Riotintobolus Wesener, 2009
|
publication ID |
https://doi.org/10.3897/zookeys.19.221 |
|
publication LSID |
lsid:zoobank.org:pub:C473F9F6-1AE7-4B3F-B17F-CA1C2709010C |
|
DOI |
https://doi.org/10.5281/zenodo.3791405 |
|
persistent identifier |
https://treatment.plazi.org/id/2D3A99A5-E680-4E8D-BFD4-B10BD240636D |
|
taxon LSID |
lsid:zoobank.org:act:2D3A99A5-E680-4E8D-BFD4-B10BD240636D |
|
treatment provided by |
Plazi |
|
scientific name |
Riotintobolus Wesener |
| status |
gen. nov. |
Riotintobolus Wesener View in CoL , gen. n.
urn:lsid:zoobank.org:act:
Type species: Riotintobolus mandenensis View in CoL sp. n.
Other species included:
R. minutus View in CoL sp. n.
R. aridus View in CoL sp. n.
R. anomalus View in CoL sp. n.
Diagnosis: only Malagasy Spirobolida genus where males in some species still have apodous rings in front of the telson. Body rings dorsally with a very wide stripe of flashy colour ( Figs 24A View Figure 24 , 40A View Figure 40 ), a unique feature, although slim stripes occur in several genera of Spirobolida . The posterior gonopods differ from other Spirobolida in the presence of a unique, finger-shaped process located laterally on the telopodite ( Fig. 26M, x, y View Figure 26 ), and the presence of a large, very thin membrane located apically like a flag ( Fig. 26M, z View Figure 26 ). Anal valves with extraordinary thick lips (absent in R. anomalus sp. n., Figs 26B View Figure 26 , 27G View Figure 27 ), a unique character for this genus. Living specimens of Riotintobolus mandenensis sp. n. and R. minu- tus sp. n. often remain stiff like a stick instead of rolling into a spiral when disturbed, a unique behaviour for Spirobolida . Telson with a well-developed, sharp-edged preanal process (absent in R. anomalus sp. n., Figs 26B View Figure 26 , 27G View Figure 27 , 28A View Figure 28 ), a feature only shared with Pseudocentrobolus gen. n. and Granitobolus gen. n. Collum not greatly enlarged, similar to Spiromimus and other small-bodied genera of Spirobolida . Gnathochilarium with a subdivided mentum and a single sclerotized ledge on each stipites ( Figs 26F, G View Figure 26 , 27C View Figure 27 ), like in Flagellobolus , Pseudocentrobolus gen. n., Granitobolus gen. n., Caprobolus gen. n., Alluviobo- lus gen. n., Ostinobolus gen. n. Vulva simple, bivalve-like, similar to all other small-bodied genera of Spirobolida from Madagascar. Posterior plate apically overlapping anterior one. Anterior gonopods of a very general shape ( Fig. 26K View Figure 26 ), similar to those of numerous other, probably not closely related genera, like Aphistogoniulus Silvestri, 1897 (Wesener et al. in press). Telopodite of posterior gonopod with a torsion: the sperm canal runs basally along the mesal margin, but is apically rotated, so that the opening is located at the lateral margin ( Fig. 26M View Figure 26 ). A torsion also exists in Zehntnerobolus and Alluviobolus gen. n.
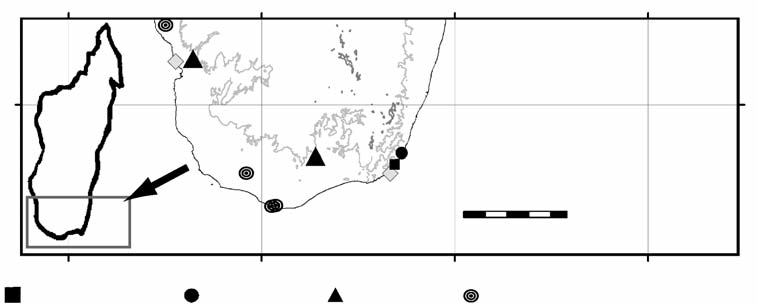
Distribution and ecology: two species, Riotintobolus mandenensis sp. n. and R. minutus sp. n. were collected in the littoral rainforest. The two other species occur in the spiny forest. R. aridus sp. n. was recorded from the spiny forest of Angavo, circa 20 km East of Antanimora, as well as Antafoky ( Fig. 25 View Figure 25 ), while R. anomalus sp. n. is recorded from the driest parts of Madagascar ( Battistini 1972, Moat & Smith 2007), the Mahafaly plateau and the Cap Sainte Marie ( Fig. 25 View Figure 25 ). The genus Riotintobolus is the first example of a relationship between spiny forest and littoral rainforest species ( Fig. 25 View Figure 25 ). R. mandenensis sp. n. and R. minutus sp. n. are microendemic, R. mandenensis is most probably restricted to the littoral rainforest of Mandena, while R. minutus could only be found in Sainte Luce ( Fig. 25 View Figure 25 ). Both species from the littoral forest were collected inside the wet leaf litter, mainly on the bottom of the thin layer of leaves slightly above the root horizon. In both species the eyes are partly reduced, featuring only 12–20 fused ocelli ( Figs 26A, C View Figure 26 , 27E View Figure 27 ). Legs are also shorter than in the other two congeneric species (reaching only 0.7 times the diameter of body rings, Fig. 27D View Figure 27 ). No explanation can be given for the unique and highly unusual defence behaviour of R. mandenensis and R. minutus . Both species turn stiff and motionless like a stick and rarely curl into a spiral like most other species of the Spirobolida .
Description. Males: length up to 45 mm, diameter up to 4.3 mm. 38–45 podous and up to two additional apodous rings. Females: length up to 51 mm, diameter up to 4.6 mm. 39–45 podous and up to two additional apodous rings.
Colour highly species-specific, ventrally always with a distinct wide stripe, see species for accurate descriptions.
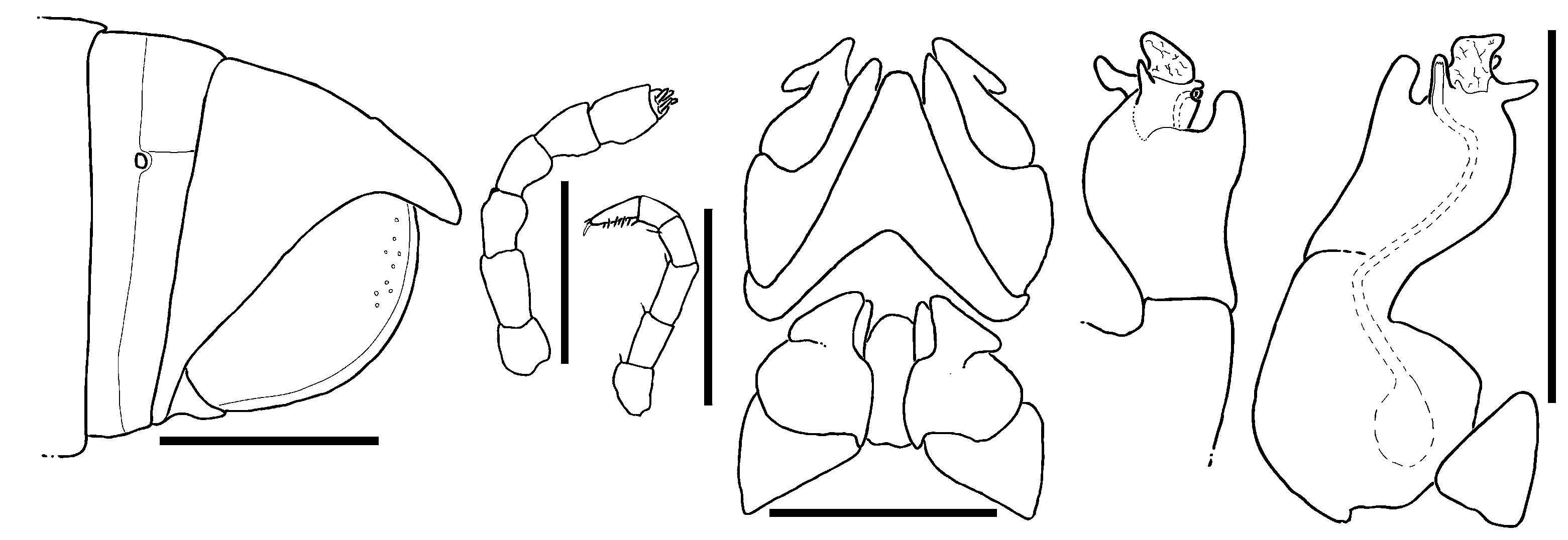
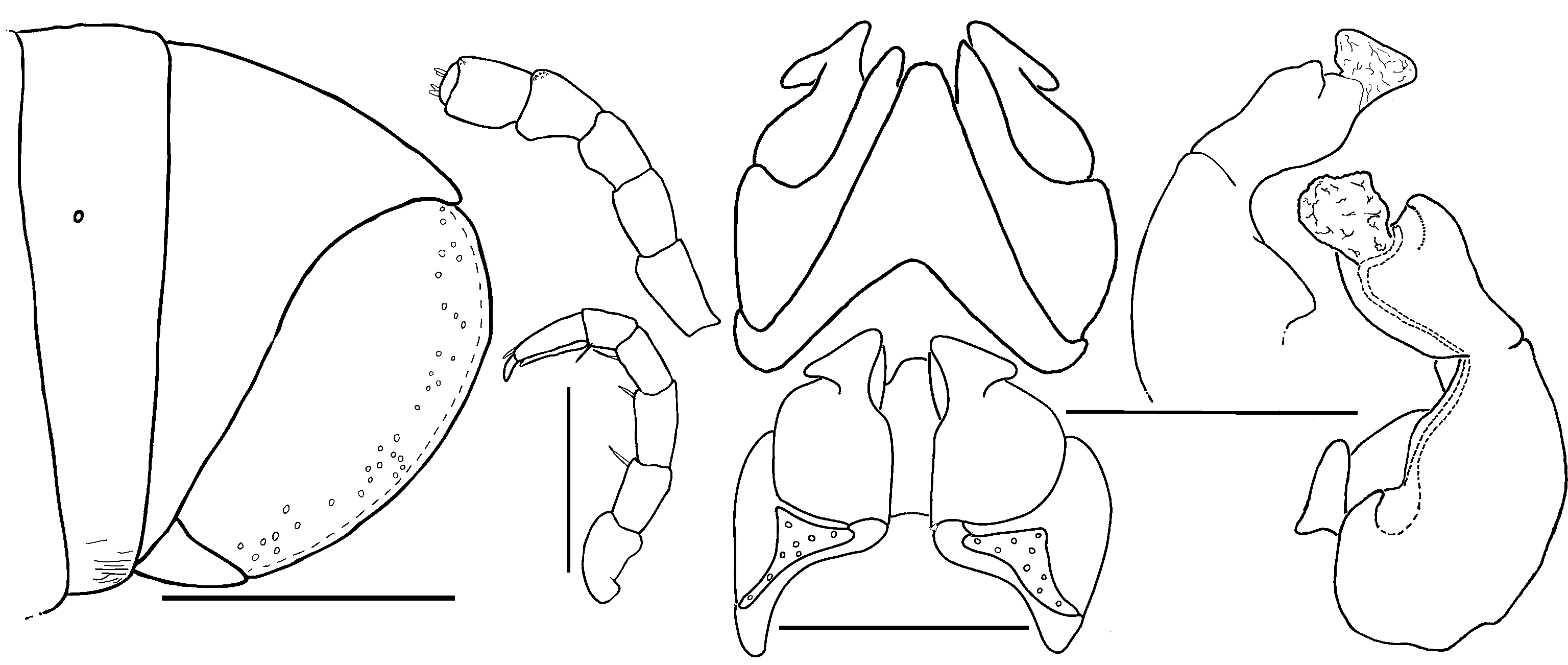
Head: each eye either with circa 28–34 ( R. aridus and R. anomalus ), or 12–20 ( R. mandenensis and R. minutus ) partly fused ocelli arranged in 3–5 vertical rows ( Figs 26C View Figure 26 , 27E View Figure 27 ). Labrum with standard three irregular teeth and a row of 10–12 stout marginal setae. Clypeus with two (rarely three) setiferous foveolae on each side ( Fig. 26D View Figure 26 ). Antennae short or of medium length, reaching back to ring 2 or 5 ( Figs 26A View Figure 26 , 27B View Figure 27 ). Relative lengths of antennomeres: 1<<2>3=4=5<6 ( Figs 26A View Figure 26 , 28B View Figure 28 , 29B View Figure 29 ). Terminal antennomere with four large sensory cones located together inside a membranous area. Antennomere 5 latero-apically with a field of four rows, antennomere 6 with a field of two rows of sensilla basiconica.
Gnathochilarium unusual ( Fig. 26F View Figure 26 ). Lamellae linguales each with two standard setae located behind one another. Stipites each with three apical setae ( Fig. 26F View Figure 26 ). Mentum basally subdivided by a wide suture ( Figs 26G View Figure 26 , 27C View Figure 27 ). Stipites each towards mentum with a large sclerotized ledge ( Fig. 26G View Figure 26 ) Palpi of gnathochilarium with numerous sensilla. Hypopharyngeal crest with a field of spine-like structures. Central pads of endochilarium separated by a step into two levels, each carrying sensory cones, number of cones not counted.
Mandible: external tooth simple, rounded; mesal tooth with three shallow cusps ( Fig. 26H View Figure 26 ). Four or five pectinate lamellae. Molar plate with few (five) transverse furrows, anterior two furrows enlarged, posterior furrows minute.
Collum: smooth, laterally not protruding as far as ring 2 ( Fig. 26A View Figure 26 ).
Body rings: dorsally and laterally smooth, meso- and metazona ventrally with numerous transverse impressions. Ozopores starting at ring 6, touching suture between mesozona and metazona ( Fig. 27B View Figure 27 ).
Telson: preanal process protruding, sharp-edged (except for R. anomalus sp. n.). Anal valves with well-visible lips and micropunctation ( Fig. 26B View Figure 26 ). Deep groove present anteriorly to sharp-edged lips in R. mandenensis sp. n. and R. minutus sp. n. ( Figs 26B View Figure 26 , 27G View Figure 27 ).
Legs: coxae 1 and 2 elongated and fused with sternum, podomeres from prefemur to tarsus in both sexes each with 4–10 ventral/mesal setae. Length of midbody legs species-specific ( Figs 27D View Figure 27 , 28C View Figure 28 , 29C View Figure 29 ). Each podomere with an apical ventral seta. Coxae 3 and beyond of cylindrical shape ( Figs 28C View Figure 28 , 29C View Figure 29 ). Tarsus with a stout dorso-apical seta and three pairs of ventral setae. Tibia short, half as long as tarsus ( Figs 28C View Figure 28 , 29C View Figure 29 ).
Male sexual characters: male legs in some species with tarsal pads. Male coxae 3–7 unmodified ( Fig. 27F View Figure 27 ).
Anterior gonopods: median sternal projection triangular, broadly rounded ( Fig. 26I View Figure 26 ). Sternite longer than coxite, but slightly shorter than mesal process of coxite ( Fig. 26I View Figure 26 ). Process of coxite long, but shorter than telopodite ( Fig. 26I View Figure 26 ). Telopodite always with a large, triangular retrorse process. Retrorse process laterally and apically extending beyond telopodite and coxite ( Fig. 26K View Figure 26 ).
Posterior gonopods coxite and telopodite clearly separated ( Figs 26J, M View Figure 26 ). Sternite sclerotized and well-visible ( Fig. 26M View Figure 26 ). Coxite mesally with a single groove, coxite protruding into a short stem towards telopodite. Telopodite slightly shorter than coxite ( Fig. 26M View Figure 26 ). Sperm canal discharging apically and laterally instead of mesally because of a torsion ( Figs 26J, M View Figure 26 ). Telopodites laterally always with a fingershaped process ( Fig. 26M, x, y View Figure 26 ), apically with a large, thin membrane ( Fig. 26M, z View Figure 26 ). Width of membrane apically larger than basally. Sperm canal first running along inner margin of coxite and sternite, than apically running through the telopodite before discharging laterally on finger-shaped process and often also apically into thin membrane ( Fig. 26M View Figure 26 ).
Female sexual characters: vulva simple, with a small, poorly sclerotized operculum at base, bivalve-like. Posterior valve apically overlapping anterior valve. Both valves smooth, lacking sensory cones. Towards opening basally on each valve with two or three rows of setae.
Etymology: Riotintobolus , masculine, refers to the mining company Rio Tintoº. Rio Tinto’s subsidiary, Qit Madgascar Minearlsº, now owns the littoral rainforests of Mandena and Sainte Luce ( Vincelette et al. 2003). This encompasses all the remaining distribution area of Riotintobolus mandenensis sp. n., which only occurs in Mandena, and of R. minutus sp. n., a species only recorded from Sainte Luce. Conservation zones will be the only remaining natural habitat after a large-scale mining action is undertaken by Riotinto in the coming years (Ganzhorn et al. 2003, Bollen and Donati 2006). These conservation zones will be directly managed by Qit Madagascar Minerals in order to conserve the biodiversity of the forests. The proposed conservation zones are 160 ha (but divided by a swamp) for the littoral forest of Mandena, and Riotintobolus mandenensis sp. n. The conservation zone for R. minutus sp. n., the S9 fragment of Sainte Luce is circa 200 ha large. The area between both forests is already degraded to pseudosteppe ( Fig. 24B View Figure 24 ). The conservation zones, at least of the Sainte Luce fragment, are still under human pressure ( Fig. 24C View Figure 24 ). Since Rio Tintoº owns and is responsible for the only remaining habitat where two Riotintobolus gen. n. species exist, the whole genus is named after the company. The author hopes that this move will increase the probability of the survival of R. mandenensis sp. n. and R. minutus sp. n.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Trigoniulidea |
|
Family |
Riotintobolus Wesener
| Wesener, Thomas, Enghoff, Henrik & Sierwald, Petra 2009 |
R. minutus
| Wesener & Enghoff & Sierwald 2009 |
R. aridus
| Wesener & Enghoff & Sierwald 2009 |
R. anomalus
| Wesener & Enghoff & Sierwald 2009 |