Colpodidium zelihayildizae, Yildiz & O & V, 2021
|
publication ID |
https://doi.org/ 10.3906/zoo-2101-20 |
|
persistent identifier |
https://treatment.plazi.org/id/303887BF-2941-9346-709A-2B62FA96F976 |
|
treatment provided by |
Felipe |
|
scientific name |
Colpodidium zelihayildizae |
| status |
|
Class: Nassophorea Small and Lynn, 1981 View in CoL View at ENA
Order: Colpodiidida Foissner et al., 2002 Family: Colpodiidae Foissner, 1995
Genus: Colpodidium Wilbert, 1982
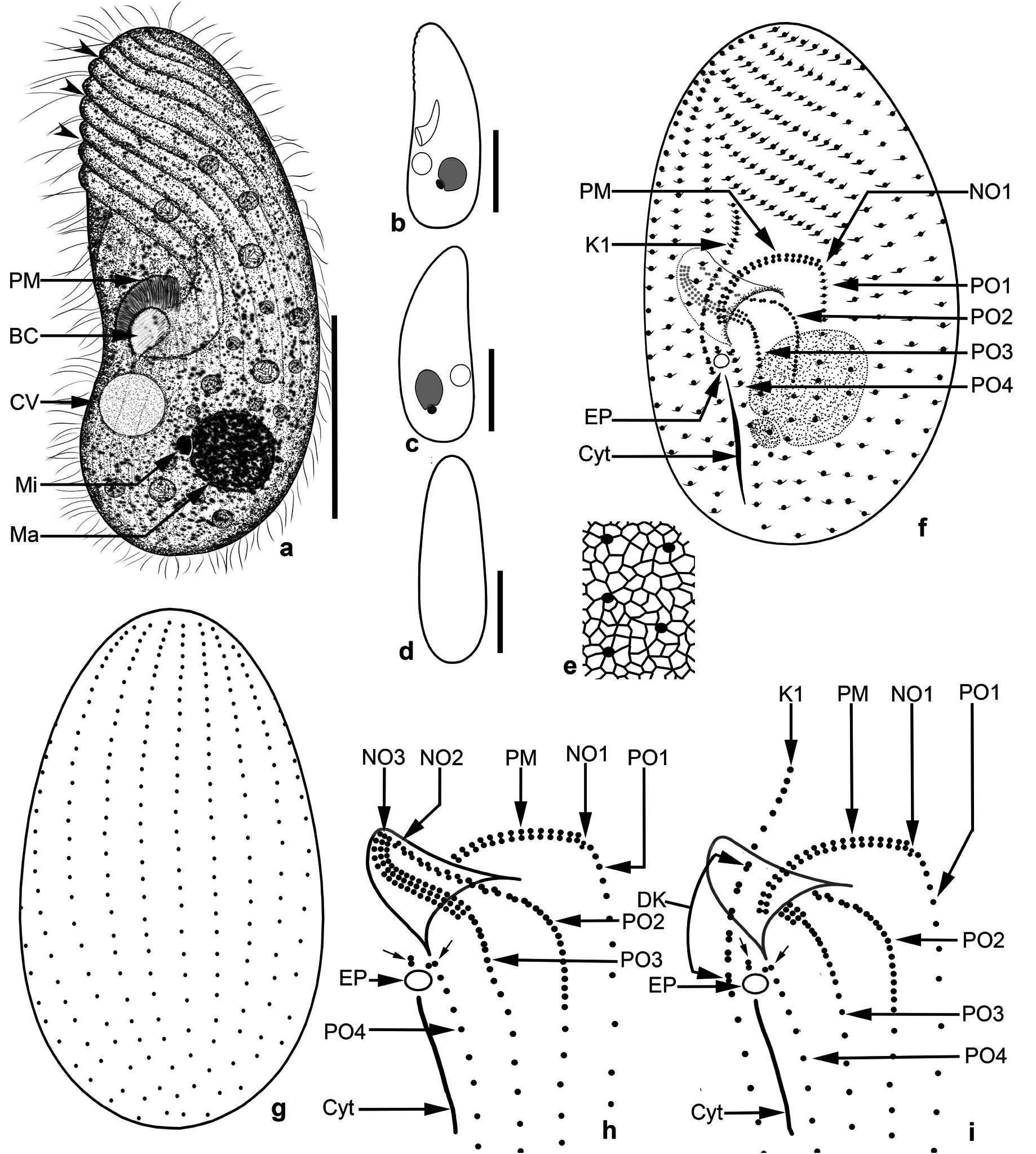
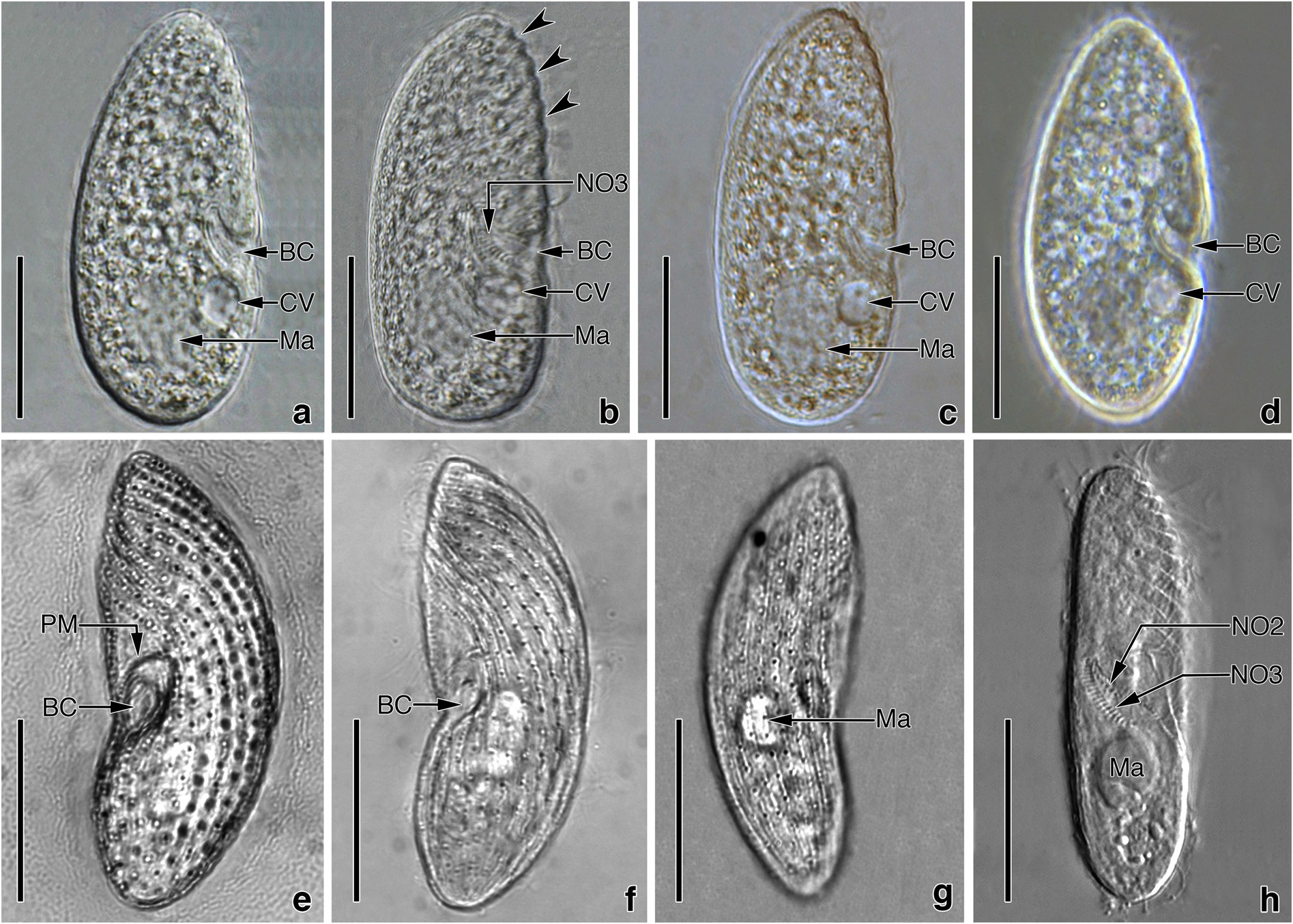
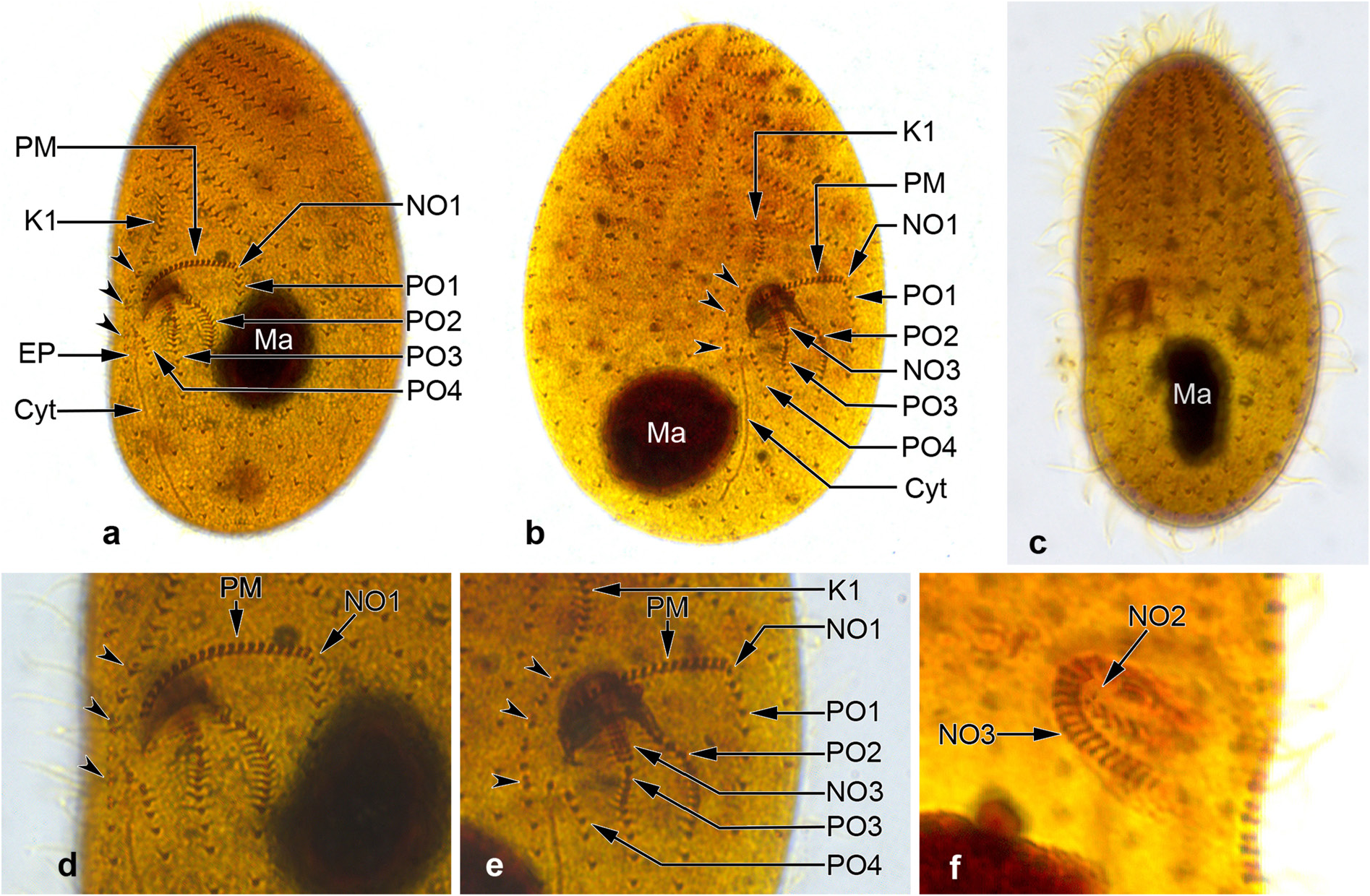
Colpodidium zelihayildizae n. sp. ( Figures 1a–1i View Figure 1 , 2a–2h View Figure 2 , and 3a–3f View Figure 3 ; Table)
Diagnosis: Size about 50 × 20 µm, in vivo; macronucleus in the last third of the cell. Cytoplasm colorless and densely granulated. No extrusomes. On average, 21 somatic ciliary rows and paroral membrane has 20 dikinetids. Buccal opening slightly below the equatorial plane. NO3 composed of 16−17 ciliary rows, each with 3 basal bodies.
Type locality: A dry irrigation channel located in the grassed area of the Faculty of Agriculture of Van Yüzüncü Yıl University (38°34′22′′ N, 43°17′36′′ E) GoogleMaps .
Type slides: The holotype (2020/H01) and 2 paratypes (2020/P01 and 2020/P02) slide with silver nitrate impregnated specimens were deposited in Van Yüzüncü Yıl University, Ciliate Research Collection, Van, Turkey .
Dedication: The name of the species, zelihayildizae , was chosen in dedication to the author’s mother, Zeliha Yıldız, who recently passed away.
Description: Size 40−60 × 15−20 mm, usually 50 × 20 µm in vivo. Shape fairly constant, lateral view elongated-ellipsoidal with more or less flat ventral and distinctly convex dorsal side ( Figures 1a–1d View Figure 1 and 2a–2h View Figure 2 ); length:width ratio 2.3−3.4:1, usually 3: 1 in silver nitrate impregnated specimens (Table). Laterally slightly flattened, ventral and dorsal view elongated ellipsoidal ( Figure 1d View Figure 1 ). Macronucleus underneath the mid-body, usually in the last quarter, ellipsoidal to spherical ( Figures 1a–1c, 1f View Figure 1 , 2a– 2c, 2h View Figure 2 , and 3a–3c View Figure 3 ), about 9 × 6 µm in size (Table), with the roundish nucleolus. Micronucleus globular to ellipsoidal ( Figures 1a–1c View Figure 1 ), difficult to recognize because of the small and in shallow indentation of the macronucleus, about 1.75−2.0 µm across. Contractile vacuole subequatorial, underneath the buccal cavity ( Figure 1a View Figure 1 ), surrounded by small vesicles during diastole; excretory pore in line with the paroral membrane underneath the right edge of buccal entrance ( Figures 1f, 1h, and 1i View Figure 1 ). Cytopyge commences from underneath the excretory pore and extends up to almost the posterior pole ( Figures 1f, 1h, and 1i View Figure 1 ). No extrusomes recognizable in either the live or impregnated specimens. Cortex distinctly furrowed by ciliary rows, especially in the anterior half ( Figures 1a View Figure 1 , 2b, and 2h View Figure 2 ). Cytoplasm colorless, contains many bright fat globules and food vacuoles with bacterial remnants ( Figures 1a View Figure 1 , and 2a–2d View Figure 2 ). Swims by rotation around the main body axis.
Somatic ciliary rows consist of 19−22 monokinetal ciliary rows and are equidistantly arranged ( Figures 1f, 1g View Figure 1 , and 3a–3c View Figure 3 ; Table), except for more widely spaced postoral kineties ( Figures 1f, 1h, 1i View Figure 1 , 3a, 3b, 3d, and 3e View Figure 3 ). Left antero-lateral kineties on the ventral surface (about 8−9 rows) sinistrally twisted, gradually shortened from the anterior body end to oral apparatus, and form a prominent preoral suture ( Figures 1f View Figure 1 , 2e, 2f, 2h View Figure 2 , 3a, and 3b View Figure 3 ). Right ventro-lateral kineties almost parallel to the main body axis, except for K1, which is sigmoidally curved ( Figures 1f, 1i View Figure 1 , and 3a–3c View Figure 3 ). K1 commences pre-equatorially with closely spaced monokinetids, extends along the right edge of the oral apparatus with dikinetids restricted to the area between the right side of the paroral membrane and excretory pore of the contractile vacuole, and terminates close to the posterior pole with ordinarily spaced (5−7 kinetids) monokinetids ( Figures 1f, 1i View Figure 1 , 3a, 3b, 3d, and 3e View Figure 3 ). Three or 4 of the right ventro-lateral kineties shortened gradually from the anterior end to the oral apparatus ( Figures 1f View Figure 1 , 2e, 2f, 2h View Figure 2 , 3a, and 3b View Figure 3 ). A blank area without kineties above the paroral membrane ( Figures 1f View Figure 1 , 2a, and 2b View Figure 2 ). Four postoral kineties ( PO 1− PO 4): PO 1 at the left edge of the buccal cavity, almost parallel to the main body axis, commences beneath NO1 and the proximal end of the paroral membrane, monokinetal throughout; PO 2 commences in the proximal region of the buccal cavity, dikinetidal in the anterior half, monokinetidal in the posterior; PO 3 commences at the distal end of NO3, dikinetidal anteriorly, monokinetidal posteriorly; and PO 4 commences on the left side of the excretory pore of the contractile vacuole, monokinetidal throughout; 2 dikinetid pairs above the excretory pore ( Figures 1f, 1h, 1i View Figure 1 , 3a, 3b, 3d, and 3e View Figure 3 ).
Somatic cilia about 8−10 mm long in live specimens ( Figure 1a View Figure 1 ), cilia very closely spaced in the anterior region of PO 2 and PO 3, and K1 ( Figures 1f View Figure 1 , 2a, 2b, 2d, and 2e View Figure 2 ).
The distance from the apex to the anterior end of paroral membrane and oral cavity is 43% and 50% of the body length, respectively (Table). Considering that the buccal opening is 5−6 µm in size, the oral apparatus is slightly under the equatorial plane. Buccal cavity horn-shaped ( Figures 1a View Figure 1 , and 2a–2d View Figure 2 ) and about 8−9 µm deep, contains NO2 and NO3, and the anterior portion of PO 2 ( Figures 1f, 1h, 1i View Figure 1 , 3b, 3d, and 3e View Figure 3 ). NO1 between the left edge of paroral membrane and anterior end of PO 1, small because it consists of only 2 kinetosomes, difficult to distinguish from the kinetosome pairs of the paroral membrane ( Figures 1f, 1h, 1i View Figure 1 , 3a, 3b, 3d, and 3e View Figure 3 ). NO2 is at the proximal end of PO 2 and difficult to recognize ( Figures 1h View Figure 1 and 3f View Figure 3 ). NO3 extends on the dorsal wall of the CV: coefficient of variation; M: median; Max: maximum value; Min: minimum value; N: number of specimens evaluated; SD: standard deviation of arithmetic mean; SE: standard error of arithmetic mean; x: arithmetic mean.
buccal cavity to the cytostome, orientated obliquely to the main body axis, proximal end bent in a hook-like shape ( Figures 1h View Figure 1 , 2h View Figure 2 , 3b, and 3e View Figure 3 ), composed of about 16−17 ciliary rows with 3 basal bodies each. Paroral membrane extends in the flat bow along the right and upper margins of the buccal opening conspicuously, composed of 20 dikinetids that are orientated perpendicularly to the kinety axis, equidistantly spaced ( Figures 1a, 1f, 1h, 1i View Figure 1 , 2e View Figure 2 , 3a, 3b, 3d, and 3e View Figure 3 ). Silverline system tight and irregularly meshed ( Figure 1e View Figure 1 ).
3.1. Phylogenetic analyses
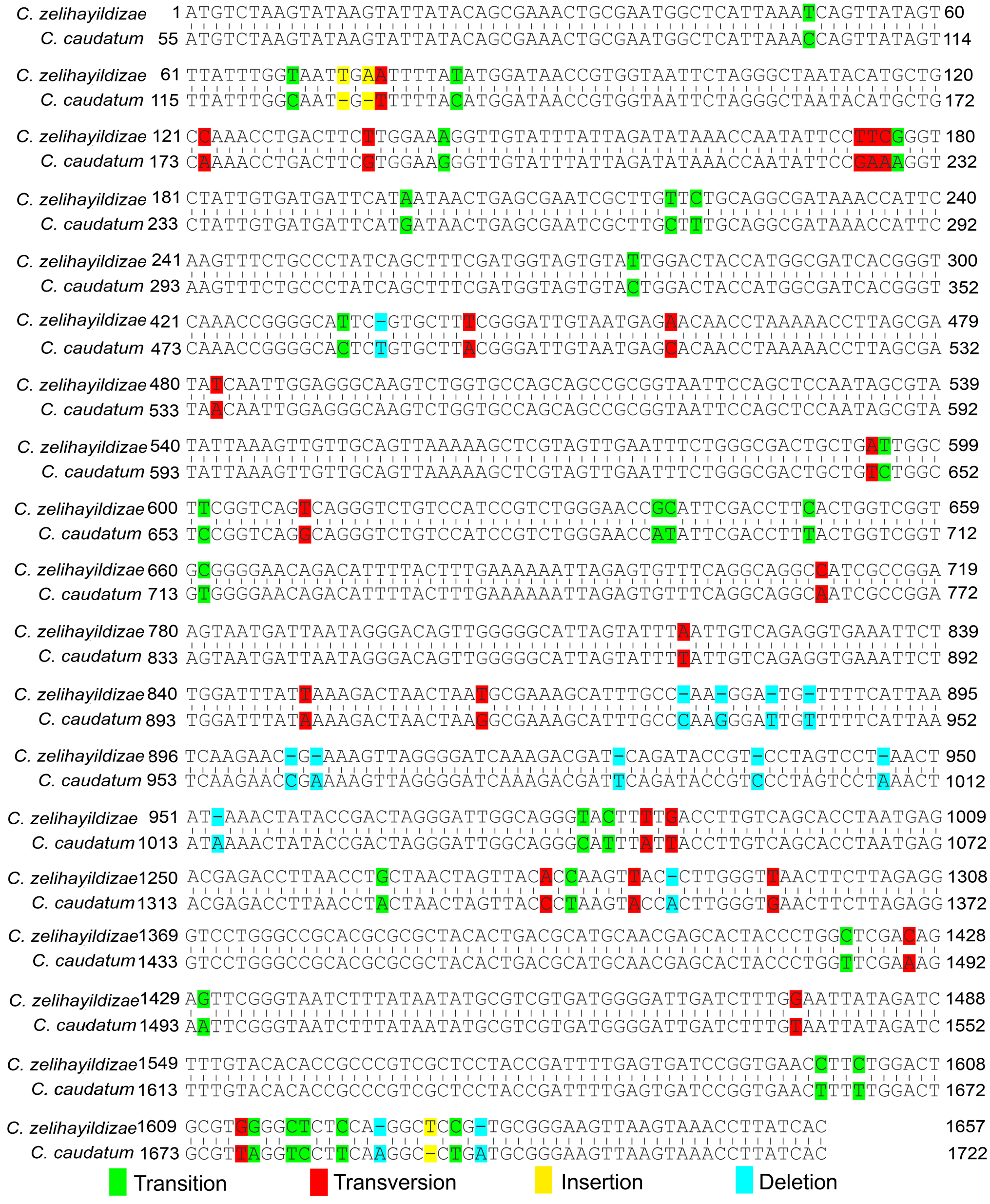
The small subunit rDNA sequence of C. zelihayildizae n. sp. was recorded in GenBank under accession number MW 411350. The length and GC ratio of the current sequence were 1657 bp and 44.72%, respectively. With the current new species included, the genus Colpodidium now comprises 6 species. Of these, the SSU rDNA gene sequences of the other species, except for C. caudatum , are currently unavailable. When the SSU-rDNA gene sequences of C. caudatum and C. zelihayildizae n. sp. were aligned, it was observed that this organism had a difference of 69 base positions ( Figure 4 View Figure 4 ) (3 insertions, 14 deletions, 29 transitions, and 23 transversions).
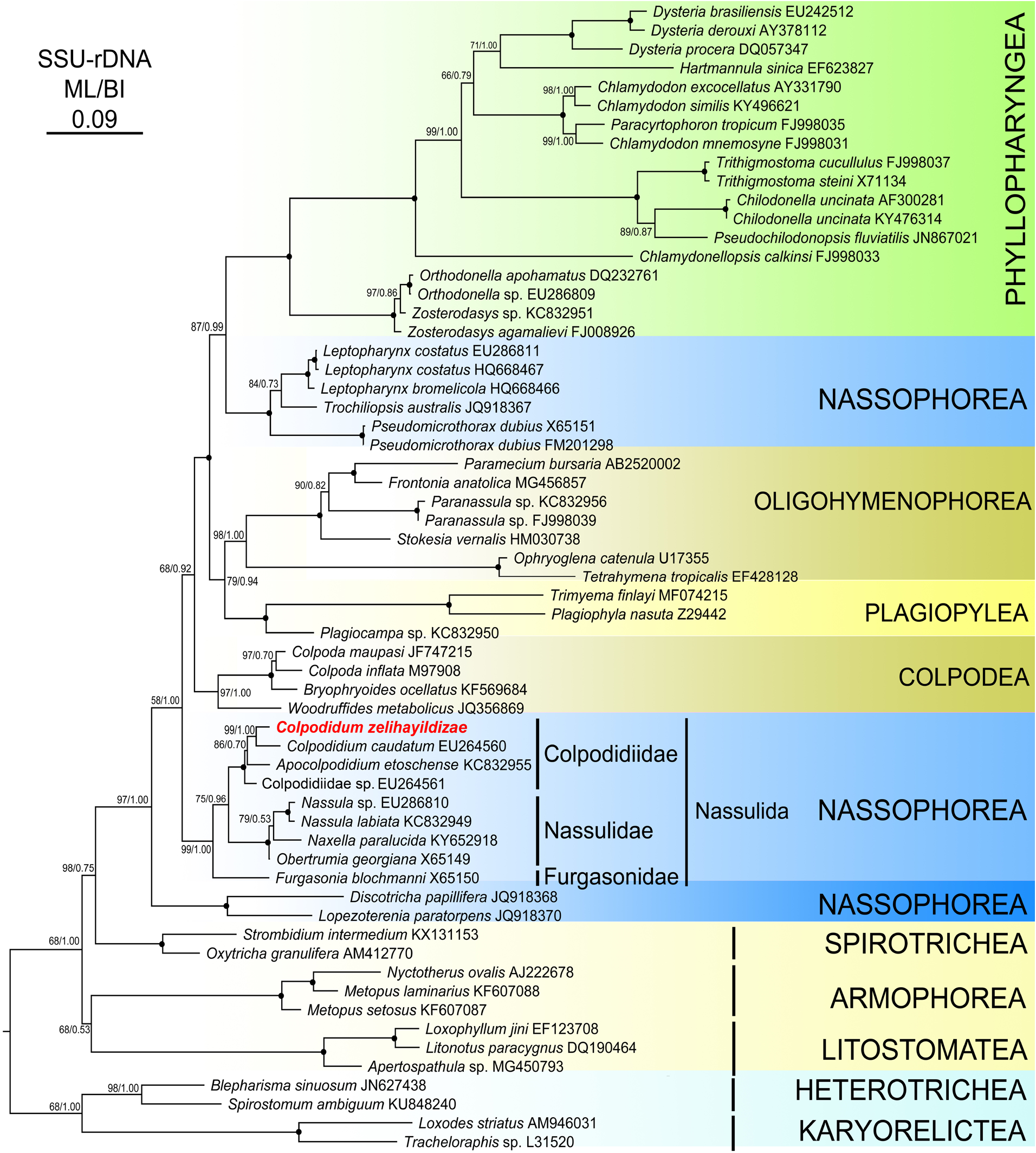
The topologies of both the maximum likelihood ( ML) and Bayesian inference (BI) phylogenetic trees were similar, thus only the ML tree was presented with the bootstrap and posterior probability values on the branches in Figure 5 View Figure 5 . The phylogenetic tree topologies obtained using the different algorithms strongly supported the finding of monophyletic groups at the class level (except for Nassophorea) of taxa in phylum Ciliophora. Nassophorea was distributed into 3 clades and most of its members were clustered with different lineages. The clade Nassulida , where Colpdidium zelihayildizae n. sp. was included, was separated into 3 subclades, namely Colpodidiidae , Nasuliidae, and Furgasonidae . Apocolpodidium etoschense was branched as a sister group with the C. caudatum and C. zelihayildizae n. sp. clade ( ML /BI, 86/0.70) and Colpodidae sp. formed a sister taxon to the branch that comprised the above 3 species ( ML / BI, 100/1.00). C. zelihayildizae n. sp. showed the nearest relationship to C. caudatum ( ML /BI 99/1.00), with a genetic distance of 3.6% based on the Kimura-2 parameter model.
| PO |
Collection of the Zoological Institute of the Russian Academy of Sciences |
| CV |
Municipal Museum of Chungking |
| MW |
Museum Wasmann |
| GC |
Goucher College |
| SSU |
Saratov State University |
| ML |
Musee de Lectoure |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |