Paleaequor Watson Russell, 1986
|
publication ID |
https://doi.org/10.1080/00222933.2017.1395919 |
|
persistent identifier |
https://treatment.plazi.org/id/03E91002-8710-1376-FE0B-FB3AFE14FE4A |
|
treatment provided by |
Felipe |
|
scientific name |
Paleaequor Watson Russell, 1986 |
| status |
|
Genus Paleaequor Watson Russell, 1986 View in CoL
( Figure 11a–d View Figure 11 , 12a, b View Figure 12 ; Tables 1, 2)
Type species: Paleaequor setula Watson Russell, 1986
Material examined
Two specimens Paleaequor nicoyensis Watson Russell, 1986 NTM . W. 25482, Peru, Eastern Pacific (mCT-00152, mCT-00170); 4 specimens Paleaequor setula NTM W . 2053, Queensland, Australia, South West Pacific (mCT-00017, mCT-00090, mCT-00095, mCT- 00107); Paleaequor breve ( Gallardo, 1968) NTM W . 25392, Malaysia, Eastern China Sea .
Distribution
Paleaequor species are found in tropical to sub-tropical waters in world oceans between 37°N and 30°S.
Habitat
The taxon is frequently found in tropical estuaries, sand flats and submarine banks at the entrances to rivers; intertidal to> 80 m. Paleaequor species have been almost exclusively recorded from sediments ranging from shell and gravel to fine sand and muds with a high organic content as well as from silty reefs ( Watson Russell 1986). Large numbers of Paleaequor nicoyensis were recorded from the eastern side of the Gulf of Nicoya from stations located off river mouths with high freshwater and detrital run-off during the wet season ( Maurer and Vargas 1984).
Within soft substrates Paleaequor species are associated with biogenic reef habitats made up of tube-dwelling polychaetes. Wilson (1979) describes Paleaequor heteroseta ( Hartman, 1945) as one of the most dominant species found in maldanid sedimentary reefs. Polgar et al. (2015) categorize Paleaequor breve as an opportunistic species present in the pre-settlement phase of sabellariid reef building when the sedimentary surface is characterized by mud deposits being reworked by the dominant surface deposit feeder, the terebellid Loimia verrucosa Caullery, 1944 .
General morphology
Paleaequor species are relatively slender chrysopetalids with the dorsum completely covered by flattened, golden-brown coloured, notochaetal paleal fans; main paleae possess raised ribs and tubercules ( Figure 11a View Figure 11 ). Paleaequor species attain short to moderate lengths, e.g. Paleaequor heteroseta , 7 mm length for 63 segments; Paleaequor breve , 18 mm length for 118 segments. Sensory structures include a small retractile prostomium (fused with anterior segments) in association with a nuchal fold, two pairs of complex eyes, cylindrical palps, lateral organs and retractile styles of dorsal cirri. Ventral pads are present ( Watson Russell 1986, figures 2–7; CW pers. obs.).
Pharynx and jaws
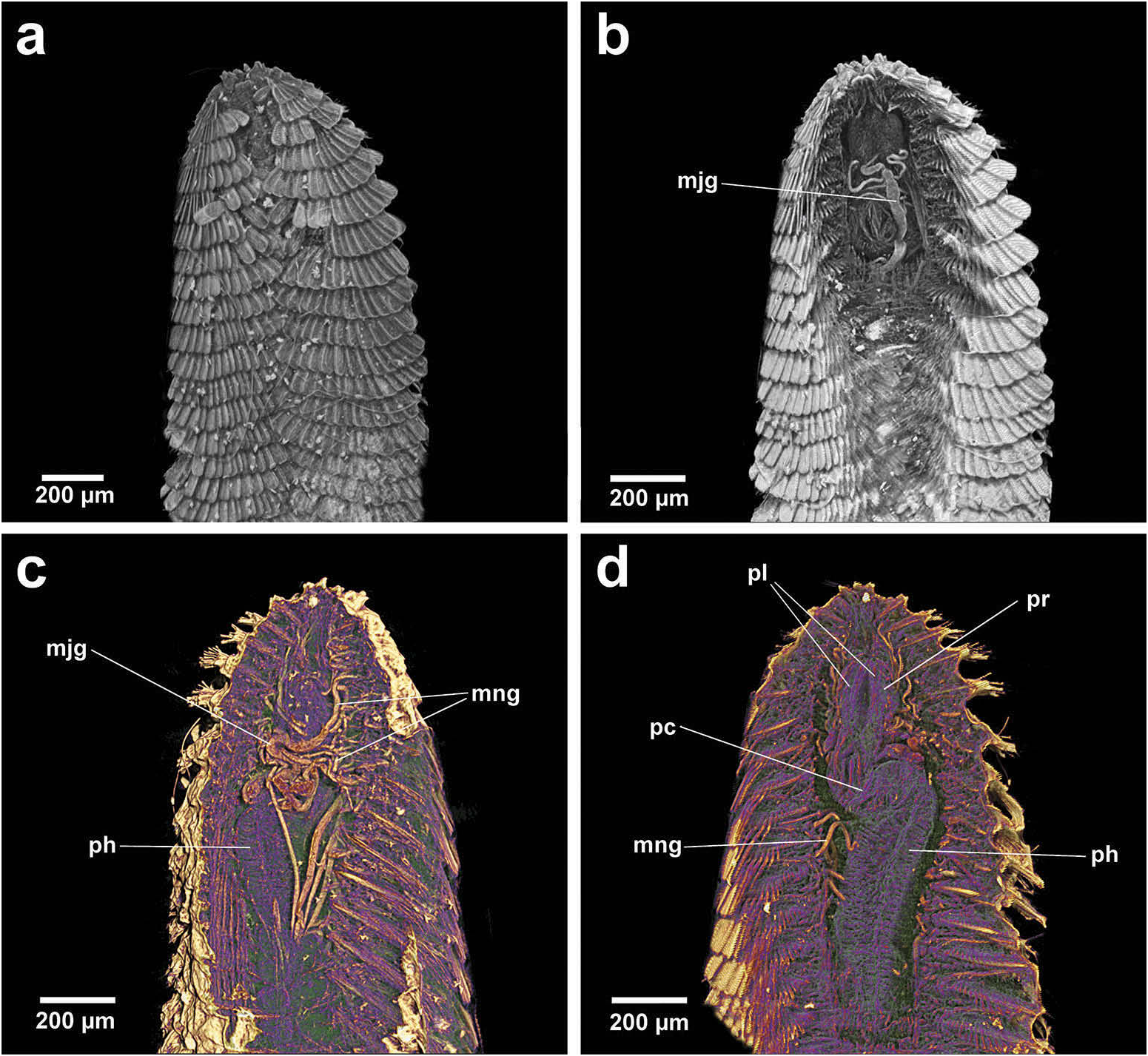
Paleaequor possesses a proboscis that when retracted appears to be divided, with possibly two associated lobes ( Figure 11d View Figure 11 ), and when closer to eversion appears more slender ( Figure 12a, b View Figure 12 ). A long, slender partly differentiated pharynx extends to approximately segment 20 and posterior caeca are present. CT scans illustrate a short rounded anterior pharynx wherein the stylets sit (stylets not visible in CT scans), followed posteriorly by a tight constriction that takes the form of a characteristic ‘kink’ ( Figure 11d View Figure 11 ; Watson Russell 1986). The pharyngeal juncture formed by this constriction is overlain with extensive, twisted glands: both major (mj. gl.) and minor (mn. gl.) in diameter and length. In dorsal view, the major glands extend longitudinally both anteriorly and posteriorly from the juncture along the pharynx ( Figure 11b, c View Figure 11 ); segmental minor glands extend horizontally into the parapodia along the length of the pharynx ( Figure 11c, d View Figure 11 ).
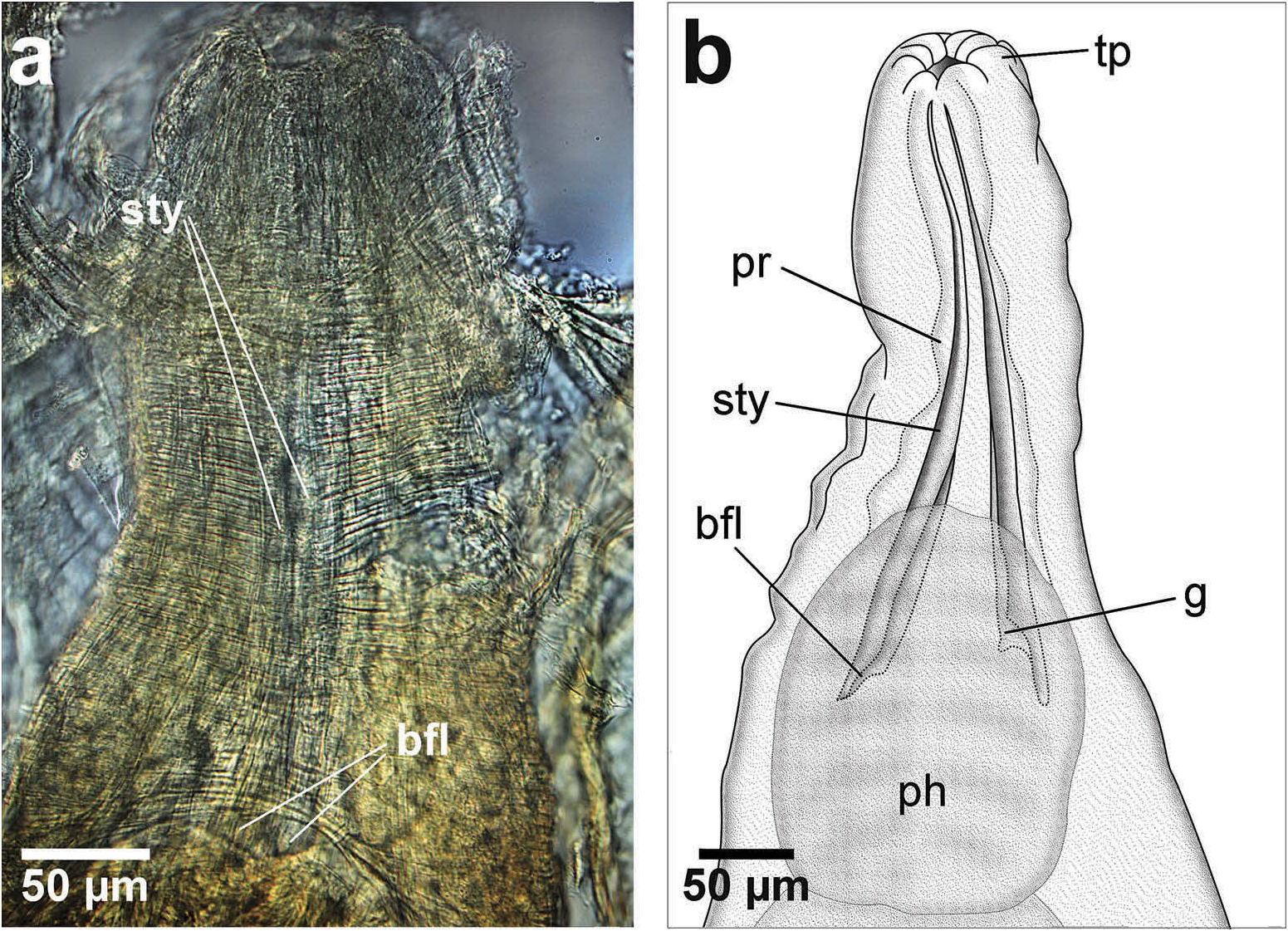
Jaws are fragile in composition with no identifiable calcification and are only visible under a compound microscope; small stylets are very slender with attenuated distal tips (lacking serration), straight margins, slightly flaring basal ends and moderately wide inner grooves ( Figure 12a, b View Figure 12 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.