Leptochryseus kuwaitensis ( Jones & Clayton, 1983 )
|
publication ID |
https://doi.org/10.5281/zenodo.191909 |
|
DOI |
https://doi.org/10.5281/zenodo.6226801 |
|
persistent identifier |
https://treatment.plazi.org/id/03F58791-D334-0C0A-C9CF-9980FC59FE4E |
|
treatment provided by |
Plazi |
|
scientific name |
Leptochryseus kuwaitensis ( Jones & Clayton, 1983 ) |
| status |
|
Leptochryseus kuwaitensis ( Jones & Clayton, 1983)
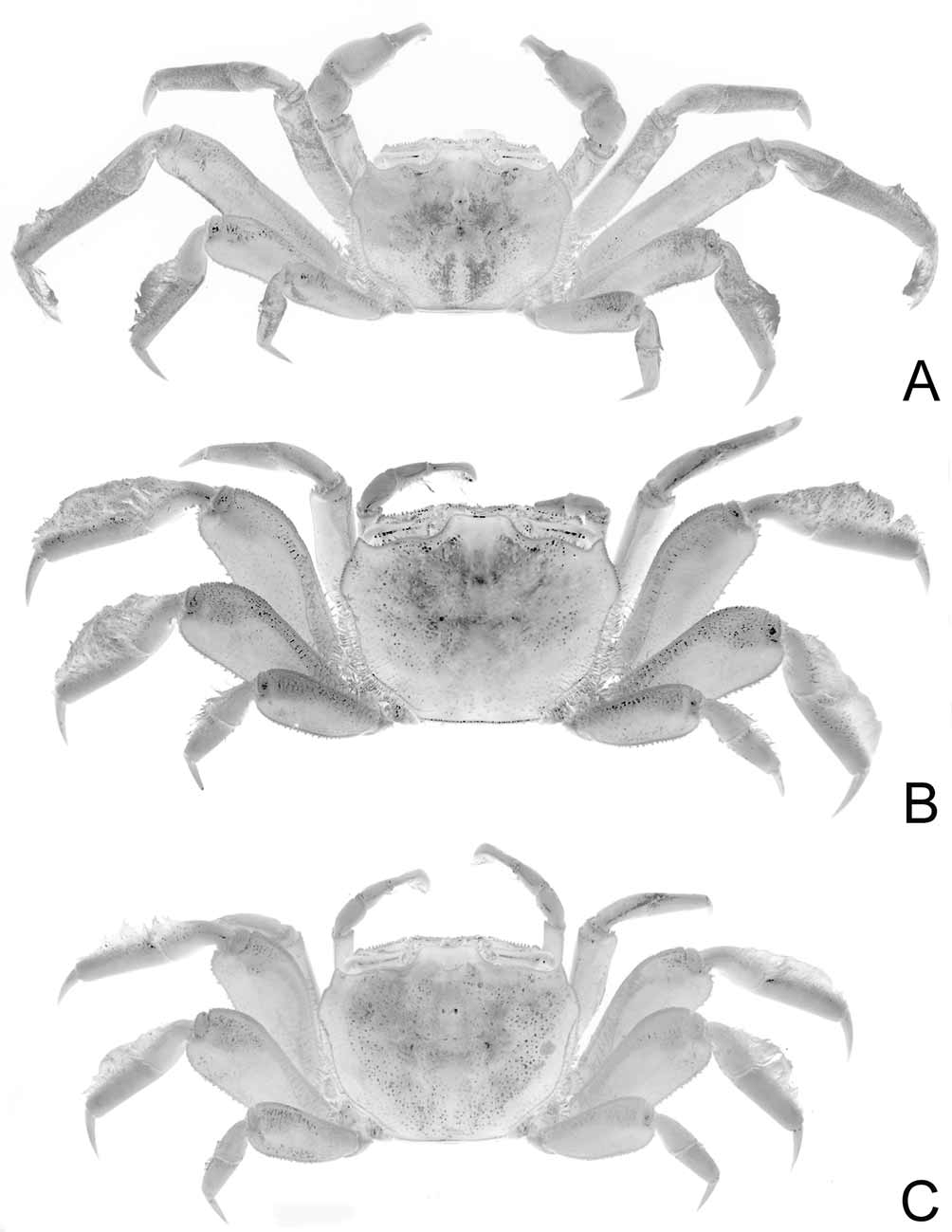
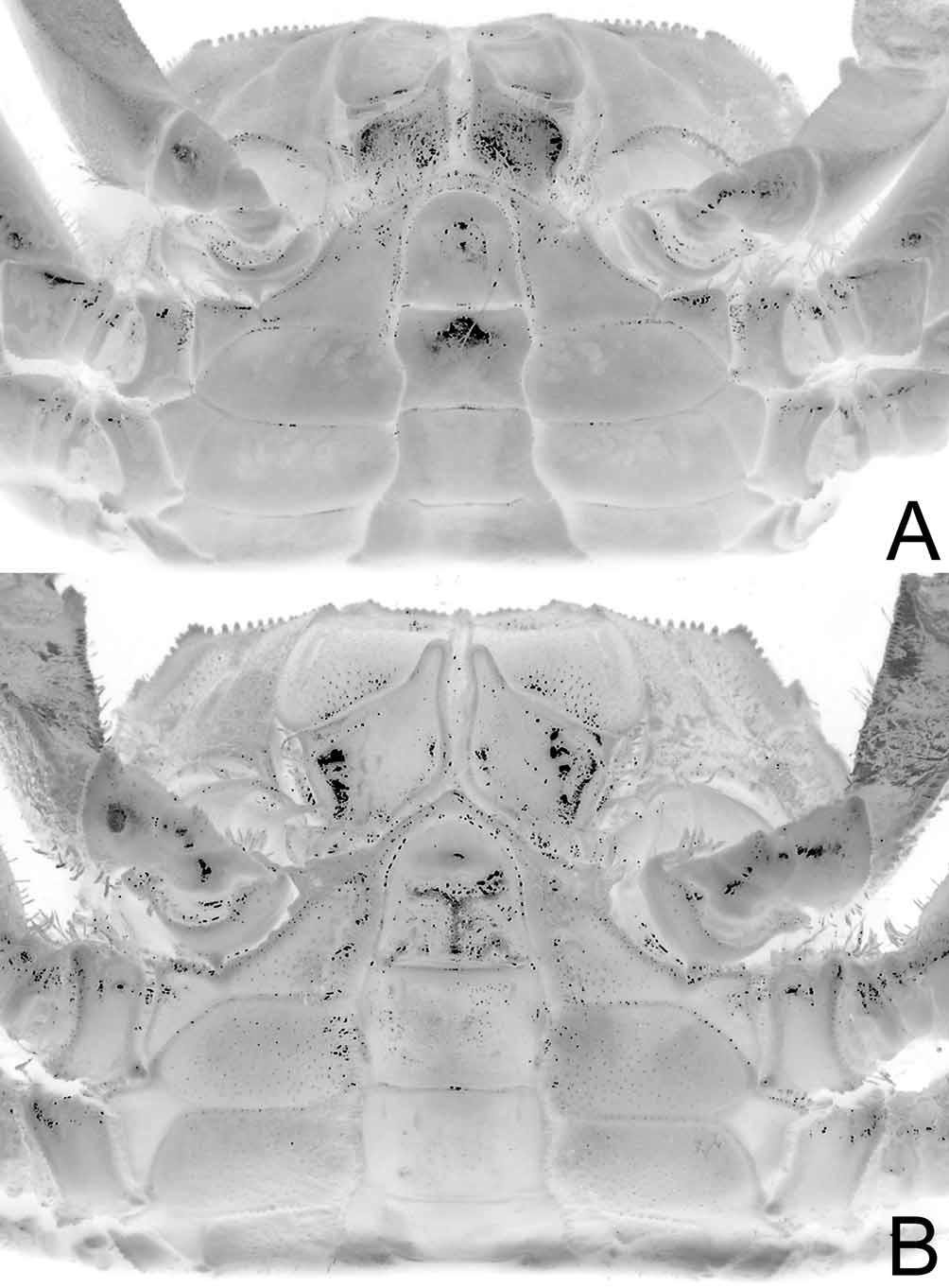
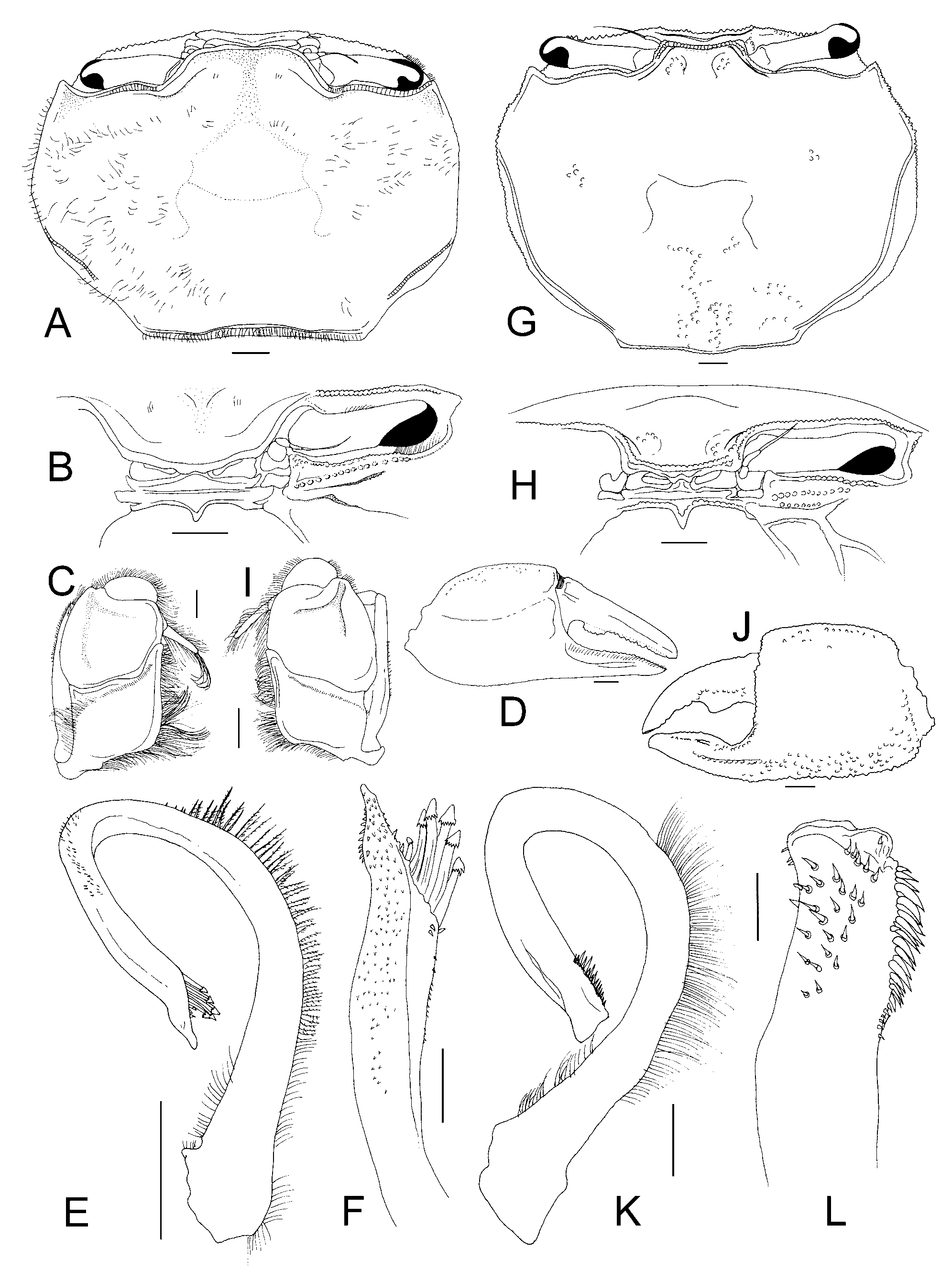
( Figs. 7 View FIGURE 7 , 8 View FIGURE 8 , 9 View FIGURE 9 B, 10B, 11G–L)
Cleistostoma kuwaitense Jones & Clayton, 1983: 185 View in CoL , fig. 2. – Ng et al. 2008: 233. Leptochryseus kuwaitense View in CoL – Al-Khayat & Jones 1996: 798.
Material examined. KUWAIT: holotype male (18.0 x 24.1 mm) (NHM 1981.498), Kathmah mudflats, coll. 10 March 1981; paratypes: 1 male (damaged), 2 females (19.1 x 26.1 mm, 20.2 x 27.4 mm) (NHM 1981.499), Kathmah mudflats, 10 March 1981; 2 males (21.1 x 28.6 mm, 21.7 x 28.8 mm) (NHM 1984.493), coast, 1979; 6 males (smallest 15.5 x 20.5 mm, largest 21.7 x 28.8 mm), 2 females (larger 19.0 x 24.6 mm) (NHM 1979.269), 1 male (22.7 x 28.6 mm), 1 female (16.8 x 22.1 mm) ( ZRC 1999.1208), Seulibinkhat Bay, coll. Vaughan, Clayton & Tytlev, 27 February 1979; 1 male (NHM 1981.498), Kathmah mudflats,; 1 male (16.8 x 21.5 mm) (NHM 1984.490), coast, 1979; 1 ovigerous female (20.8 x 27.0 mm) (NHM 1984.491), Al-Jahara mudflats, coll. D. A. Clayton, 14 March 1978; 2 males (NHM 1984.494), coast, 1979; 1 male, 1 female (NHM 1984.495), coast, 1979; 2 males (NHM 1984.454), coast, coll. 1979.
Type locality. Kuwait.
Description. Carapace width to length ratio 1.26-1.37 (average 1.32); dorsal surface with short setae, granules scattered on branchial regions; epigastric lobes coarsely granular, groove parallel to lateral margins of front shallow; posterior part of cardiac region weakly cristate, coarsely granular; dorsolateral margin distinct as row of large granules reaching to above P5. Anterolateral margin with sharp, high granules immediately after external orbital tooth, forming indistinct lobe; posterolateral margin rounded; posterior margin straight, with distinct submarginal row of large granules. Front beaded submarginally, approximately one-third of width of anterior carapace width, anterolateral angles quadrate from dorsal view; frontomedial portion distinctly bilobed. Supraorbital margin with sharp high granules, middle third weakly convex; infraorbital margin with finely granular ridge placed within orbital shelf; suborbital ridge well developed, with high, sharp granules; 2 rows of granules below suborbital ridge. Interantennular septum with median region ridged; posteromedial tooth triangular, beaded submarginally; posterior margin almost straight. Pterygostomian region finely granular, setose, prominent, broad Y-shaped sulcus. Inner edge of third maxilliped merus thick, with anterior distal angle strongly produced, submarginal oblique row of short setae close to distal edge; ischium roughly circular, moderately deep Y-shaped groove running from outer distal margin to midline, outer distal angle rounded.
Chelae globular, equal; longer, stouter in large males; surface of carpus coarsely granular, inner margin with projecting, rounded lobe covered with pointed granules; propodus with short row of denticles on lower, outer borders; fingers with rows of setae on inner surface, both terminating in rounded, slightly spatulate tips, better developed in females; movable finger with large, triangular tooth on cutting edge close to proximal end; female chelae generally similar to male but relatively more elongated, with more pronounced rows of setae.
Ambulatory legs (P2–P5) relatively robust in small males (carapace width less than 25 mm), females; anterior margins granular, carpi, propodi with thick tomentum; P3, P4 longest, P5 shortest; meri in small males, females distinctly flattened laterally, appears foliaceous; dorsoposterior margins of P3, P4 meri with relatively more prominent sharp granules, ventral margins lined with numerous short spines; second, third ischia with granular, robust tooth on posterior margin; P3 dactylus with distinct comb of long setae in large males, other dactyli glabrous; large males (carapace width exceeding 25 mm) with disproportionately elongated P3, merus, propodus especially long, slender.
Surface of male thoracic sternum sparsely granular; sternites 1–3 fused, highly compressed together, anterior edge drawn out to form broad but distinct triangle, narrow groove separating sternites 1, 2 from narrow sternite 3; anterolateral margin of sternite 3 angular, with shallow subangular depression. Male abdomen relatively broad; somite 1 subtruncate, subequal in width to somite 2, not reaching P5 coxae, separated by part of thoracic sternite 8 even when abdomen closed; somites 2–5 immobile, sutures between somites incomplete to shallow, never complete, suture between somites 3, 4 visible only as short, transverse, median groove; somite 5 broadly hexagonal with gently convex lateral margins; somite 6 immobile or with limited mobility with somite 5, suture complete but shallow; telson sub-semicircular, free. Female abdomen subcircular, covering most of thoracic sternum; all somites free; somite 6 with short, transverse median ridge close to distal margin.
G1 gradually recurved, no distinct junction between recurved, proximal portions, subdistal part of inner (sternal) surface with low but distinct, longitudinal fold, which may have distal edge forming a low lobe (in smaller males); tip broad, truncate, with strong, robust, with numerous low subterminal spines on one side.
Remarks. In addition to the generic characters discussed earlier, Cleistostoma dilatatum and Leptochryseus kuwaitensis can also be separated by the ornamentations of the carapace, cheliped and ambulatory legs, with L. kuwaitensis being a relatively more granular species ( Figs. 7 View FIGURE 7 , 8 View FIGURE 8 , 11 View FIGURE 11 G), while C. dilatatum is generally smoother ( Fig. 11 View FIGURE 11 A). The tooth on the dactylar finger of the male chela is distinctly triangular in L. kuwaitensis ( Fig. 11 View FIGURE 11 J) but truncate in C. dilatatum ( Fig. 11 View FIGURE 11 D). The comb of setae on the P3 dactylus is present in large specimens of L. kuwaitensis but absent in C. dilatatum . In addition, the pterygostomian sulcus in L. kuwaitensis ( Fig. 11 View FIGURE 11 H) is relatively broader compared to that of C. dilatatum ( Fig. 11 View FIGURE 11 B). These differences, however, are only useful at the species level.
According to Jones & Clayton (1983: 186, 188, fig. 2h), smaller specimens of L. kuwaitensis (less than 25 mm carapace width) lack the heavily setose leg patches and the comb of setae on the dactylus of the “third leg”. Examination of the present specimens indicates that they were actually referring to the second ambulatory leg (or third pereopod, P3) ( Figs. 7 View FIGURE 7 , 8 View FIGURE 8 A). We have also observed this pattern. Smaller males and juveniles also have relatively small and slender chelipeds as in females, and the proportions of their ambulatory legs are similar, i.e. more slender ( Fig. 8 View FIGURE 8 B, C). What Jones & Clayton (1983) did not discuss, but did figure, was the remarkable ambulatory leg dimorphism between adult and apparently dominant males compared to smaller males. Large males of L. kuwaitensis (carapace width of about 25 mm) have the ambulatory legs distinctly more slender ( Fig. 8 View FIGURE 8 A). In smaller males (and females), the ambulatory legs are relatively short and stout, with the merus appearing foliaceous ( Fig. 8 View FIGURE 8 ). The most dramatic difference is in the P3 of large males, which is much longer than the other legs with the merus and propodus being especially elongated ( Fig. 8 View FIGURE 8 A) (see also Jones & Clayton 1983: fig. 2h). This extreme ambulatory leg dimorphism between large and small males is not known for any other camptandriid species.
Although Al-Khayat & Jones (1996) used “ Leptochryseus kuwaitense ”, they stated that the gender of Leptochryseus was masculine. As such, the species name should be “ kuwaitensis ”.
Habitat and Biology. Jones & Clayton (1983) gave a detailed account of the biology of L. kuwaitensis . They found that it is a deposit feeder which uses its spatulate chelae bearing long fine setae to "spoon up and retain finer particles from the mudflats" ( Jones & Clayton 1983: 197). They postulated that the operculiform third maxillipeds may be an adaptation to fine particle feeding and noted that L. kuwaitensis is remarkably eurythermal and euryhaline, "surviving burrow water salinities of over 100‰ and below 20‰ near sewage effluent channels".
Geographical distribution. So far known only from Kuwait.
| ZRC |
Zoological Reference Collection, National University of Singapore |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
Family |
|
|
Genus |
Leptochryseus kuwaitensis ( Jones & Clayton, 1983 )
| Ng, Peter K. L., Rahayu, Dwi Listyo & Naser, Murtada D. 2009 |
Cleistostoma kuwaitense
| Al-Khayat 1996: 798 |
| Jones 1983: 185 |