Amphicticeps shackelfordi Matthew and Granger, 1924
|
publication ID |
https://doi.org/10.1206/0003-0082(2005)483[0001:AAAACF]2.0.CO;2 |
|
DOI |
https://doi.org/10.5281/zenodo.5637257 |
|
persistent identifier |
https://treatment.plazi.org/id/03F85E56-FFF4-E850-FD6F-FAD9467A6E52 |
|
treatment provided by |
Felipe |
|
scientific name |
Amphicticeps shackelfordi Matthew and Granger, 1924 |
| status |
|
Amphicticeps shackelfordi Matthew and Granger, 1924
Figures 2–7 View Fig View Fig View Fig View Fig View Fig View Fig ; Tables 2–4
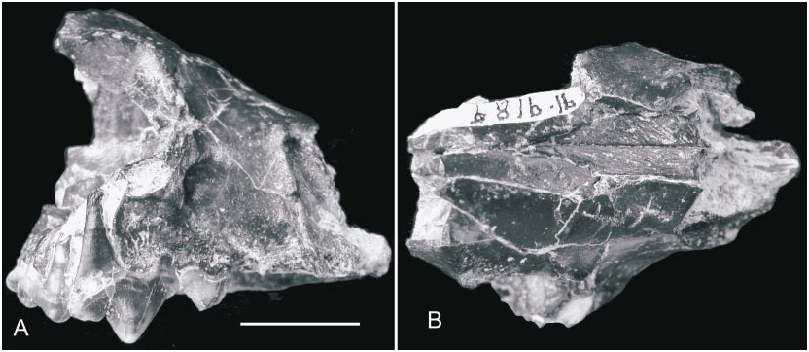
HOLOTYPE: AMNH 19010 About AMNH , nearly complete skull with left and right P1–2, P4–M1, and alveoli of left and right C1, P3, and M2 ( Matthew and Granger, 1924: figs. 4–5).
TYPE LOCALITY: Originally designated as from ‘‘Hsanda Gol formation, Loh’’ ( Matthew and Granger, 1924: 4), AMNH 19010 (field no. 89) was collected from about 2 mi southwest of the Loh campsite, in Tsagan Nor Basin, eastern Valley of Lakes, OborKhangay Province, in northcentral Mongolia.
REFERRED SPECIMENS: AMNH 19017 About AMNH , partial left ramus with p2–m1 and alveoli of c1– p1 and m2, field no. 69, from Loh ; AMNH 19127 About AMNH , partial right ramus with p3, m1, and alveoli of c1–p2, p4, and m2, field no. 84, from Loh ; AMNH 19128 About AMNH , right ramal fragment with m1 and alveoli of c1–p4 and m2, field no. 92, from 2 mi southwest of Loh ; AMNH 21695 About AMNH , left ramal fragment with m1 and alveoli of c1–p4, field no. 536, from 2 mi west of the ‘‘ Grand Canyon’ ’ area, which is 10 mi west of Loh ; AMNH 83610 About AMNH , left ramal fragment with p4–m2 and m3 alveolus, field no. 531, ‘‘ Grand Canyon’ ’; AMNH 81336 About AMNH , left ramal fragment with m2 and m3 alveolus ; AMNH 85749 About AMNH , right ramal fragment with m2–3, field no. 548 ; MAE BU.91.9187–90 ( AMNH cast 129686), partial rostrum with right alveoli of C1–P2, right P3–M2, and left P3–M1, partial mandible with left p2, p4–m2, and alveoli of c1–p1 and p3, and right p1–m2 and root of m3, collected by Perlé Altangerel in 1994, field no. M152, from 2 mi southwest of Loh ; MAE SG.95.7518, right ramal fragment with p2, p4, and m1 (broken); MAE SG.95.8919, posterior skull fragments with top of the inion and left basicranial region, 458179490N 1018379130E, collected by Khosbayar on 10 August 1995; and MAE M217, isolated left m1, field no. M217, from 2 mi southwest of Loh .
DISTRIBUTION: Early Oligocene of northcentral Mongolia. An undescribed record was mentioned in the early Oligocene Khatan Khayrkhan locality of Altai Province of Mongolia by Russell and Zhai (1987: 324).
GEOLOGY AND AGE: The above referred specimens of Amphicticeps shackelfordi come from three localities (some specimens lack a detailed locality record): (1) general vicinity of Loh for AMNH 19017 and 19127; (2) 2 mi southwest of Loh for AMNH 19010, AMNH 19128, MAE BU.91.9187–90, and MAE M217; (3) general vicinity or 2 mi west of the Ulaan Khongil (‘‘Grand Canyon’’ or Tatal Gol) for AMNH 21695 and 83610.
While field studies are currently pursued by the ongoing joint expeditions of the MAE and formal stratigraphic revisions will have to wait for that result (see Höck et al., 1999, for a recent summary), it is relevant to note here that the above three Amphicticeps producing localities fall within a more restricted concept of the Hsanda Gol Formation close to the level of a discontinuous but approximately contemporary basaltic lava (Basalt I of Höck et al., 1999).
Historic collections are largely concentrated in the Ulaan Khongil fauna in the lower part of the Hsanda Gol Formation below the prominent basaltic lava and immediately above. Amphicticeps specimens from near the Loh campsite and 2 mi southwest of Loh (including the holotype) are darkly stained due to the percolation of ground water, and they all belong to the Ulaan Khongil fauna. AMNH 21695 and 83610 from near the ‘‘Grand Canyon’’ area, on the other hand, are lightcolored and may belong to the Zavlia fauna in the upper part of the Hsanda Gol Formation above the lava. This presumed younger age of AMNH 21695 and 83610 relative to the rest of the A. shackelfordi hypodigm is also consistent with the former’s wider m1 and more prominent lingual cingulum on the m1 trigonid, tendencies that indicate a slightly more advanced stage of evolution for the species.
EMENDED DIAGNOSIS: Amphicticeps shackelfordi is distinguishable from the more derived A. makhchinus and A. dorog by its smaller size, smaller angle between the labial borders of P4 and M1, more enlarged M1 parastyle, larger M1 metaconule, more reduced anterior cingulum of M1, and more lingually located M2. In addition, the P4 protocone of A. shackelfordi is larger than in A. dorog , but is less well developed than in A. makhchinus .
DESCRIPTION: Matthew and Granger’s (1924) original report of Amphicticeps shackelfordi consisted of a brief diagnosis only. A full description is furnished here for the holotype and the newly referred materials.
Skull (figs. 2–4): The holotype, AMNH 19010 About AMNH , is still the only nearly complete skull available, although additional referred cranial fragments supplement the holotype in a number of important ways. Its rostral part is slightly crushed, such that the left cheek region is uplifted by approximately 3 mm. The reconstructed skull illustrated by Matthew and Granger (1924: fig. 5) is mostly accurate in overall proportions except for a more posteriorly displaced mastoid process (relative to the nuchal crest) in the dorsal view .
For a small carnivoran, the skull is rather strongly built, with a short and broad rostrum. The incisorbearing part of the premaxillary is broken off and only the posterior processes of the premaxillary between the nasal and maxillary are preserved; they extend slightly behind the level of the P2. The posterior tip of the nasal reaches nearly to the level of the postorbital process of the frontal. In keeping with the broad snout, the frontal shield is also wide, that is, there is a long distance between upper rims of the orbits. There is a small fossa above the antorbital rim on the frontal/maxillary suture, for the insertion of the levator nasolabialis, and this fossa is more prominent on the right side of the holotype. The postorbital process of the frontal is small but rather sharply pointed; that on MAE BU.91.9187–90 is more reduced. The distance between the postorbital constriction and the postorbital process is relatively elongated (the postorbital constriction is disjointed in the type but enough is preserved on the right side to indicate this elongation), and is approximately 12 mm, as is also seen in Potamotherium and Paragale . The temporal crests merge into the sagittal crest slightly behind the postorbital constriction. The braincase is not laterally expanded near the postorbital constriction as in Potamotherium or nearly becoming so in Paragale . Although not very high, the sagittal crest is thick and robust; so is the nuchal crest. The temporal region of the skull has a rugose surface texture. In lateral view, the skull is somewhat shallow and has a rather flat forehead.
The anterior half of the right orbital region is well preserved on MAE BU.91.9187–90 (fig. 4). The infraorbital canal is short, about 3 mm long, and has a round cross section. Immediately above the canal is a small, rounded lacrimal bone forming the inner rim of the antorbital rim. The lacrimal foramen on the lacrimal bone opens posterodorsally. About 1 mm into the orifice for the lacrimal sac, there is a small foramen on the ventral floor that opens into the dorsal wall of the infraorbital canal. A slender process of the palatine meets the lacrimal and excludes orbital contact of the frontal with the maxillary. At the palatine–maxillary–lacrimal junction, there is a small, oval fenestra, probably due to lack of ossification at this stage of the ontogeny, although a fossa for inferior oblique muscle in hyaenids has been identified at the same triple junction ( Werdelin and Solounias, 1991: fig. 26). The anterior process of the jugal is broken away in both AMNH 19010 and MAE BU.91.9187–90, leaving the jugalmaxillary suture surface well exposed in both specimens. From these sutures, it can be deduced that the anterior tip of the jugal stops just above the infraorbital canal and does not reach the lacrimal bone.
Basicranium (fig. 5): The occipital condyles are broken off on both sides of the holotype. The remaining basioccipital floor between the bullae is distinctly widened posteriorly, such that the lateral edges of the basioccipital form a 258 angle, in contrast to smaller angles in primitive arctoids such as Amphictis and to nearly parallel (08) edges in canids. A small, rounded process for the attachment of the rectus capitis ventralis muscle lies close to the lateral edges of the basioccipital and is slightly in front of the posterior lacerate foramen. Both glenoid fossae are missing. On the left side, however, the medial segment of the postglenoid process is still preserved. Behind this broken process is a small postglenoid foramen, 1 mm in diameter.
The mastoid part of the petrosal is inflated, forming a prominent, laterally protruding mastoid process. The process has a smooth and flat lateral facet and is connected to the paroccipital process via a posterior ridge and to the lambdoidal crest via a more prominent dorsal blade. Such a blade can also be seen in most North American oligobunines. The mastoid tubercle (processus hyoideus) is formed by the petrosal. The posteriorly directed paroccipital process is broadly based because of its expanded wings on each side, but shows no sign of fusion with the bulla (not preserved) at the base. There is a low, longitudinally oriented ridge on the ventral surface of the paroccipital process.
In front of the mastoid process is an ovalshaped suprameatal fossa; its long axis is transversely oriented. The fossa is not fully enclosed toward the medial side, and is thus incompletely rimmed. Approximately 1.5 mm deep, the fossa is excavated into the squamosal bone, which forms the anterior wall of the mastoid process. The suprameatal fossa is primarily developed toward the caudal direction, and is excavated slightly toward the ventrolateral aspect such that it begins to be hidden by a thin bony rim, although the degree of excavation is far less than seen in some procyonids and mustelids.
Although both bullae are missing, the presence of an ectotympanic bulla is indicated by a clearly defined scar posterior to the postglenoid foramen and by a broad, smooth depression on the alisphenoid/squamosal suture (fused) area just medial to the postglenoid process. Such surface markings leave little doubt as to where the anterior crus of the ectotympanic ring was attached (also see the description under Amphicticeps dorog for the preserved ectotympanic). A broad facet facing anteroventrally between the posterior lacerate and stylomastoid foramina at the base of the paroccipital process is apparently the site of attachment of the posterior crus of the ectotympanic. The presence of an entotympanic, on the other hand, is indicated by a 2 mmwide rugose area on the ventral surface of the promontorium immediately lateral to the petrosal/basioccipital juncture. It is not possible to ascertain whether a bony external auditory meatus was present. However, the rather distinct mark of the above mentioned ectotympanic attachment behind the postglenoid foramen suggests that the anterior crus of the ectotympanic does not wrap around to superimpose on the squamosal around the dorsal bony passage of the meatus to form a complete ring by the ectotympanic, as happens in many arctoids that have a tubular external bony auditory meatus.
The promontorium of the petrosal is prominently domed ventrally, particularly near the fenestra cochleae and fenestra vestibuli (oval and round windows). Its ventral surface is marked by at least two indistinct grooves (more clearly shown on the left side) that begin posteriorly at a small tubercle near the entotympanic/promontorium contact facet. These grooves make small arches laterally at a level slightly in front of the fenestra cochleae and then turn medially toward the entotympanic/promontorium suture. Despite the superficial resemblance of the course of these grooves to the sulcus of the promontorial branch of the internal carotid artery and nerve in primitive caniforms ( Wang and Tedford, 1994), its occupant is unlikely to be a promontorial artery, contrary to Cirot (1992: fig. 2), who postulated a promontorial artery in the primitive musteloid Amphictis , and to SchmidtKittler (1981), who implied the existence of an internal carotid artery on the promontorium of Amphicticeps . In taxa with a promontorial artery, there is usually a stapedial branch leading transversely toward the oval window. In Amphicticeps there is no such transverse sulcus medial to the oval window. Instead, there is a distinct groove along the ventral rim of the oval window. Such a longitudinal groove can also be found in Promartes and in living procyonids. In the case of the latter group, the soft structures that left the grooves are fine branches of the caroticotympanic artery and the accompanying caroticotympanic nerves ( Story, 1951: fig. 83). The caroticotympanic artery, which is a minor component in the internal carotid artery, arises from the main internal carotid artery within the carotid canal, and after looping across the promontory, anastomoses with the tympanic arteries. The inferior tympanic artery and the tympanic nerve loop around the posterior edge of the round window instead the anterior position as in a promontory artery. The surface sulci on the promontorium of Amphicticeps are thus best reconstructed as left by an arterial and nervous configuration similar to that of extant procyonids, that is, no promontory artery is present. The main course of the internal carotid artery is assumed to be in the typical arctoid fashion enclosed within the medial bullar wall (see the description under A. teilhardi for further evidence of a medially positioned internal carotid artery).
At the posteromedial corner of the promontorium, there is a distinct posterior process protruding toward the posterior lacerate foramen. This process is broken off on the left side, showing pneumatic spaces beneath the bony surface.
The fossa for the tensor tympani is deep and located anteromedial to the epitympanic recess. There is a thin sheet of bone covering the canal for the facial nerve; some segments of this sheet are so thin that the bone is rather transparent along the nerve canal. The epitympanic recess is shallow, and walled by squamosal ventrolaterally and petrosal dorsolaterally.
Medial to the Eustachian canal at the level of the presumed anterior end of the entotympanic, the basisphenoid is deeply excavated into a large pit just anterior to the median lacerate foramen. This space marks the turnaround point of the internal carotid artery (cica of fig. 5; Wang and Tedford, 1994). The inferior petrosal vein is excavated into the lateral wall of the basioccipital but remains rather thin (approximately 1 mm in diameter) within a canal formed by the petrosal and basioccipital (ips of fig. 5; best seen near the posterior lacerate foramen). The caliber of the inferior petrosal vein is such that it is unlikely to be able to accommodate a doublelooped internal carotid artery hypothesized to be present in many ursoids ( Hunt, 1977; Hunt and Barnes, 1994).
The area anterior to the Eustachian canal is damaged on both sides of the skull, and it is not possible to ascertain the status of the alisphenoid canal except by indirect inferences. SchmidtKittler (1981: 784) stated, without elaboration, that Amphicticeps has an alisphenoid canal on each side of the skull. On AMNH 19010, only the anterodorsal roof of the foramen rotundum (shared with the anterior opening of the alisphenoid canal) is partially preserved on each side of the skull, and the ventral floor of the canal (if present) is missing. Further preparation on the better preserved left side of the holotype reveals a short segment of bone, about 2 mm in length, between the posterior aspect of the foramen rotundum and the foramen ovale. In Canis ( Evans and Christensen, 1979) and Ailurus ( Story, 1951) , the maxillary artery enters the posterior opening of the alisphenoid canal (caudal alar foramen in Evans and Christensen, 1979) and emerges from the foramen rotundum (rostral alar foramen). In Procyon (which lacks the alisphenoid canal), on the other hand, only a small branch of the internal maxillary artery, the medial meningeal artery, enters the foramen ovale ( Story, 1951: fig. 82). On AMNH 19010, the bony bridge flooring the orbital fissure and roofing the alisphenoid canal forms a halfpipe structure on the ventral view, possibly because of a broken ventral floor for the alisphenoid canal. A tiny foramen is present on the medial wall of the alisphenoid canal at the level of the presumed posterior entrance of the canal, as is also seen in Amphicynodon teilhardi . While structural damages do not permit us to state with certainty the existence of a posterior opening of the alisphenoid canal, we agree with SchmidtKittler that an alisphenoid canal is likely present.
Bulla of MAE SG.95.8919 (fig. 6): We tentatively refer a left bulla and associated posteriormost portion of the skull, MAE SG.95.8919, to Amphicticeps shackelfordi . Although the bullar size and overall basicranial morphology seems to be compatible with the holotype, there are a number of differences that prevent us from being certain of our reference. Furthermore, in the absence of associated dental materials in MAE SG.95.8919, it is prudent to describe this bulla separately in order to highlight the conflicting morphologies in the basicranial area from those in the holotype.
MAE SG.95.8919 consists of a crushed posterolateral aspect of the skull, preserving much of the left bulla as well as the occipital condyles and the top of the skull. Although much of the bony relationships between various elements are intact, crushing has distorted some areas so that full restoration of their original relationships is no longer possible.
The top of the braincase is well preserved, including about 25 mm of the posteriormost segment of the sagittal crest and a complete nuchal crest. The sagittal crest is 7 mm high at its deepest point just in front of the nuchal crest. The posterior segment of the sagittal crest on the holotype is missing, but based on the height of its nuchal crest, seems to be slightly lower than that in MAE SG.95.8919. The temporal foramen at the suture of parietal and supraoccipital is more posteriorly located than in the holotype. The profile of the nuchal crest, viewed from the caudal end, is also different from that of the holotype; instead of a rather flat top in the holotype, MAE SG.95.8919 has a rather pointed inion with a more steeply sloped nuchal crest on either side. The nuchal crest is also slightly thinner than in the holotype.
The most prominent difference between MAE SG.95.8919 and the holotype is in the size and lateral extrusion of the mastoid processes, although the overall construction of the mastoid process in MAE SG.95.8919 is similar to that in the holotype. A laterally expanded mastoid forms a conspicuous, rounded (in dorsal view) crest continuous from the lambdoidal crest. In lateral view, the outline of the mastoid process is roughly triangular. A posteroventral facet for attachment of the obliquus capitis cranialis muscle ( Antón et al., 2004) is the largest surface of the process, and this facet is less posteriorly oriented than in the holotype. The depth of the mastoid process in MAE SG.95.8919 is also significantly less than in the holotype, resulting in a smaller area of the lateral facet of the mastoid for attachment of the sternomastoideus muscle. Overall, one gets the impression that the much enlarged and laterally extruded mastoid process in the holotype is mostly related to the increased size and leverage of the m. obliquus capitis cranialis, and thus presumed more powerful head rotation ( Antón et al., 2004), although ontogenetic variation may also be responsible for such differences.
Associated with its smaller mastoid process, the paroccipital process in MAE SG.95.8919 is also narrower in ventral view—the broader process in the holotype is apparently the result of a proportional lateral expansion due to its greatly expanded mastoid process. Otherwise, the paroccipital process is of the same general construction, with a dorsally convex and ventrally flat process that is completely posteriorly oriented without any hint of a ventral bending toward the bulla. Such a ‘‘free’’ paroccipital process, without hugging the bulla, is often a primitive condition for all caniforms (e.g., see Wang, 1994; Wang and Tedford, 1994; Wang et al., 1999).
The bulla is more or less intact with the exception of a crack on the ventrolateral aspect. The lateral half of the ectotympanic ring is slightly caved in by approximately 1 mm along this crack. Other than such a distortion, the bulla seems to maintain its original proportions. The form of the bulla is quite inflated for a basal arctoid, more so than the modern ursid ‘‘ type A’’ bulla ( Hunt, 1974). The axis along the ventralmost rim of the bulla forms a slight angle with the parasagittal axis of the skull, in contrast to the canid condition of mostly parallel bullar axes. Composition of the bullar elements is difficult to ascertain due to extensive fusions and fine cracks on the bullar surface. An extremely subtle groove seems to run from the base of the paroccipital process across the posterior aspect of the bulla, crossing slightly behind the ventral floor of the bulla and reaching toward the anterior carotid foramen (the anterior extent of this groove is less well defined because surface marks are becoming less clear). This narrow band of slightly roughened area may be one possible interpretation of the rostral entotympanic– ectotympanic contact, although such an interpretation is highly speculative and other alternatives are just as likely. The posterior carotid foramen is located on the anterior rim of the large posterior lacerate foreman.
Toward the medial aspect of the bulla, the basioccipital–basisphenoid region is fractured, and anatomic relationships are difficult to interpret. The nearly vertical medial wall of the bulla is buttressed by a thickened lateral wall, up to 6 mm in depth, of the basioccipital. The lateral surface of this lateral wall of the basioccipital is essentially flat and hugs the medial wall of the bulla, although there is a narrow gap between these two walls toward the posterior aspect of their contact. The medial wall of the basioccipital lacks a prominent invagination for the embayment of the inferior petrosal sinus seen in many ursoids such as ursids, amphicyonids, and basal pinnipeds (e.g., Hunt, 1977; Hunt and Barnes, 1994). The above mentioned gap between the lateral wall of the basioccipital and the medial wall of the bullapetrosal seems too small to accommodate an enlarged inferior petrosal sinus. On the medial side of the lateral basioccipital wall a small canal is embedded within the basioccipital bone. This canal, probably for a nutrient blood vessel, emerges anteriorly into the braincase slightly behind the level of the anterior carotid foramen.
The external auditory meatus is quite well developed for a basal arctoid. The ventral lip of the meatus is 3–4 mm long, much longer than those in European basal arctoids such as Amphicynodon leptorhynchus (FSP ITD 312) and Amphictis ambiguus (FSP PFRA 28). Areas inside the meatus were prepared. A suprameatal fossa is vaguely developed on the posterodorsal aspect of the meatal wall of the squamosal. Such a weak fossa is in contrast to that in the holotype, on which it is not only substantially deeper but also better defined by a sharp rim along its lateral and ventral aspects. The fossa in MAE SG.95.8919 is also less well developed than in Amphicynodon teilhardi (see description below). The postglenoid process is broken off, exposing the canal for the retroarticular vein, which is of relatively small caliber.
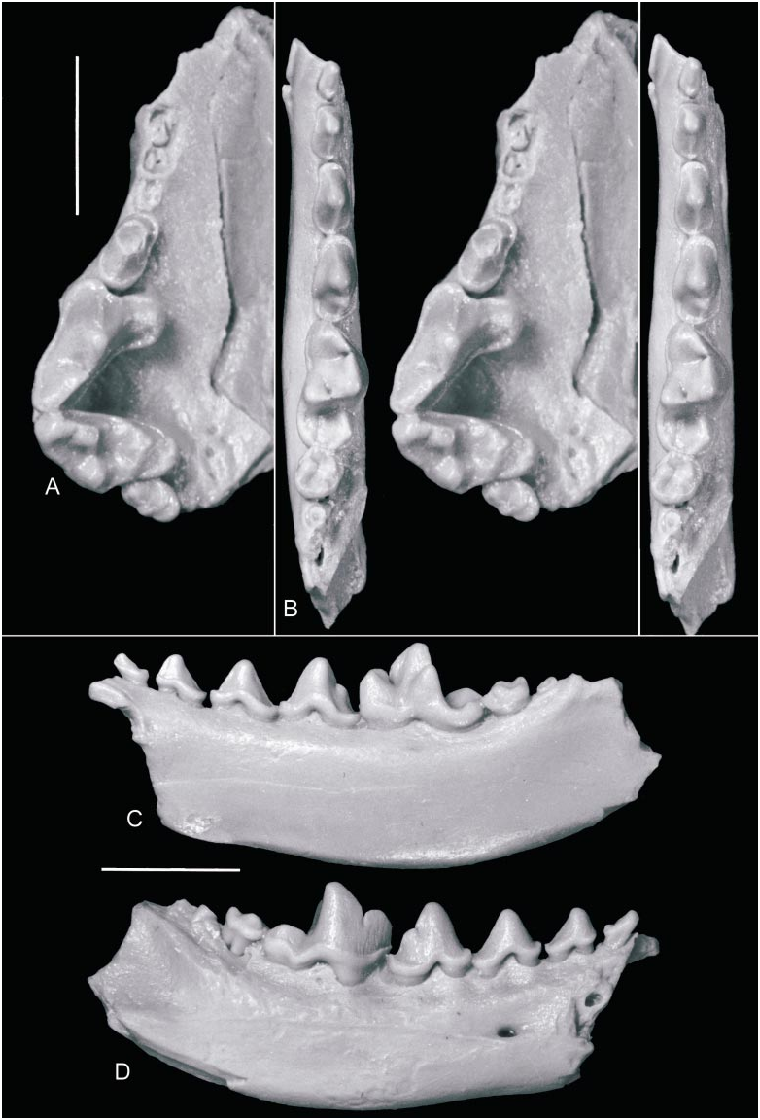
Mandible (fig. 7B–D): Discovery of the associated upper and lower jaws of MAE BU.91.9187–90 allows us confidently to refer several ramal fragments to Amphicticeps . Nonetheless, our knowledge of the angular process and the ascending ramus is still incomplete.
The mandible is short, thick, and deep, with an average thickness of 6.0 mm and depth of 11.0 mm (both measured at the level of the talonid basin of m1; N 5 5). On AMNH 19127 About AMNH , the remaining ascending ramus suggests a rather erect anterior border, forming a 1258 angle with the horizontal ramus. There are two mental foramina, one below the anterior edge of the p2 and another between the two roots of the p3.
Teeth (figs. 2, 7): No upper incisor is preserved. Only the root of right I3 is partially intact on AMNH 19010 About AMNH . A robust upper canine can be inferred from the large alveoli on both sides of the holotype. Immediately behind the canine is a small, singlerooted P1 with a single main cusp. The doublerooted P2 also has a single main cusp, which is surrounded by a weak cingulum on the lingual side. This cingulum thickens on the posterior end and shows an incipient development of a cingular cusp. Both P 3s are missing on the type, but are well preserved in MAE BU.91.9187–90. Like P 2, P3 is single cusped, although its cingulum is stronger than that on P2. The upper carnassial, P4, is transversely broad due to a lingually extended protocone, which is near the anterolingual corner of the tooth. The apex of the P4 protocone is relatively low and formed by a raised lingual cingulum. This crestlike protocone contrasts with that of the North American oligobunines, which have a primitively tall, cusplike protocone (i.e., the apex is not associated with the cingulum). A low crest is present on the labial aspect of the protocone. There is a narrow cingulum on the labial side, which continues in front of the tooth and thickens slightly to become an indistinct parastyle. A carnassial notch is present.
M1 is transversely elongated, and its labial border forms a steep angle, averaging 1128, with that of the P4. The M 1 parastyle is strong, rising to nearly the same height as the paracone. In MAE BU.91.9187–90, there is a faint notch (absent in the holotype) separating the parastyle from the paracone. The paracone is much higher than the metacone. The preprotocrista is low and lacks a protoconule on the holotype but is swollen slightly at the base of the paracone to indicate an indistinct protoconule in MAE BU.91.9187– 90. The postprotocrista is clearly present and is oriented somewhat posteriorly. The postprotocrista ends at the posterior border of the M1 rather than at the base of the metacone. The lingual cingulum is moderately developed. It is rather low as compared to the protocone, and is thickest along the posterolingual border of the M1. The cingulum quickly tapers off anterior and posterior to the protocone, in contrast to a welldeveloped posterior ridge bordering a deep talon basin in the oligobunines. M2 is doublerooted and is located lingually such that its lingual border is at the same level as that of M1 whereas its labial border only reaches the middle of M1. The ovalshaped M2 has a prominent paracone toward the labial margin, and a posterolingually located, but much smaller, metacone at the posterior border of the tooth. A crestlike protocone is near the middle of the tooth, and is surrounded lingually by a lingual cingulum.
No lower incisors or canines are preserved on the holotype or referred specimens. The lower premolars are as robust as their upper counterparts. The p1 is single rooted and has a single main cusp. A cingulum is present around the anterior and posterior borders of p2–p3, which are single cusped. The p4, however, has a small posterior accessory cusp behind the main cusp. The p4 cingulum nearly completely surrounds the tooth except the region between the roots on the labial side. The m1 is rather broad (transversely) and its trigonid is short. The trigonid cusps are low and blunt, and the metaconid is not greatly reduced. The lingual border between the paraconid and metaconid is slightly concave to give a somewhat sigmoid appearance in occlusal view. Most individuals have a labial cingulum on the trigonid, whereas the lingual cingulum is more reduced. But the lingual cingulum is usually present at the level of the carnassial notch and may extend along the entire trigonid as in AMNH 21695 About AMNH , which occurs stratigraphically higher than the rest of the sample. The talonid of m1 is narrower than the trigonid, and consists of a dominant hypoconid bordered lingually by a low entoconid crest much like a cingulum. The anterior hypoconid crest, the cristid obliqua, is oriented parasagittally. There is a weak cingulum on the labial side of the hypoconid. The entoconid crest does not have a notch at the base of the trigonid as in Potamotherium and the oligobunines. The doublerooted m2 is shortened and nearly quadrate in outline. The protoconid and metaconid are large and distinct. There is no paraconid anterior to the protoconid and metaconid; in its place there is a low, triangular platform. The greatly reduced talonid consists of a small hypoconid along the posterolabial border of the tooth. The entoconid takes the form of a narrow cingulum. A narrow cingulum is also present along the labial border of m2. The presence of a tiny m3 is indicated by a small root in MAE BU.91.9187–90, but it is absent in other individuals (definitely in AMNH 19127 About AMNH and probably in AMNH 19017 About AMNH ) .
COMPARISON: Although Matthew and Granger’s (1924) diagnosis of Amphicticeps shackelfordi indicated the presence of lower jaws, they did not elaborate the exact nature of the specimens, nor did they illustrate a lower jaw of this species. Four lower jaws were probably available at the time of their study (specimens that were collected during the 1922 season), and what Matthew and Granger had in mind were probably AMNH 19017 and 19128 because these two jaw fragments possess an m1 but are missing the m2, a combination that matches their descriptions. Their descriptions of the lower teeth, although very brief, must have been important in their attempt to delineate various species. In particular, their contrasts between lower carnassials of Amphicticeps and Amphicynodon (their Cynodon ) must have been based on these referred lower jaws.
With the naturally associated upper and lower jaws of MAE BU.91.9187–90, our confidence in the references of isolated lower dental materials to Amphicticeps shackelfordi is considerably increased. In light of the new materials, Matthew and Granger’s (1924: 4) comparisons about the lower carnassials having ‘‘a narrower and shorter heel with more distinct hypoconid crest’’ relative to those of Amphicynodon are still correct and their concept of the hypodigm still valid. However, specimens from the 1922 collection lack an m3, which naturally led Matthew and Granger to conclude that absence of this last molar is one of the main distinctions between Amphicticeps and Amphicynodon .
Our new discovery that MAE BU.91.9187– 90 has an unmistakable m3 adds a new wrinkle to the interpretation of this character. As pointed out in the above descriptions about specimens that have preserved the posterior dental battery, an m3 is definitely absent in AMNH 19127 and is probably absent as well in AMNH 19017. Given the tiny size of the m 3 in MAE BU.91.9187–90, it is quite possible that individuals, such as AMNH 19217, could have had an m 3 in an earlier part of their life, which was later broken and fully healed without leaving traces of its root, a situation common in carnivores with small p1s (personal obs.). Whatever the actual situation with AMNH 19217, taken at the face value of existing materials, 30%–50% of individuals have retained an m3, although our sample size is obviously too small to allow a true statistical sense of the ratios. A similar situation is better documented in the loss of the M 3 in the basal canid Hesperocyon gregarius , in which about 7% of the Chadronian individuals still retain a small, nonfunctional M3 and by Orellan time all have lost it (Wang, 1994: 30). However, the actual ratio may not be an important point in the present analysis. The important phylogenetic implication is that Amphicticeps represents a small clade that in its most basal species, A. shackelfordi , is on its way to losing its last molars, and this loss is yet another independent disappearance of this molar among carnivorans.
It is also worth noting that AMNH 21695, the only referred specimen of A. shackelfordi from the Zavlia fauna well above the level of the persistent basalt, is also the most robust individual known, both in terms of ramal construction and width of the m1. In addition, it is the only individual with a nearly complete cingulum on the lingual side of the trigonid, and its premolar alveoli indicate an individual with a relatively shorter rostrum compared to individuals from the Ulaan Khongil fauna below or immediately above the lava. The reliability of these features as indications of a later stage of evolution of the species remains to be verified by further samples from the Zavlia fauna.
MAE SG.95.8919 offers the only bulla for Hsanda Gol carnivorans, and despite its less than certain taxonomic status, is of considerable importance in our understanding of the basal arctoids. That it belongs to the basal arctoids is certain. Of the main two arctoid lineages in the Shand Gol that are likely candidates, Amphicynodon teilhardi , the only species so far known for the genus in Mongolia, is too small for MAE SG.95.8919. Species of Amphicticeps , on the other hand, encompass a size range that is consistent with that of MAE SG.95.8919. More specifically, the holotype of A. shackelfordi has the same bulla size (judging from the attachment sites for the bulla) and general basicranial morphology as the MAE SG.95.8919. We thus cautiously place MAE SG. 95.8919 in A. shackelfordi .
Overall, the holotype of Amphicticeps shackelfordi has a more robust construction than in MAE SG.95.8919, particularly in its thicker nuchal crests and larger and more laterally extruded mastoid process. These proportional differences may seem conspicuous, but probably are all attributable to a stronger development of the head–neck musculatures in the holotype. Even more extreme lateral expansions of the mastoid process can be seen in Allocyon . In the absence of contradicting evidence, we tentatively treat such differences as variations due to sexual dimorphisms in Amphicticeps . If our treatment is correct, the variations in the size of the suprameatal fossa are also considerable.
| AMNH |
American Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Caniformia |
|
InfraOrder |
Arctoidea |
|
ParvOrder |
Ursida |
|
SuperFamily |
Ursoidea |
|
Family |
|
|
Genus |