Crematogastrini, Forel, 1893
|
publication ID |
https://doi.org/ 10.1093/isd/ixy013 |
|
persistent identifier |
https://treatment.plazi.org/id/03E7E05F-CE64-FFCD-981B-E5FE2B95FC11 |
|
treatment provided by |
Felipe |
|
scientific name |
Crematogastrini |
| status |
|
Diversification of Crematogastrini View in CoL
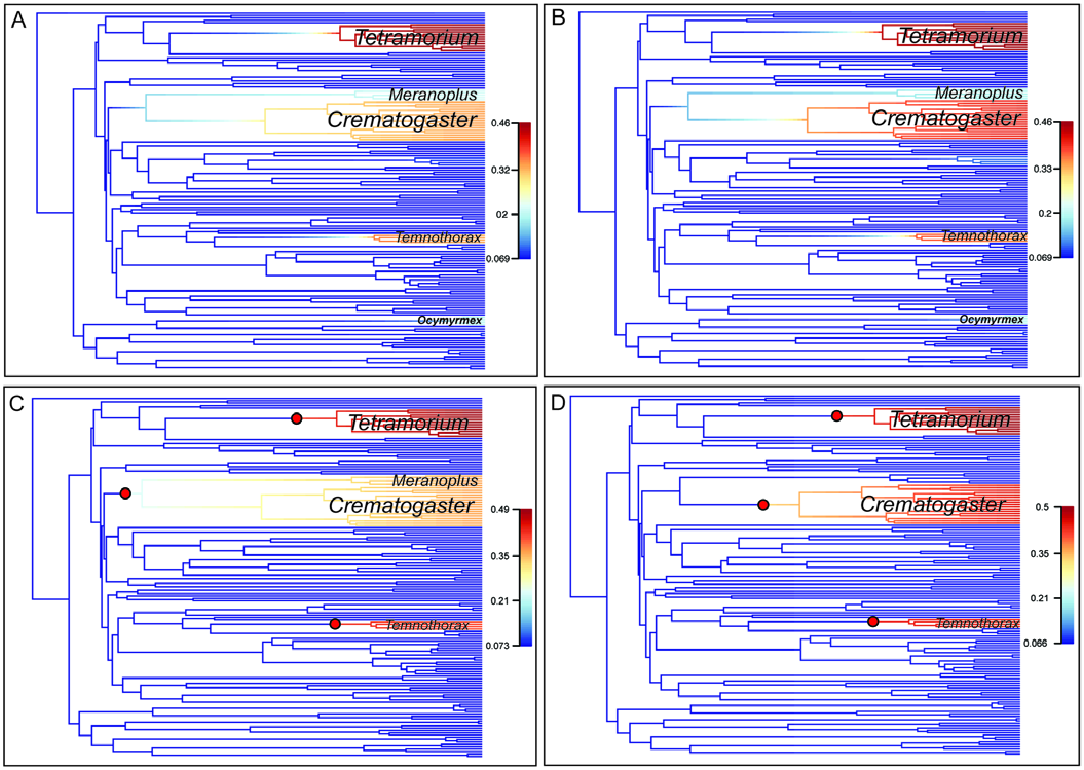
The subfamily Myrmicinae is the most species-rich and diverse lineage of ants in the world. A majority of this diversity is represented by the tribe Crematogastrini (together with the Attini), which contains three of the largest, most species-rich genera within the Myrmicinae : Tetramorium , Crematogaster , and Temnothorax . Three diversification rate shifts were inferred by our study and these shifts are associated unsurprisingly with these same three genera, which are also among the geographically most widespread taxa within Crematogastrini . A moderate diversification rate increase is further estimated for Meranoplus and Ocymyrmex , but with no distinct shifts supported. These two genera are fairly small, with 90 described species for Meranoplus and 34 species for Ocymyrmex . One explanation is that Ocymyrmex is estimated with a very young age (5.8 Ma), an estimate that could have been influenced by incomplete taxon sampling in our dating analyses (see above). We suspect that the evolutionary rate for this lineage is overestimated by our BAMM analyses. Meranoplus is the sister lineage to Crematogaster , and in one of the analyses (50-best-CP analysis excluding subspecies; Fig. 4C View Fig ), a rate shift is placed on the branch subtending both Meranoplus and Crematogaster . Possibly Meranoplus shares some (but not all) of the characteristics with Crematogaster that led to increased diversification in this clade. Interestingly, in a larger-scale analysis estimating diversification rate across the ants (Blanchard and Moreau 2016), a positive shift in diversification rate is also placed on the branch leading to the Crematogaster and Meranoplus clade (rather than just Crematogaster ). Further rate shifts in this study are placed in very similar locations on the Crematogastrini subtree; for example, as in our analysis, a positive shift in diversification rate for Tetramorium is recovered (Blanchard and Moreau 2016). However, the positive shift placed in our estimations on the Temnothorax branch is located in that study on the branch leading to a more inclusive clade of Temnothorax , Leptothorax , Formicoxenus , and Harpagoxenus , and is further estimated to be negative, resulting in a decreased diversification rate for the clade.
That study also recovers another negative shift leading to a clade comprised of Proatta , Dacatria , and Tetheamyrma (Blanchard and Moreau 2016) . Presumably these differences in results compared to our study are influenced by taxon sampling, divergence dating, and the different methods used to estimate diversification rates (Medusa was used in Blanchard and Moreau 2016). The accuracy of BAMM in estimating diversification rates and rate shifts under certain circumstances has been recently debated. Moore et al. (2016) raised concerns about the sensitivity of BAMM analyses to the selected rate shift prior, which were convincingly countered by Rabosky et al. (2017). Additional criticism has been raised regarding a tendency to overestimate diversification rates in smaller clades, thereby resulting in a potential underestimation of rate shifts overall ( Meyer and Wiens 2018). We conclude that our results are most likely not affected by potential erroneous diversification rate estimates, given that we recovered statistically significant shifts for the three most species-rich genera within Crematogastrini only—a result that essentially confirms observations based on taxonomic species diversity.
Positive shifts in diversification rate have usually been associated with either increased ecological opportunity, e.g., dispersal to and colonization of a new environment, or with the evolution of a key innovation, such as a novel trait (be it morphological, physiological, or genetic) that confers a competitive advantage to an organism or allows it to expand into a previously inaccessible environmental niche space. For example, turtle ants, genus Cephalotes , show an increase in diversification rate after colonizing a new habitat in the Chacoan region of South America ( Price et al. 2014, Price et al. 2016). This positive rate shift was associated with phenotypic diversification in turtle ants ( Price et al. 2016). Likewise, diving beetles (genus Exolina) were found to show a diversification rate shift after dispersing to Melanesia from Australia ( Toussaint et al. 2015). Our results did not enable us to discern an association of the diversification rate shifts leading to Crematogaster and Tetramorium with a biogeographic dispersal event or an evolutionarily significant geological time period. However, potential biogeographic dispersal events within these genera may be responsible for these patterns, which would be masked by our generic-level approach to estimating diversification rates. With respect to the rate shift estimated for Temnothorax , if this shift would be more correctly located on the branch leading to the larger clade that includes Leptothorax , Formicoxenus , and Harpagoxenus (as estimated in Blanchard and Moreau 2016), a possible association with a dispersal of this clade from the Indomalayan to the Palearctic and Nearctic regions and a burst of diversification in these temperate regions is conceivable. Beyond a biogeographic dispersal, the colonization of additional nesting space could have led to increased diversification in these three groups. Crematogaster , Temnothorax , and Tetramorium have all evolved to nest both in ground and in arboreal habitats from an ancestrally ground-nesting state, and it seems plausible that the exploitation of the arboreal habitat has led to increased species diversification. For Crematogaster , morphological innovations also have been proposed to have led to the success of this genus. Synapomorphies for the genus are the dorsal attachment of the postpetiole to the fourth abdominal segment (first segment of gaster) and the absence of a dorsal petiolar node. These features give the ants the ability to flex the gaster forwards over the mesosoma while the petiole can be pressed tightly against the propodeum for protection ( Buren 1959). Crematogaster workers also use this posture in defense paired with another unique feature: their spatulate sting applies venom topically instead of piercing the skin of the attacker ( Marlier et al. 2004). Unfortunately, while hypotheses of diversification associated with dispersal and ecological opportunity could be tested with extended species-level phylogenetic and biological data, hypotheses regarding the evolutionary significance and influence of innovative features are difficult to test and must remain somewhat speculative.
In summary, we here present an improved genus-level phylogeny for the tribe Crematogastrini that provides the basis for recognizing 10 genus-group lineages. Our estimates show that these lineages have evolved over a relatively short time frame (50–70 Ma) from a Paleotropical ancestor. The position of the monotypic enigmatic Indomalayan genus Rostromyrmex as the sister lineage to all remaining lineages within the tribe prevents us from pinpointing a specific biogeographic region that acted as the cradle for the diversification of the tribe. However, we have strong support for an early diversification of Crematogastrini in the Afrotropical region within the Cardiocondyla and Carebara genus-groups, and then for a shift in emphasis toward the Indomalayan region as the center for crematogastrine diversification. We discerned several shifts in diversification rates related to the evolution of large, widespread genera, but were not able to associate these definitively with key evolutionary events such as dispersal or novel morphological characteristics. Arboreal habitats have been successfully colonized by only a few clades within Crematogastrini , but it appears that members of the tribe have remained faithful to their lofty nest sites once they adapted to utilize this habitat. Our genus-level study sets the scene for more detailed investigations of diversification and evolution in the delineated genus-group lineages using species-level phylogenies and biological data.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |