Dasypus hybridus ( Desmarest, 1804 )
|
publication ID |
https://doi.org/ 10.1093/mspecies/sew001 |
|
publication LSID |
lsid:zoobank.org:pub:0928AD0C-7E42-4757-9F6F-BEF5A5F70D2F |
|
DOI |
https://doi.org/10.5281/zenodo.4589123 |
|
persistent identifier |
https://treatment.plazi.org/id/1F1A879E-CF1C-2E7B-40D9-526AFDEF88CC |
|
treatment provided by |
Felipe |
|
scientific name |
Dasypus hybridus ( Desmarest, 1804 ) |
| status |
|
Dasypus hybridus ( Desmarest, 1804) View in CoL Southern Long-nosed Armadillo
lor [icatus]. hybridus Desmarest, 1804:28 View in CoL . Type locality unknown; based on “ Le tatou mulet de d’Azara ,” which was from “ Paraguay et dans celle des Missions, sans s’approcher beaucoup de la rivière de la Plate.... vers le Sud par les Pampas de Buenos Ayres” ( de Azara 1801:186); type locality restricted to San Ignacio, Misiones, Paraguay, by Cabrera (1957:223).
[ Dasypus] hybridus: G. Fischer, 1814:126 View in CoL . Name combination.
[Dasypus] auritus Illiger, 1815:108. Nomen nudum.
T [atus]. auritus Olfers, 1818:221. Type locality “ Paraguay ”; based solely on “ T. mulet ” of de Azara (1801).
Tatusia hybridus: Lesson, 1827:311 . Name combination.
T [atusia] hybrida: Turner, 1851:213 . Corrected gender agreement.
Praopus hybridus: Burmeister, 1861:428 . Name combination.
Tatu hybridus: Lahille, 1899:203 . Name combination.
[ Tatusia (Muletia) ] hybrida: Trouessart, 1898:1140 . Name combination.
[ Tatus (Muletia)] hybridus: Trouessart, 1905:814 . Name combination.
Muletia hybrida: A. Miranda-Ribeiro, 1914:46 . Name combination.
D [asypus]. Brevi-cauda Larrañaga, 1923:344. Type not stated, but Uruguay implied (p. 242); based on de Azara’s (1802:156) “Mulita.”
Dasypus hibridus: Azevedo, El Achkar, Martins, and Ximenez, 1982:95 . Incorrect subsequent spelling of Loricatus hybridus Desmarest, 1804 .
CONTEXT AND CONTENT. Context as for genus. Synonymy is modified from Wetzel et al. (2008). Dasypus hybridus View in CoL is monotypic.
NOMENCLATURAL NOTES. The generic name, Dasypus , means hairy or rough-footed (dasy from dasys, Greek, meaning hairy, rough + pus from pous, Greek, meaning foot; see Braun and Mares 1995). The species name, hybridus (from hybridis, meaning a hybrid), possibly refers to the 7 movable bands that gave the species the appearance of being a hybrid between the 9-banded armadillo ( Dasypus novemcinctus ) and the 7-banded armadillo ( Dasypus septemcinctus —Braun and Mares 1995).
Other common names in Spanish are mulita, mulita orejuda, mulita chica, and mulita pampeana, and in Portuguese tatuíra and tatú-mulita (Superina and Aguiar 2006; Abba and Vizcaíno 2011). It is sometimes called the southern lesser long-nosed armadillo in English.
DIAGNOSIS
Dasypus hybridus is a small armadillo that usually bears 7 movable bands on its gray carapace and has a relatively long tail, long rostrum, and long, parallel ears that are, however, relatively shorter than in other Dasypus ( Fig. 1 View Fig ; Wetzel and Mondolfi 1979). D. hybridus lacks the protruding scales on the hind legs characteristic of the greater long-nosed armadillo ( D. kappleri ) and the dense, light brown hair on the dorsum of the woolly armadillo ( D. pilosus ). It has a smaller head and shorter body length, a proportionally shorter tail (<70% compared to ≥ 70% of head and body length), fewer movable bands, and a more uniform carapace color than the partially sympatric D. novemcinctus (whose carapace is sometimes yellow on the sides—Wetzel 1985a). Palate length of D. hybridus is 66% of naso-condyle length, compared to 69% in D. novemcinctus ( Wetzel 1985b) . D. hybridus is larger, has shorter ears and fewer movable bands, and is distributed farther south than D. septemcinctus . The llanos long-nosed armadillo ( D. sabanicola ) is similar in size, but has a greater number of movable bands and is restricted to Colombia and Venezuela ( Wetzel 1985b). D. hybridus is slightly smaller than Yepes’s mulita ( D. yepesi —Vizcaíno 1995) and much smaller than D. kappleri .
GENERAL CHARACTERS
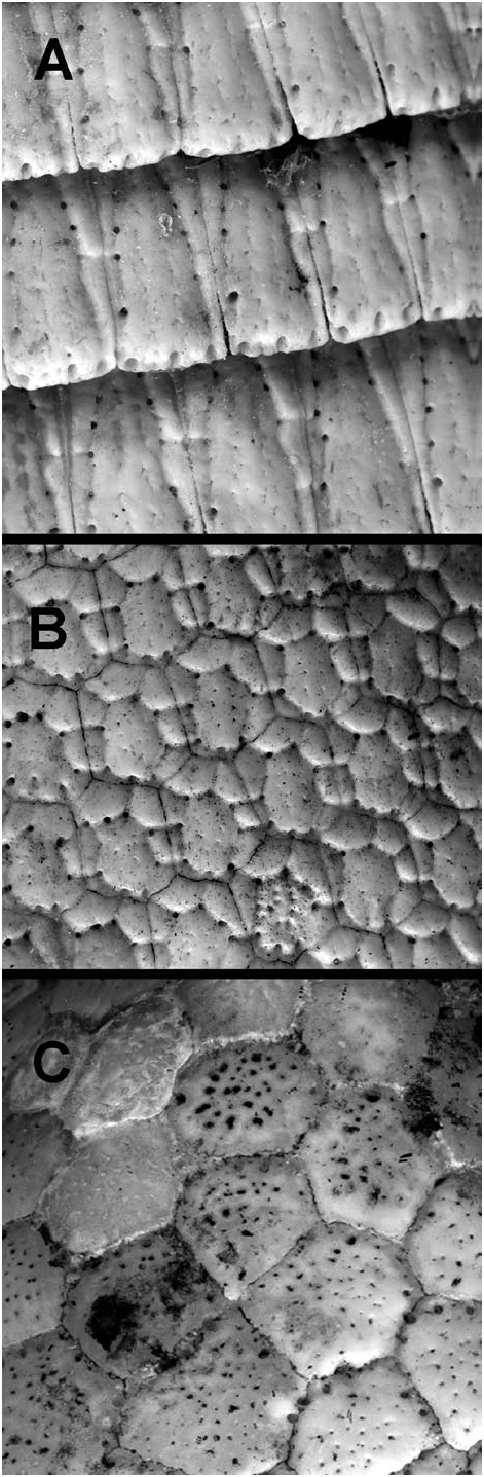
Like all Cingulata , Dasypus hybridus bears a carapace consisting of ossified dermal scutes (osteoderms) that are covered by epidermal scales ( Figs. 2 View Fig A–C). Six to 7 (sometimes 8) bands separate the scapular and pelvic shields ( Wetzel 1985b). The tail and top of the head are also covered with scutes. The dark gray carapace is sparsely haired. Each osteoderm of the movable bands is about 20 mm long and 5 mm wide ( Fig. 2A View Fig ) and bears 3 triangular figures (sectors), the central one with an apex pointing anteriorly and the lateral ones posteriorly. There are 6–7 perforations in the groove that separates the figures. Two parallel, longitudinal lines of small perforations are present on the central figure. Usually there are 3–4 perforations in the posterior border (Vizcaíno and Bargo 1993). The osteoderms of the scapular and pelvic shields are about 5 mm long and 5 mm wide ( Fig. 2B View Fig ). They are predominantly hexagonal and bear a subcircular main figure that is somewhat displaced toward the rear and surrounded by up to 8 small, peripheral figures. One perforation in each intersection of the groove delimits the main figure from the grooves that separate the small peripheral figures from each other (Vizcaíno and Bargo 1993). The osteoderms of the head shield vary in size and shape and lack figures ( Fig. 2C View Fig ).
The tail is 67–70% of body length, bears distinct rings on the proximal two-thirds, and has a slender tip ( Hamlett 1939; Wetzel and Mondolfi 1979). The head is slender, with the rostrum forming 55–58% of the length of the skull ( Fig. 3 View Fig ), and bears a trapezoidal head shield. The relatively long ears reach 25–33% of the head length and are closer together than in other armadillo genera ( Hamlett 1939; Wetzel and Mondolfi 1979). The 4 toes (see “Form and Function”) on the forefeet and 5 on the hind feet are equipped with large decurved claws (Wetzel and Mondolfi 1979).
Mean external measurements (mm ± SD, with parenthetical n) given by Wetzel (1985b) for adult specimens (mixed sexes) from Argentina (n = 10), Uruguay (n = 11), and Brazil (Rio Grande do Sul; n = 5) were total body length (from the tip of the snout to the base of the tail), 297.3 ± 10.6 (11); tail length, 169.0 ± 9.8 (11); hindfoot length, 66.3 ± 3.9 (7); ear length, 27.4 ± 2.6 (11). Range of measurements (mm, with parenthetical n) of animals from several localities (Feijó and Cordeiro-Estrela 2014) were tail length, 150–185 (9); ear length, 20–27 (8); skull length, 69–81.07 (24); condylobasal length, 66.3–75.5 (22); nasal length, 20.67–25.51 (21); zygomatic width, 28.69–34.04 (24); braincase width, 23.96– 27.3 (24). Mean measurements (mm, of 39 adults— 14 males and 25 females of Buenos Aires province, Argentina—Abba et al. 2011) were tail length, 163.10; ear length, 26.17; head length, 83.03; length of scapular shield, 162.21; length of front band, 269.08; length of back band, 264.05; total body length, 255.57; tail base circumference, 104.95 (for details about the measurements, see Loughry and McDonough 1996; Abba et al. 2011). No sexual dimorphism has been observed (Abba et al. 2011). Adult D. hybridus have a mean body mass of about 2 kg ( Wetzel 1985b, 2.04 kg, n = 4; Abba et al. 2011, 2.01 kg, n = 39).
The number of movable bands given by Wetzel (1985b, mean ± SD, with parenthetical n) is 6.9 ± 0.6 (20); that of 28 D. hybridus from Buenos Aires is 6.5 ± 0.5 (Abba 2008); and de Azara (1801) described 6 adults with 6 bands, 1 with 7 bands, and 8 fetuses with 5 bands. The number of scutes on the 4th movable band given by Feijó and Cordeiro-Estrela (2014, n = 20) is 46–55, and the number given by Wetzel (1985b) is 52.6 ± 2.2 (n = 20).
Condyle-nasal length is 70.2 ± 2.2 mm (mean ± SD, n =26), the adjusted rostral length (distance along midline of rostrum from a line through lacrimal foramina to tip of nasal bone) is 39.1 ± 1.6mm (n = 26), and the ratio of the adjusted rostral length to condyle-nasal length is 0.56 ± 0.01 (n = 26— Wetzel 1985b). The palate is long, extending posteriorly beyond the margin of the toothrows. The pterygoids contribute to the posterior surface of the palate, except at the midventral point of the posterior border. The mandible is proportionally more slender than in other armadillos. The proximal segment of the anterior wing of the hyoid points toward the midline of the palate (Wetzel and Mondolfi 1979).
DISTRIBUTION
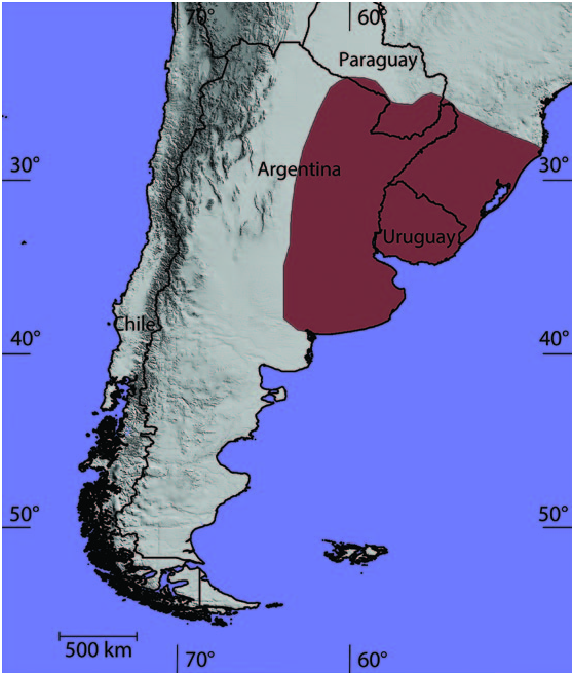
Dasypus hybridus is found in Argentina, Uruguay, Paraguay, and southern Brazil ( Fig. 4 View Fig ). The exact northern limit of its range is uncertain due to its morphological similarity with D. septemcinctus , and the western limit is unclear due to its similarity with D. yepesi . Reported from the provinces of Buenos Aires, Chaco, Córdoba, Corrientes, Entre Ríos, Formosa, La Pampa, Misiones, Santa Fe, and Santiago del Estero (Abba and Superina 2010; Abba and Vizcaíno 2011; Abba et al. 2012), it is widely distributed in Argentina. It also occurs in all of Uruguay except Montevideo (Fallabrino and Castiñeira 2006; Ameneiros et al. 2015). The records from Paraguay are limited to 1 specimen lacking specific locality data and another from Departamento Canindeyú ( Smith 2012). In Brazil, D. hybridus occurs in the provinces of Paraná ( Reis et al. 2010), Rio Grande do Sul ( Corrêa et al. 2013; Oliveira et al. 2013), and Santa Catarina ( Cherem et al. 2004; Spezia et al. 2013). Its presence in São Paulo State, Brazil, is uncertain: the single record of Jorge et al. (1985) and those of Anacleto et al. (2006) may be D. septemcinctus .
FOSSIL RECORD
The genus Dasypus is known from the early Pleistocene ( Vizcaíno et al. 1995). Dasypus hybridus is known from the late Pleistocene (Lujanian Stage/Age) to Recent of South America (Carlini and Scillato-Yané 1999). The earliest record of this species is from Buenos Aires Province, where it was found in the local mammalian biozone Equus (Amerhippus) neogaenus (about 130,000 years ago—Cione and Tonni 1999, 2005). In summary, the existence of D. hybridus dates back about 100,000 years.
FORM AND FUNCTION
Form. —Armadillos of the genus Dasypus are the only living Xenarthra that retain 2 functional generations of teeth and have enamel ( Ciancio et al. 2012). The thin enamel layer is present only at the tip and can be observed only in unworn permanent molars ( Ciancio et al. 2014). The adult dentition of Dasypus typically consists of 8/8 homogeneous molariform teeth (Mf/mf), although many specimens have only 7 teeth, the anteriormost locus being empty. None of the upper teeth are placed in the premaxilla. The anterior 7 loci in both the maxilla and dentary are replaced; only the posteriormost tooth (Mf/mf 8) erupts later ( Ciancio et al. 2012). The order of dental eruption in Dasypus is variable. One specimen studied by Ciancio et al. (2012) had a full complement of deciduous teeth along with the erupted molar in each series. Another specimen had most upper and lower permanent premolars (P/p3–P/p7) but lacked fully erupted molars in the dentary and had only 1 upper molar on the left side. In some individuals, the molar fails to erupt on one side, without a sign of an alveolus. Eruption of the permanent dentition generally occurs from posterior to anterior, with upper and lower molars erupting first, followed by P/p4–7, P/p3, P/p2, and finally P/p1 ( Ciancio et al. 2012). The permanent teeth are euhypsodont (ever-growing, high-crowned teeth, with an open pulp cavity), conical when first erupted, and become cylindrical with growth and wear ( Ciancio et al. 2012). The eruption of permanent teeth is considered to occur relatively late, as previously observed in afrotherians ( Ciancio et al. 2012).
The skin of D. hybridus on the ventral side bears small, dark brown prominences (hair follicles and glands) of 1–3 mm diameter with 1, 2, or more hairs in their central part. The skin is thinnest on the neck, tail, and immediately below the external carapace edge. It is also thin and smooth on the inner side of the legs, although it is thick and wrinkled on the abdomen (Cuba- Caparó 1978). The hair follicles in the areas of thin skin are typical but smaller (Cuba-Caparó 1979).
Both lungs have apical, medial, and diaphragmatic lobes (Cuba-Caparó 1979). The digestive tract follows the typical structure of monogastric mammals. Histological features of D. hybridus have been described by Cuba-Caparó (1978). The mean length of the small intestine is 145.6 ± 12.64 cm (n = 9—A. M. Abba and M. C. Ezquiaga, in litt.). The cecum is histologically similar to the large intestine ( Estecondo et al. 1996). The liver consists of 4 lobes, 2 of which are visible only from the abdominal side (Cuba- Caparó 1978). The spleen, a relatively simple structure (Casanave and Galíndez 2008) with a smooth, deep red surface, weighs <1% of body mass in adults but 2.3–3.9% of body mass in newborns ( Galíndez et al. 2000). The spleen consists of red pulp formed by sinuses and with little lymphoidal tissue (Cuba-Caparó 1978) and has a bilayered fibromuscular capsule and numerous trabeculae ( Galíndez et al. 2000). Its hemopoietic function is limited to erythrocytic and megakaryocytic lines; there is no evidence for granulopoiesis ( Galíndez et al. 2000). The main lymph nodes are the cervical superficial, axillary, inguinal, cervical, prescapular, tracheobronchial, abdominal, mesenteric, and preaortic (Cuba- Caparó 1978, 1979). The olfactory mucosa follows the typical mammalian organization ( Ferrari et al. 2000).
Blood can be collected from the coccygeal vein ( Luaces et al. 2011). Hematological parameters given by Cuba-Caparó (1976) for males (n = 10) and females (n = 16), respectively, were (mean ± SEM): packed cell volume, 40.47 ± 1.26 and 39.67 ± 1.40 ml/ dl; sedimentation rate, 15.3 ± 3.26 and 10.6 ± 2.01 mm /h; red blood cell count, 6.001 ± 0.128 and 5.963 ± 0.170· 106 /mm 3; white blood cell count, 8.425 ± 1.417 and 10.084 ± 1.001· 103 / mm 3; hemoglobin, 16.14 ± 0.66 and 16.84 ± 0.52 g /dl; mean cell volume, 67.30 ± 1.94 and 68.04 ± 3.65 μ 3; mean cell hemoglobin, 26.87 ± 1.11 and 28.66 ± 1.15 μμg; mean corpuscular hemoglobin concentration, 40.12 ± 1.93% and 42.74 ± 1.64%. Absolute leukocyte values per mm 3 given by Cuba-Caparó (1976) for males (n = 10) and females (n = 16), respectively, were (mean ± SEM): banded neutrophils, 456 ± 6.7 and 195 ± 52.90; polymorphonuclear neutrophils, 4,461 ± 650.0 and 5,716 ± 749.4; eosinophils, 173 ± 62.4 and 138 ± 26.0; basophils, 81 ± 30.9 and 50 ± 15.9; monocytes, 369 ± 103.2 and 527 ± 80.3; lymphocytes, 2,877 ± 673.3 and 3,455 ± 419.1. Gender differences were not statistically significant. The lymphocytes lack receptors for the Fc-segments of immunoglobulins ( Sasiain et al. 1977).
Dasypus females have 4 inguinal mammary glands (Wetzel and Mondolfi 1979). The simple pear-shaped uterus is flattened dorsoventrally, with a convex surface of the fundus. It measures from 2.5 to 3.0 cm at its widest point and is 4.0–5.0 cm long ( Cetica et al. 2005). “The cervix leads to a tubular structure where the columnar epithelium changes abruptly to transitional epithelium, forming a urogenital sinus rather than a true vagina” ( Cetica et al. 2005:60). The urogenital sinus is large, slightly arched, and 1.5–2.0cm long. The large vulva ranges from 0.8 to 1.0 cm ( Cetica et al. 2005). Disk-shaped ovaries with a slight central concavity facing the infundibulum, and a slight convexity facing the mesovarium, are enclosed in a bursa ovarica and lie parallel to the longitudinal axis of the body, with the concavity corresponding to the parenchymatous zone and the convexity to the vascular zone of the ovary (Codón and Casanave 2000; Cetica et al. 2005). The diameter of the ovary varies from 6 to 9 mm and its thickness is about 5 mm ( Cetica et al. 2005). The ampulla of the 2.0– 2.5 cm ( Cetica et al. 2005) oviduct coils around the tubal extremity of the ovary and continues caudally along the concave side up to the middle of the ovary. Primordial follicles are mainly concentrated in the concavity. A corpus luteum was observed in 1 out of 5 apparently nonpregnant individuals collected in March (Codón and Casanave 2000).
The intra-abdominal testes produce spermatozoa that average 63.07 µm ( Cetica et al. 1993). Their heads are relatively small (6.37 µm long and 3.76 µm wide), oval in the frontal view, and with an ensiform profile ( Cetica et al. 1993; Cetica and Merani 2008). The penis is similar to that of D. novemcinctus and D. sabanicola (see Wetzel and Mondolfi 1979: figure 2), and somewhat thickened at its base. It has a trifid appearance due to a distal pair of lateral lobes flanking a pointed tip (Wetzel and Mondolfi 1979).
The forefoot of D. hybridus has a reduced 5th digit. Digits II and III are similar in size and longer than digits I and IV. A series of modifications in the carpus, including a reduced contact among carpals and metacarpals, separate this species from the typical form attributed to scratch-diggers ( Galliari 2014). Vizcaíno and Milne (2002) and Milne et al. (2009) classified the Dasypodini tribe as generalized diggers, with only Tolypeutes being less fossorial (armadillo tribes in increasing order of fossoriality: Tolypeutini , Dasypodini , Euphractini , Priodontini , Chlamyphorini ). Vertebral formula of D. hybridus is 7 C, 9–11 T, 4–5 L, 9 S, and 22 Ca, total 51–54 ( Fitzinger 1871; Galliari et al. 2010).
Function. —Like all Xenarthra , Dasypus hybridus has poor thermoregulatory abilities. The rectal temperature of wild D. hybridus varies from 33.1°C to 38.4°C, with a mean of 36.0°C (Abba et al. 2011). At a constant ambient temperature of 21°C, the morning body temperature of D. hybridus is between 29.5°C and 32°C (Cuba-Caparó 1976). Abba et al. (2011) proposed that D. hybridus might enter torpor and hibernation, as observed in another armadillo species, the pichi, Zaedyus pichiy (Superina and Boily 2007) . Abba et al. (2011) observed a positive correlation of the rectal temperature of wild individuals with environmental temperature. In Buenos Aires province, Argentina, rectal temperatures are higher in summer than in winter, in young than in juveniles (yearlings) and adults, and in active than in resting individuals (Abba et al. 2011).
ONTOGENY AND REPRODUCTION
According to observations of mating behavior in captive individuals, the reproductive season is initiated in March ( Ferrari et al. 1997). Dasypus hybridus young are born from October to the beginning of December ( de Azara 1801; Ferrari et al. 1997). Females give birth inside their burrows, in nests made of plant material (Abba et al. 2011). Neonates average 47.54 g (n = 11—F. Galliari, in litt.).Young are born with their eyes open and continue to nurse until weaned at 2 months of age (Cuba-Caparó 1979). When emerging to forage, the female leaves her offspring inside the burrow, carefully closing the burrow entrance with plant material or soil, presumably to reduce the risk of predation on the young ( de Azara 1801). Juveniles are mainly observed in the austral spring–summer (Abba 2008; Abba and Cassini 2010a). The osteoderms are partially developed at birth, which together with other tissue characteristics of the postcranial skeleton suggests that the young of this species are precocial ( Krmpotic et al. 2012).
Dasypus hybridus has a hemochorial placenta, the maternal face of which is uniformly villous except in the areas of insertions of the umbilical cords ( Adamoli et al. 2001). Gestation length is about 7 months (Cuba-Caparó 1979). Polyembryony results in 6–10 or even up to 12 presumably monozygotic (genetically identical) offspring ( Fernandez 1915; Mañé-Garzón 1977; Wetzel and Mondolfi 1979). The embryology of D. hybridus is described in detail by Fernandez (1915).
ECOLOGY
Dasypus hybridus mainly inhabits grassland habitats on humic soils from near sea level up to 2,300 m above sea level ( Abba et al. 2007, 2012; Abba and Cassini 2010b). Dependent on natural and seminatural grasslands, it appears to prefer areas of low disturbance with high vegetation cover and avoids cultivated pastures ( Abba et al. 2007; Abba and Cassini 2010b). D. hybridus is more common in farmlands with extensive cattle ranching than in crop fields ( Abba et al. 2015) and is rarer in agricultural lands, corn and soybean stubble, and on roadsides (Abba and Vizcaíno 2011); it has been reported to use woodlands in some areas (Abba and Cassini 2008). In Brazil, it is predicted to occur in the Pampa and, marginally, the Atlantic forest biomes ( Anacleto et al. 2006). Its distribution is limited by the precipitation levels during the driest quarter of the year ( Abba et al. 2012). Based on a species distribution model for Argentina, 27% of the population inhabits the “Pampas” grasslands, 20% the “Espinal” ecoregion, 16% the “Monte” of plains and plateaus, 15% the arid Chaco, and 7% the humid Chaco. Seven different ecoregions account for the remaining 15% ( Abba et al. 2012).
Dasypus hybridus digs and uses burrows for shelter, resting, and thermoregulation. The burrows are usually built on flat or gently sloping ground, more commonly in open fields than in ravines or near rocks (Redford and Eisenberg 1992; González et al. 2001). Usually a single (sometimes 2) cylindrical entrance of less than 25 cm in diameter is hidden under roots, shrubs, or rocks (Redford and Eisenberg 1992; Superina 2000; González et al. 2001). The entrance may point to any cardinal direction but often is oriented away from the direction of dominant winds ( González et al. 2001). More than one-half of burrow entrances have dried plant matter in the mouth or in the first 30 cm of the tunnel ( González et al. 2001). Burrows are linear without branches, with a conical end. Measurements (cm; mean ± SD) of 20 excavated burrows in Uruguay were length, 118.8 ± 105.69; width, 15.3± 5.15; depth, 43.4 ±10.22 ( González et al. 2001). One individual dug a 43-cm burrow in 1 night ( de Azara 1801). Nests made of plant matter have been observed near the burrow entrance, at its end, and also in the middle of the tunnel. As observed by one of us (AMA) and González et al. (2001), sometimes the nest is placed in a widened section of the burrow (A. M. Abba, in litt.).
Dasypus hybridus is described as an opportunistic insectivore ( Redford 1985) or an omnivore with a strong tendency to myrmecophagy (Abba et al. 2011). It digs small and shallow foraging holes, called “hozaduras,” to feed. Stomachs contained mainly ants (49%) and termites (9%), but also some other invertebrates and even young mice ( Barlow 1965). All 32 fecal samples analyzed by Abba et al. (2011) contained remains of arthropods, mainly ants and coleopterans, and large amounts of plant material. Traces of vertebrates (small mammals, amphibians, and reptiles) were detected in less than 1% of D. hybridus feces.
There is no published information about the natural predators of D. hybridus . However, our field observations suggest that this species, particularly when young, is prey of blackchested buzzard eagles ( Geranoaetus melanoleucus ), southern crested caracara (Caracara plancus), lesser grison ( Galictis cuja ), Pampas fox ( Lycalopex gymnocercus ), and Geoffroy’s cat ( Leopardus geoffroyi ). Other potential predators are mediumto large-sized carnivorous mammals, such as crab-eating fox ( Cerdocyon thous ), cougar ( Puma concolor ), jaguar ( Panthera onca ), and ocelot ( Leopardus pardalis ); and raptors such as great black hawk ( Buteogallus urubitinga ), crowned eagle ( Buteogallus coronatus ), and white-tailed hawk ( Geranoaetus albicaudatus ). Juvenile D. hybridus could also be prey of raptors of open areas, such as Parabuteo , long-winged hawk ( Circus buffoni ), zone-tailed hawk ( Buteo albonotatus ), roadside hawk ( B. magnirostris ), and savanna hawk ( Buteogallus meridionalis ), among others. Domestic and feral dogs ( Canis lupus familiaris ) are among the most relevant predators of D. hybridus , and the presence of these domestic animals is negatively correlated with D. hybridus abundance ( Abba et al. 2007).
Dasypus hybridus may be a reservoir host for Salmonella ( Quevedo et al. 1978) . The fungus Histoplasma capsulatum has been isolated from individuals in Argentina ( Bogado et al. 1983). In Uruguay, the saprophytic fungus Sporothrix schenckii is known as “hongo mulita” (armadillo fungus), and 50–80% of patients treated for sporotrichosis were confirmed as having been in contact with armadillos or their burrows ( Mackinnon et al. 1969; Bonasse et al. 1987). However, none of 15 examined D. hybridus from Uruguay (erroneously identified as D. septemcinctus by Mackinnon et al. 1969) were infected with Sporothrix . Nevertheless, S. schenckii has caused symptoms of disease in other armadillo species ( Kaplan et al. 1982; Wenker et al. 1998). Infection of armadillo hunters probably occurs when the animals defend themselves with their claws and the resulting skin lesions are contaminated with soil or plant material bearing S. schenckii ( Kaplan et al. 1982) . D. hybridus is one of the few mammals susceptible to Mycobacterium leprae , the causative agent of leprosy ( Storrs et al. 1975; Storrs and Burchfield 1985) and thus has been used in leprosy research in the past ( Baliña et al. 1980, 1985; Franco et al. 1999).
Ectoparasites include the fleas Tunga penetrans ( Ezquiaga et al. 2008) and Rhopalopsyllus lutzi cleophontis (Mauri and Navone 1993) , the mites Dasyponyssus neivai (listed as Dasypus sp. from Corrientes, Argentina in Mauri 1982) and Xenarthronyssus furmani (Lavalleja, Uruguay—Radovsky and Yunker 1971), and the ticks Amblyomma auricularium and A. pseudoconcolor ( Venzal et al. 2002) . D. hybridus is host to a few species of intestinal parasites, including Mazzia bialata ( Navone 1990) , Delicata abbai ( Ezquiaga et al. 2012) , Pterygodermatites (Paucipectines) chaetophracti (Navone and Lombardero 1980) , Aspidodera fasciata ( Ezquiaga 2013) , and undetermined Strongyloides ( Ezquiaga 2013) . Delicata cameroni , Macielia macieli , and Moennigia lutzi are documented in D. hybridus from Brazil ( Vicente et al. 1997), but doubts remain about the correct identification of the Dasypus species (based on the distribution, it was probably D. septemcinctus ). A. fasciata is common with a prevalence of up to 80%, but other parasites do not exceed 10% prevalence ( Ezquiaga 2013). Trypanosoma cruzi has been isolated from the blood of a single D. hybridus from the Artigas Department of Uruguay ( Salvatella 1986).
Miscellaneous. —Similar to virtually all armadillo species, Dasypus hybridus cannot be caught with common live traps. Hence, the best method to capture this species is by hand and/ or using long dip nets. Cone-shaped traps wedged directly into the mouth of the burrow sometimes are effective.
HUSBANDRY
Dasypus hybridus is rarely kept in zoological institutions because it is difficult to maintain due to its excitable temperament ( Superina 2000). However, it has been successfully housed in pens of 6 by 2 m with concrete floor and walls. A 2 by 2 m section of the pen was covered with a roof and communicated through a door with the external part, which measured 4 by 2 m and had 1-m walls ( Ferrari et al. 1997). Inside the covered part of the pen, a nesting box of 1 m 3 made of bricks and a removable wooden cover allowed manipulation and observation of the animals. The box, filled with linen hay for nesting, had an L-shaped roofed entrance of 30 cm height to ensure absolute darkness ( Ferrari et al. 1997).
For temporary visual identification, D. hybridus can be marked with various shapes and colors of reflective tape glued onto different areas of the carapace. It can be permanently marked by injecting a passive induced transponder (PIT) tag under the scapular shield, at its juncture with the neck. Also, a combination of ear notches at different locations around the periphery of each ear allows reliable identification of individuals (Abba 2008).
BEHAVIOR
Dasypus hybridus is diurnal, being most active between 1200 and 1500 h (Abba et al. 2011). Active all year long (Abba and Cassini 2010a), it shows reduced activity during the cold season (Abba and Cassini 2010b). Adult D. hybridus are solitary and observed in pairs only during the reproductive season (Abba et al. 2011). When threatened, D. hybridus runs to hide in an existing burrow, digs a new one, or hides in areas with high vegetative cover (see Abba et al. 2011). Vocalization is limited to a purring sound when captured animals are being handled. D. hybridus shows nest building and bathing behaviors (dipping head under water and rolling in shallow puddles) similar to those described for D. novemcinctus by Eisenberg (1961) and Taulman (1994). One frequently observed behavior is the removal of moist plant material from the burrow, letting it dry in the sun, and then returning it as nest material to the burrow.
GENETICS
The diploid number (2n) for Dasypus hybridus is 64 chromosomes, consisting of 7 pairs of metacentric (2 large, 5 medium) and 24 pairs of acrocentric (4 large, 14 medium, 6 small) autosomes. The X chromosome is submetacentric and the Y is acrocentric ( Saez et al. 1964; Jorge and Pereira 2008). The fundamental number (FN) is 81.
The genetic distinction between D. hybridus and D. novemcinctus has been determined by electrophoresis of hemoglobins and plasma proteins (Ramsey and Grigsby 1985; Ryder and Davis 1985). Gibb et al. (2016) provide mitogenomic data for a D. hybridus sample from Uruguay. The sequences are almost identical (99.3% identity) to those of a D. septemcinctus sample from Argentina but this is probably due to a misidentification of the latter animal because it originated from a potential area of sympatry of the 2 species (see Hamlett 1939; Abba et al. 2012).
CONSERVATION
Dasypus hybridus is listed as “Near Threatened” by the International Union for Conservation of Nature and Natural Resources Red List of Threatened Species (International Union for Conservation of Nature and Natural Resources 2014). It is also listed as “Near Threatened” in the Red List of Threatened Mammals of Argentina ( Superina et al. 2012). Populations of D. hybridus have decreased by about 20–25% over the past 3 generations (with a suspected generation length of around 4 years) mainly due to habitat loss and (illegal) hunting for food and sport (International Union for Conservation of Nature and Natural Resources 2014). Individuals are also killed by dogs and hit by cars.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Dasypus hybridus ( Desmarest, 1804 )
| Abba, AGUSTíN M. & Superina, Mariella 2016 |
Dasypus hibridus:
| Azevedo, El Achkar, Martins, and Ximenez 1982: 95 |
Muletia hybrida:
| A. Miranda-Ribeiro 1914: 46 |
Tatus (Muletia)] hybridus:
| Trouessart 1905: 814 |
Tatu hybridus:
| Lahille 1899: 203 |
hybrida:
| Trouessart 1898: 1140 |
Praopus hybridus:
| Burmeister 1861: 428 |
hybrida:
| Turner 1851: 213 |
Tatusia hybridus:
| Lesson 1827: 311 |
Dasypus] hybridus
| : G. Fischer 1814: 126 |
Dasypus hybridus
| : G. Fischer 1814 |
hybridus
| Desmarest 1804: 28 |
Loricatus hybridus
| Desmarest 1804 |