Trichillum, Harold, 1868
|
publication ID |
https://doi.org/ 10.1649/1132.1 |
|
persistent identifier |
https://treatment.plazi.org/id/31602359-5B46-FFCA-8369-FDF9FEEDE335 |
|
treatment provided by |
Valdenar |
|
scientific name |
Trichillum |
| status |
|
Nesting by Trichillum View in CoL and Related Genera
Ohaus (1909) indicated that Trichillum species live in fresh horse or cattle dung, but oviposit in older dung where its larvae excavate tunnels as they feed. As in many Aphodiini, pupation occurs within the same dung pat. This is the first reference to a scarabaeine that reproduces without preparing a nest. The two species of Trichillum collected by Ohaus were described by Arrow (1931) as T. ohausi Arrow and T. cristatum Arrow. These two species were recently included in the genus Onoreidium (Vaz-de-Mello 2008).
Martínez (1959) reports T. externepunctatum as a kleptoparasite on the brood masses of a larger scarabaeine, Dichotomius bosqui Pereira , although it also feeds on the excrement that it encounters on the soil surface. As indicated below, other species also take advantage of a large mass of dung gathered by a larger beetle. This behavior does not require nesting given that the kleptoparasite takes advantage of the mass of dung gathered by the larger beetle. This is common in Aphodiini and has also been observed for other small Scarabaeinae .
Boucomont (1928) made the first reference to a species of this group being associated with a sloth ( Bradypus sp. ). The species was Trichillum bradyporum Boucomont (transferred to Pedaridium by Ferreira and Galileo 1993) and the description was based on a specimen found near the anus of a sloth in Santa Clara province, Costa Rica. Balthasar (1939) indicated that the same species is frequently found near the anus of Bradypus infuscatus Riqueimis and cited another specimen with the same characteristics as those described by Boucomont. This relationship is further explored in an interesting study by Ratcliffe (1980), in which he describes Trichillum adisi Ratcliffe (also transferred to Pedaridium by Ferreira and Galileo (1993)) which is phoretic on a sloth (around the anus) until the animal climbs down the tree to defecate and leaves a compact mass of excrement on the ground which it then buries. At that moment, P. adisi oviposits on the sloth dung. Ratcliffe found larvae and pupae in the buried excrement. The two species mentioned above were recently transferred to Bradypodidium , a genus formed exclusively by species associated with sloths (Vaz-de-Mello 2008).
According to Verdú and Galante (2001), Pedaridium brasiliense Ferreira and Galileo (currently a synonym of Genieridium bidens (Balthasar) (Vaz-de-Mello and Génier 2005; Vaz-de-Mello 2008)) and P. almeidai Pereira (currently included in Pereiraidium (Vaz-de-Mello 2008)) exhibit similar behavior. One by one, the female deposits eggs in the dung without any previous preparations. All development occurs in the dung mass (as occurs in the majority of Aphodiini). The authors observed that the larvae stridulate and indicated that this could be a way of marking their territory to avoid contact with other larvae and possible cannibalism. Verdú and Galante (2001) revisit the possibility of primitive nesting in ‘‘ Pedaridium ’’ and Trichillum .
According to field observations by one of us (FZVM), several species belonging to this group of genera can be collected from dung or carrion on the soil surface, but they also make use of the provisioned galleries of larger beetles such as Dichotomius Hope , Isocopris Pereira and Martinez , and Ontherus Erichson. This occurs in G. bidens , Genieridium cryptops (Arrow) , G. margareteae Génier and Vaz-de-Mello, T. externepunctatum , T. heydeni Harold , and T. adjunctum Martínez.
Also, according to field observations reported by Vaz-de-Mello (2003) and Vaz-de-Mello and Génier (2005) and references in the literature ( Martínez 1959, 1969), a group of species belonging to Trichillum live in association with the debris accumulated in the nests of Acromyrmex ants. Among these are Trichillum tishechkini Vaz-de-Mello and Génier (misidentified as T. heydeni by Martínez 1959, 1969) and other species currently being described.
The genera that are closest to the monophyletic group comprised of the genera mentioned are Scatimus Harold and Scatrichus Génier and Kohlmann , the latter apparently being the sister group (Vaz-de-Mello 2003; Génier and Kohlmann 2003; Vaz-de-Mello 2007; Vaz-de-Mello 2008). For the latter genus, there is little field information beyond that obtained by trapping (from dung, carrion, or with light). Scatimus ovatus Harold has been collected from the feeding and brood masses of Dichotomius (G. Halffter, personal observation). It has also been found on the brood balls of Phanaeus MacLeay (Halffter and Edmonds 1982) and detritus of Acromyrmex Mayr (Halffter and Matthews 1966) . Halffter and Edmonds (1982) provided a brief description of the nest –paracoprid, pattern I– with the egg placed in the upper part of the brood mass, which, apart from this detail, is similar to the nests of the genera Ateuchus Weber and Canthidium Erichson. Edmonds and Halffter (1972, 1978) give a brief description of the larva of Scatimus and compare it with those of Canthidium .
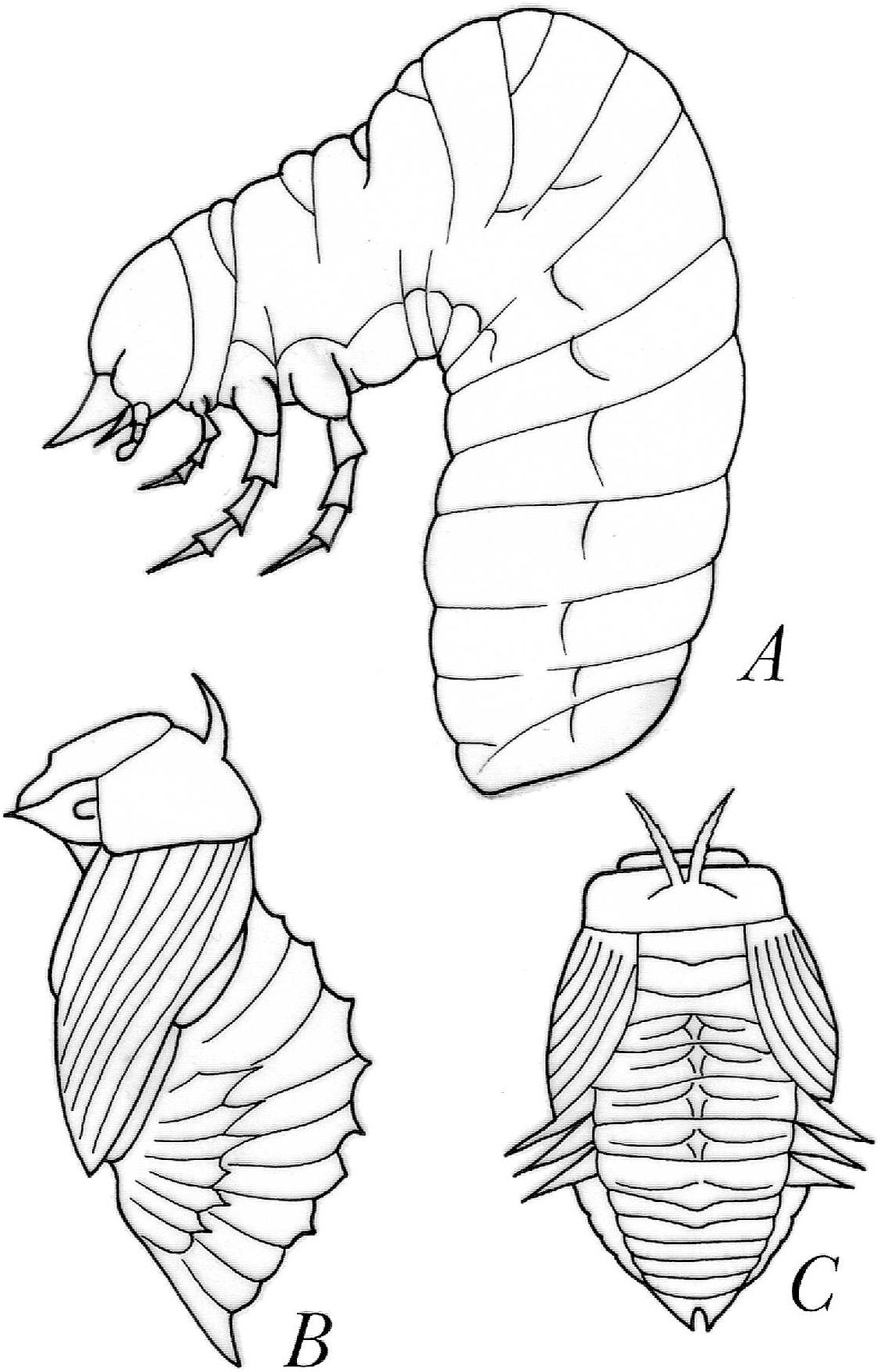
The larvae and pupae of Bradypodidium were described by Ratcliffe (1980) and those of Genieridium and Pereiraidium by Verdú and Galante (2001). Those of Trichillum are currently being described by one of us (FZVM). In summary, the larvae exhibit the characteristic bump and the relatively reduced base of the abdomen ( Fig. 1 View Fig ), although it is not much more reduced than that of larvae that lead a sessile life, such as those of Canthidium . (Species examined: B. adisi , G. bidens , P. almeidai , T. heydeni , and T. externepunctatum ). The pupae ( Fig. 1 View Fig ) have the pronotal support projections described by Edmonds and Halffter (1978), notably developed, paired, pointing antero-laterally and passing over the head as documented for B. adisi ( Ratcliffe 1980) and T. externepunctatum . The caudal support projections are notably developed, paired, pointed and aligned along the back and outwards in T. externepunctatum , but are apparently reduced in B. adisi ( Ratcliffe 1980) . The lateral tergal projections (three pairs), dorsal pteronotal and tergal, are normally developed in T. externepunctatum , while only the first of these are present in B. adisi ( Ratcliffe 1980) . A first analysis allows us to conclude that the pronotal and caudal projections that would have the function of protecting the pupa from contact, as indicated by Edmonds and Halffter (1978), are notably developed as an adaptation to the lack of a proper pupal chamber.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.