Acanthonyx petiverii H. Milne Edwards, 1834
|
publication ID |
https://doi.org/10.11646/zootaxa.5146.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:52C3E5E3-80B6-49DB-BC9C-194560D491F7 |
|
DOI |
https://doi.org/10.5281/zenodo.7626257 |
|
persistent identifier |
https://treatment.plazi.org/id/03E3878A-A861-FFBB-04F4-8AC5FC77FEBC |
|
treatment provided by |
Plazi |
|
scientific name |
Acanthonyx petiverii H. Milne Edwards, 1834 |
| status |
|
Acanthonyx petiverii H. Milne Edwards, 1834 View in CoL View at ENA
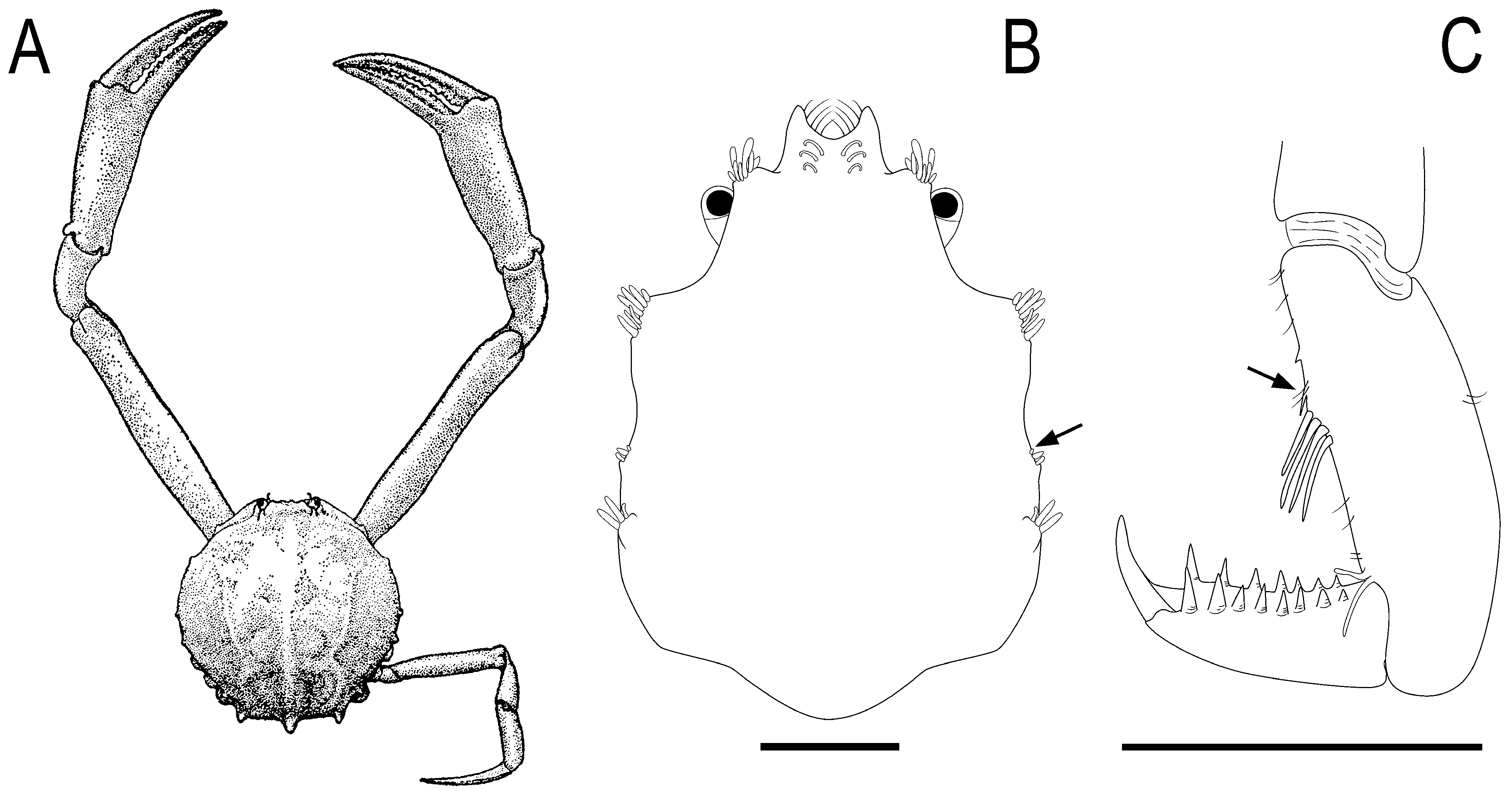
( Figs. 11B, C View FIGURE 11 , 12A–C View FIGURE 12 , 13A–D View FIGURE 13 )
Acanthonyx petiverii H. Milne Edwards, 1834: 343 View in CoL [ Type locality: Antilles].
Peltinia scutiformis Dana, 1851a: 273 View in CoL . Dana (1855) [Atlas]: pl. 5, fig. 7a–c [ Type locality: port of Rio de Janeiro].
Acanthonyx dissimulatus Coelho View in CoL in Coelho & Torres, 1993: 231, fig. 1 [ Type locality: Tambaú, João Pessoa , Paraíba].
Trindade and Martin Vaz specimens. 1 male ( MZUSP 40595 View Materials ) , 1 male juvenile ( MZUSP 40671 View Materials ), Brazil, off Espírito Santo, Trindade Island , Parcel das Tartarugas, 20°31’11.6”S, 29°18’0.6”W, J.B. Mendonça coll., 18.xi.2017, tide pool, 1.5 m GoogleMaps . 1 male ( MZUSP 40192 View Materials ), ibidem, 20°31’10.4”S, 29°17’58.4”W, J.B. Mendonça coll., 3.viii.2018, 1.0 m GoogleMaps . 1 male (39663), ibidem, 19.vi.2016, 1 m . 1 male GoogleMaps ( MZUSP 40190 View Materials ), ibidem, 20°31’11.6”S, 29°18’0.6”W, J.B. Mendonça coll., 18.xi.2017, 1.5 m. 4 males, 5 ovigerous females ( MZUSP 40201 View Materials ), 1 ovigerous female ( MZUSP 40191 View Materials ) , 1 juvenile female ( MZUSP 40615 View Materials ), ibidem, 20°31’11.6”S, 29°18’0.6”W, J.B. Mendonça coll., 15.xi.2017, 1.5 m GoogleMaps . 1 male ( MZUSP 40604 View Materials ) , 1 male, 2 ovigerous females, 4 juveniles ( MZUSP 40560 View Materials ), ibidem, 18.xi.2017, 1.5 m . 2 males, 1 young female ( MZUSP 39616 View Materials ), ibidem, 20°31’10.4”S, 29°17’58.4”W, J.B. Mendonça coll., 11.viii.2018, 1.0 m. 1 ovigerous female ( MZUSP 40726 View Materials ), GoogleMaps ibidem, 20°31’29.8”S, 29°19’52.0”W, J.B. Mendonça coll., 21.xi.2017, 11.3 m GoogleMaps . 1 male ( MZUSP 33816 View Materials ), ibidem, Enseada da Cachoeira , 20°30’55.6”S, 29°20’21.7”W, J.B. Mendonça coll., 12.vii.2012 GoogleMaps , 12 m. 1 male, 1 juvenile female ( MZUSP 39881 View Materials ) , 1 male ( MZUSP 39882 View Materials ), ibidem, Praia das Cabritas , 20°29’32.0”S, 29°19’46.5”W, J.B. Mendonça coll., 28.iv.2014, 9.2 m GoogleMaps . 1 male ( MZUSP 33817 View Materials ), ibidem, Praia dos Andradas , 20°30’45.7”S, 29°18’21.9”W, J.B. Mendonça coll., 5.vii.2013, tide pool GoogleMaps . 1 male ( MZUSP 39655 View Materials ) ibidem, Laje, Enseada Noroeste , 20°29’51.0”S, 29°20’44.3”W, J.B. Mendonça coll., 14.ii.2012, 22 m GoogleMaps . 1 male (damaged) ( MZUSP 40717 View Materials ), Martin Vaz Archipelago , 20°30’45.7”S, 29°18’21.9”W, J.B. Mendonça coll., 24.vii.2013, washed algae, 13 m GoogleMaps .
Size of largest male: cl 20.0 mm, cw 14.0 mm; largest female: cl 14.9 mm, cw 10.0 mm.
Comparative material examined. Acanthonyx brevifrons : Cape Verde: 2 ovigerous females ( MZUSP 4835 View Materials ), Porto Grande, São Vicente, Cambridge Expedition to St. Paul Rocks , stn 4.4, coll., 1.ix.1979, 1–2 m, on rock with weed. Acanthonyx dissimulatus : Brazil: holotype male, cl 20 mm, cw 13.5 mm ( MZUSP 6596 View Materials ), 1 female paratype ( MZUSP (24053), Paraíba, João Pessoa, Tambaú, 7.ix.1971. Acanthonyx lunulatus : France: 1 female ( MZUSP 41576 View Materials ), Banyuls-sur-mer, A. Anker coll., 1997 . Italy: 5 males, 4 ovigerous females ( USNM 205787 About USNM ), Sicily, Mazara Del Vallo, R.B. Manning and C. Froglia coll., 31.viii.1985, 2–4 m. 1 ovigerous female ( USNM 152264 About USNM ), ibidem, Trapani, R.B. Manning coll., 18.vi.1974. Acanthonyx minor Manning & Holthuis, 1981 : Gulf of Guinea: 2 males, 1 female paratypes ( USNM 171477 About USNM ), Annobon Island, R/V “Pillsbury”, stn 271, 19.v.1965, shore. Acanthonyx petiverii : Brazil: Piauí: 1 ovigerous female ( MZUSP 8394 View Materials ), 1 ovigerous female ( MZUSP 8395 View Materials ), 1 juvenile female ( MZUSP 8535 View Materials ), R/V “Almirante Saldanha”, Norte / Nordeste I, stn 1730, 02°37’S, 41°27’30”W, H.R. da Costa coll., 30.x.1967, 21 m. GoogleMaps Ceará: 1 male ( MZUSP 27599 View Materials ), Paracuru, P. Pachelle and A. Anker coll., 4.vii.2012, stn 12-159, low tide, under rock on algae. GoogleMaps Rio Grande do Norte : 1 male, 2 females ( MZUSP 28319 View Materials ), Maxaranguape, Parracho de Maracajaú , 05°23’39.99”S, 35°15’49”W, Tiego Coasta coll., ii.2010. GoogleMaps 3 males, 3 juveniles ( MZUSP 41127 View Materials ), ibidem, Extremoz, Praia de Genipabu , 05°41’630”S, 35°12’210” W, M. Tavares et al. coll., 16.xi.2009, from algae, intertidal at night. GoogleMaps 2 males, 5 females (3 of which juveniles) ( MZUSP 3666 View Materials ), ibidem, Natal. Pernambuco: 1 male, 1 female ( MZUSP 7535 View Materials ), Praia da Piedade , vii.1986. Espírito Santo: 1 ovigerous female ( MZUSP 32443 View Materials ), Araracruz, stn 7, 13.x.1989. 1 male ( MZUSP 8466 View Materials ), Guarapari, H.R. da Costa coll., 14.iv.1960. GoogleMaps 1 male, 1 female ( MZUSP 8507 View Materials ), ibidem, H.R. da Costa? coll., 19.iv.1960. GoogleMaps Rio de Janeiro : 1 ovigerous female ( MZUSP 8265 View Materials ), ibidem, Cabo Frio [Búzios], Armação dos Búzios, vii.1967. GoogleMaps 1 juvenile female ( MZUSP 41128 View Materials ), Ilha Grande, Praia do Funil , G.A.S. de Melo? coll., 24.vii.1966. GoogleMaps São Paulo: 1 male ( MZUSP 21063 View Materials ), Ubatuba, F. Marques coll., 3.iv.2002. Peru: 2 males, 4 ovigerous females ( USNM 71018 About USNM ), off coast just north of Paita , W.L. Schmitt coll., 7.x.1926, dredge, M.J. Rathbun det. GoogleMaps Central Atlantic: Acanthonyx sanctaehelenae Chace, 1966: 1 male, 2 ovigerous females ( USNM 134719 About USNM ), Saint Helena Island, James Bay GoogleMaps .
Distribution. Amphi-American. Western Atlantic: from southern Florida, Bahamas, through the Caribbean islands southwards to Brazil ( Piauí to São Paulo). This is the first record of Acanthonyx petiverii from Trindade and Martin Vaz. For the detailed distribution in the Caribbean Sea, see Carmona-Suárez & Poupin (2016). Eastern Pacific: from Baja California to Chile ( Valparaiso), including the Galpagos Islands ( Emparanza et al. 2007, and references therein).
Ecological notes. Adults and juveniles of Acanthonyx petiverii usually inhabits macroalgae to which they attach themselves by means of the P2–P5, whose propodi ventroposterior margins are provided with a blunt tubercle against which the dactyli fits like a claw when flexed ( Fig. 12A, B View FIGURE 12 ). The crabs acquire algal pigments (e.g. chlorophyll and phycoerythrin) from the algae they live and feed, and effectually conceals themselves by matching the background (e.g. brown crabs on brown algae) ( Wilson 1987). However, as one or more molts are required to incorporate the algal pigments into the exoskeleton, decorating itself with the host algae allows for more rapid concealment. Acanthonyx petiverii , being not a heavy decorator, attaches to itself only a few pieces of the host algae by means of hooked setae (less commonly ribbons of hydroids. See Guinot & Wicksten, 2015 and references therein). Unlike other spider crab species, A. petiverii does not use its attached algae as food ( Wicksten 1993; Guinot & Wicksten 2015). In Trindade, adults and juveniles were found together on brown ( Dictyota ) and red algae in tide pools down to 22 m ( Fig. 2C View FIGURE 2 ). Juveniles (e.g. cl 4.5 mm, cl 2.9 mm or even smaller), more commonly than adults, were carrying algae attached to the dorsal surface of the rostrum, which is provided with two longitudinal rows of hooked setae ( Figs. 11B View FIGURE 11 , 12A, C, D View FIGURE 12 , 13A, C View FIGURE 13 ). Adults and juveniles of A. petiverii have been occasionally found associated to the sea urchin Echinometra lucunter (see Vera-Caripe et al. 2019 and references therein) and in wood perforated by the wood-boring bivalve Teredo (see Rodriguez 1980).
Teixeira et al. (2009) reported on males sexually mature at 10–11 mm and 8–9 mm cw, respectively. Males reach larger sizes than females. Mature females have deeply concave, broad pleon of 4 somites reaching to the bases of the legs, and telson; somites 4–6 fused. Ovigerous females carrying large number of tiny eggs of about 1.0 mm ( Fig.13 C, D View FIGURE 13 ) were caught in August and November in Trindade. The larval phase consists of two zoeal stages and one megalopa, as usual in majoid crabs. The duration of the first and second zoea stages in laboratory conditions was about of 5 and 11 days, respectively ( Hiyodo et al. 1994). The duration of the megalopa stage is unknown.
Remarks. Acanthonyx petiverii is widely distributed along the Atlantic and Pacific coasts of the Americas ( Rathbun 1925; Garth 1958; Retamal 1981; Hendrickx 1992; Emparanza et al. 2007). This wide distribution in combination with the great morphological variability among developing and adult males and females prompted the description of new species, which later proved inseparable from A. petiverii s. str. The observed morphological variability include the shape of the carapace outline; development of the hepatic and branchial lobes, presence and conspicuousness of the setiferous tubercles on the protogastric, cardiac and intestinal regions of the carapace; density and length of the tufts of setae on the carapace and tubercles; form of fingers and gaping in the chelipeds; conspicuousness of the crest and tubercles of the cheliped carpus and merus, and number of teeth ventrally on the dactyli of the pereopods ( Fig. 11C View FIGURE 11 , 12A, B View FIGURE 12 , 13 B, C View FIGURE 13 ). The color of the specimens varies with the type of the hosting algae (see above under Ecological notes).
Acanthonyx petiverii and A. scutiformis ( Dana, 1851a) were regarded as each other’s synonyms by Rathbun (1925) and Emparanza et al. (2007), but were listed as separate species by Ng et al. (2008).
Current arguments in favor of the validity of A. scutiformis are essentially those of Coelho & Torres (1993) to whom the species is recognizable by having the hepatic lobes of the carapace curved upwards and forwards and furnished with long setae and the dorsal and lateral setiferous tubercles also crowned with long setae. Dana’s (1851a: 273) original description was too brief and provided not enough information to distinguish between species, so that Coelho & Torres (1993) surmised the aforementioned distinguishing characters from the illustration by Dana (1855: pl. 5, fig. 7a). According to Coelho & Torres (1993) the same characteristics distinguish A. scutiformis from A. dissimulatus Coelho in Coelho & Torres, 1993, whose hepatic lobes are never curved upwards and forwards, and the dorsal and lateral setiferous tubercles are provided with very short setae ( Coelho & Torres 1993). However, we submit that the purported differences between A. petiverii , A. scutiformis and A. dissimulatus ( Fig. 12 D View FIGURE 12 ) are no greater than the variations between specimens of different size and sex from the same locality.
Tamburus & Mantelatto (2016) found that neither morphological nor molecular data support the recognition of A. scutiformis and A. dissimulatus as valid species and, accordingly, regarded both as junior synonyms of A. petiverii . Tavares et al. (2017) objected by arguing that there were problems with the sequences deposited in the GenBank by Tamburus & Mantelatto (2016) and possibly the misidentification of specimens as the observed levels of genetic divergence is actually suggestive of two species.
In the absence of clear-cut characters to separate the three species from one another Acanthonyx petiverii , A. scutiformis and A. dissimulatus are here provisionally regarded as conspecific.
Five additional species of Acanthonyx are known to occur in the Atlantic Ocean, namely A. brevifrons A. Milne-Edwards, 1869 (EA); A. depressifrons Manning & Holthuis, 1981 (EA); A. lunulatus ( Risso, 1816) (ME and adjacent EA); A. minor Manning & Holthuis, 1981 (EA); and the insular A. sanctaehelenae Chace, 1966 ( AS and SH). The morphological differences among these five species have been discussed by Chace (1966) and Manning & Holthuis (1981). D’Udekem d’Acoz (2001) and Tavares et al. (2017) further discussed the differences between A. brevifrons and A. lunulatus .
The TMV specimens are herein assigned to A. petiverii , whose unique combination of characters are not found in the aforementioned five species. They differ from A. brevifrons in having always 3 distinct lateral lobes in the carapace (vs 2 lobes in A. brevifrons ); from A. depressifrons in that the rostrum is not as depressed and the ventral margins of the dactyli of P2–P5 are provided with 11–17 teeth (vs rostrum strongly depressed and 4–6 tubercles ventrally on dactyli in A. depressifrons ); from A. lunulatus in having the carapace rectangular in outline in adults and 2 tubercles on the dorsoproximal surface of the cheliped merus, being the most proximal one always inconspicuous or even absent (vs carapace broadly pear-shaped in adults and cheliped merus always with 2 strong tubercles, being the proximal smaller than the distal one in A. lunulatus ); from A. minor in being much larger with the orbital margin nearly smooth, and the ventral margins of the dactyli of P2–P5 provided with 11–17 teeth (vs a distinct projection on the orbital margin and 6–8 tubercles ventrally on dactyli, 3–4 in juveniles, in A. minor ). The TMV specimens differ from A. sanctaehelenae in the adult carapace outline, cheliped carpus with a dorsolateral crest and 2–3 setiferous tubercles, and in the number of tubercles on the dorsoproximal surface of the cheliped merus (vs carapace broadly pear-shaped in adults, lack of carpal tubercles, and 1 minute but recognizable meral tubercle in A. sanctaehelenae).
Juveniles (e.g. cl 4.5 mm, cl 2.9 mm or smaller) of A. petiverii already have well-developed rostral horns with hooked setae to which algae is commonly attached. In contrast, the developing lateral lobes of the carapace are provided with short, poorly developed setae; the first branchial lobe is inconspicuously developed ( Fig. 11B View FIGURE 11 , 13A View FIGURE 13 ). The propodi of P2–P5 lack the characteristic compressed, dilatated shape; their ventroposterior margins are deprived of tubercle but are instead provided with a few large setae directed to dactyli, whose ventral margins have two rows of 7–9 teeth, instead of 11–17 teeth as in the adults ( Figs. 11C View FIGURE 11 , 13A–D View FIGURE 13 ).
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
SuperFamily |
Majoidea |
|
Family |
|
|
Genus |
Acanthonyx petiverii H. Milne Edwards, 1834
| TAVARES, MARCOS & MENDONÇA, JOEL BRAGA DE JR. 2022 |
Acanthonyx dissimulatus
| Coelho, P. A. & Torres, M. F. A. 1993: 231 |
Peltinia scutiformis
| Dana, J. D. 1851: 273 |