small populations for such dental variations to become common ( Ducrocq et al., 1995; Lihoreau et al., 2006).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |