Abida secale ( Draparnaud, 1801 )
|
publication ID |
https://doi.org/10.11646/zootaxa.2539.1.1 |
|
DOI |
https://doi.org/10.5281/zenodo.10538112 |
|
persistent identifier |
https://treatment.plazi.org/id/03E4E904-CE65-EC49-7EEE-FA33C5AEC9F8 |
|
treatment provided by |
Felipe |
|
scientific name |
Abida secale ( Draparnaud, 1801 ) |
| status |
|
Abida secale ( Draparnaud, 1801) View in CoL
Type locality: France .
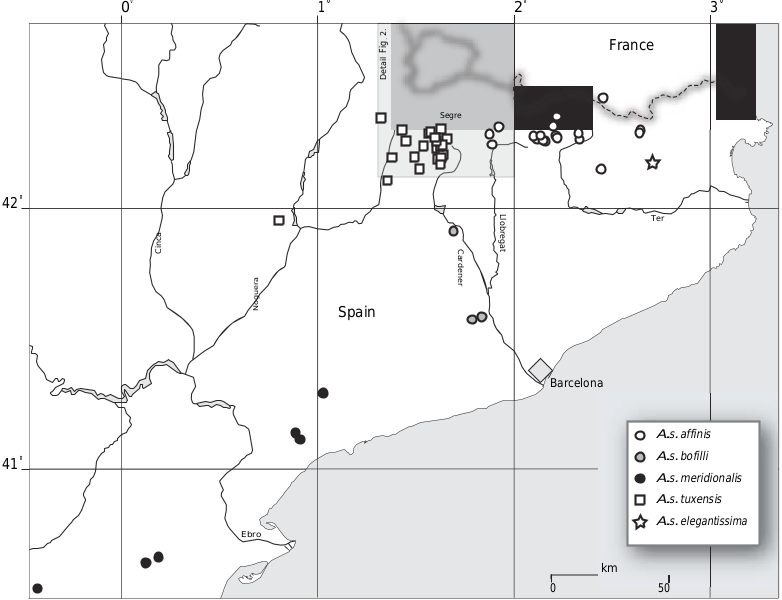
Distribution.—For the distribution of the various subspecies of A. secale , we refer to figs 2 View FIGURE 2 and 3 View FIGURE 3 .
Notes.—Since the last comprehensive study on A. secale ( Gittenberger 1973) , two subspecies have been added to this extremely polytypic species. Four more are added here. Molecular data support the monophyly of A. secale s.l. as interpreted by Gittenberger (1973) and Kokshoorn (2008: 73). These data also suggest that there has relatively recently been a hybridization between A. attenuata ( Fagot, 1886) and A. secale (see Kokshoorn 2008: 73–98), resulting in introgression of the A. attenuata mitochondrion into A. secale . This obscured the phylogenetic relations between the populations and subspecies in A. secale . Gittenberger (1973) postulated that A. secale forms a ring species complex. On the basis of samples of shells kept in museum collections, he suggested that the extreme morphological variation in the Sierra del Cadí, with a complex system of clines, locally resulted in intraspecific reproductive isolation. Using newly collected material with more precise locality data for biogeographical, morphological and molecular studies, we could neither confirm nor convincingly falsify the ring species model. For the moment being, we still prefer to unite a large group of subspecific taxa under the heading of a single species, i.e. A. secale s.l. The intra- and intersubspecific morphological variation does not prevent the delimitation of subspecies that are geographically coherent (but see Bech & Viader 1996) and that are more or less clearly interconnected.The results generated by DNA sequencing show that A. secale secale dispersed into its present range across Europe from its origin in or near the Segre valley in northern Spain. Morphologically the nominate subspecies is most closely linked to the subspecies found in southern France, i.e. A. s. boileausiana, A. s. saxicola and A. s. andorrensis. Based on extensive collections in the area, we concluded that there is a differentiation between subspecies with a more northerly and those with a more southerly distribution in northern Spain. Abida s. andorrensis (with A. s. ionicae) and A. s. brongersmai are linked by intermediates in the west and north of the Segre valley. The molecular data suggest that A. s. brongersmai and A. s. brauniopsis are linked by the high-altitude taxa A. s. cadiensis and A. s. cadica across the Cadí and Moixeró mountain ranges, although shell forms intermediate between A. s. cadiensis and A. s. cadica are not known. South of the Cadí-Moixéro mountain ranges A. s. tuxensis and A. s. lilietensis are found, linked by intermediates. Further eastwards, A. s. lilietensis gradually changes into A. s. affinis, which is linked by intermediates to A. s. margaridae, with A. s. merijni across the Moixéro range. Because of their shell morphology and distribution, and not contradicted by the molecular data, the taxa occurring further south, i.e. A. s. bofilli and A. s. meridionalis, are classified with the southern group. Since specimens intermediate between A. s. andorrensis and A. s. tuxensis are also known ( Gittenberger 1973; personal observations), this series of taxa cannot be separated from A. secale .
Based on these observations, we suggest a scenario with temporary isolation between a northern and a southern group of populations, resulting in a morphological differentiation with the unique development of a protruding aperture in the ‘ andorrensis -brongersmai -brauniopsis ’ group. There are minor climatic differences throughout the area, but we were not able to correlate any of these with potentially adaptive conchological characteristics. The scenario presented here would explain the absence of intermediates between geographical neighbouring taxa like A. s. brongersmai and A. s. margaridae, and A. s. tuxensis–A. s. brauniopsis–A. s. lilietensis. Unfortunately, there is no fossil record for Abida ; this makes it impossible to verify these hypothesized events or to suggest a timeframe. The distribution of the subspecies in the Cadí area is shown in figure 2 View FIGURE 2 . The distributional patterns in NE Spain outside this area are shown in figure 3 View FIGURE 3 . These maps are based on records from three major Dutch collections of Chondrinidae , viz. the National Museum of Natural History (Leiden), the Zoological Museum (Amsterdam) and the private collection of Mr. J. Eikenboom (Hellevoetsluis). Only those samples were included that contained 1 km UTM or other comparably accurate geographical data. A few records from the literature ( Gittenberger 1973; Bech 1993; Martínez-Ortí et al. 2004) were added if these data were sufficiently accurate.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.