Chaeridiona thailandica
|
publication ID |
https://doi.org/10.5281/zenodo.179585 |
|
DOI |
https://doi.org/10.5281/zenodo.6246385 |
|
persistent identifier |
https://treatment.plazi.org/id/03E0879F-6076-5140-ECEC-635FFD97FC6C |
|
treatment provided by |
Plazi |
|
scientific name |
Chaeridiona thailandica |
| status |
|
Chaeridiona thailandica View in CoL
Larva ( Figs 4–6 View FIGURES 1 – 8 , 9, 10, 13–45). Length of mature larva (without head): 5.00 mm, width across mesonotum: 1.60 mm (n=1). Length of younger instar larvae: 3.75–4.35 mm, width: 1.00–1.32 mm (n=3).
Colour of alcohol preserved larva, on dorsal as well as ventral side, yellowish-brown with black stemmata, brown mouth parts and brown last pair of spiracles forming posterior tip of body (Figs 9, 10).
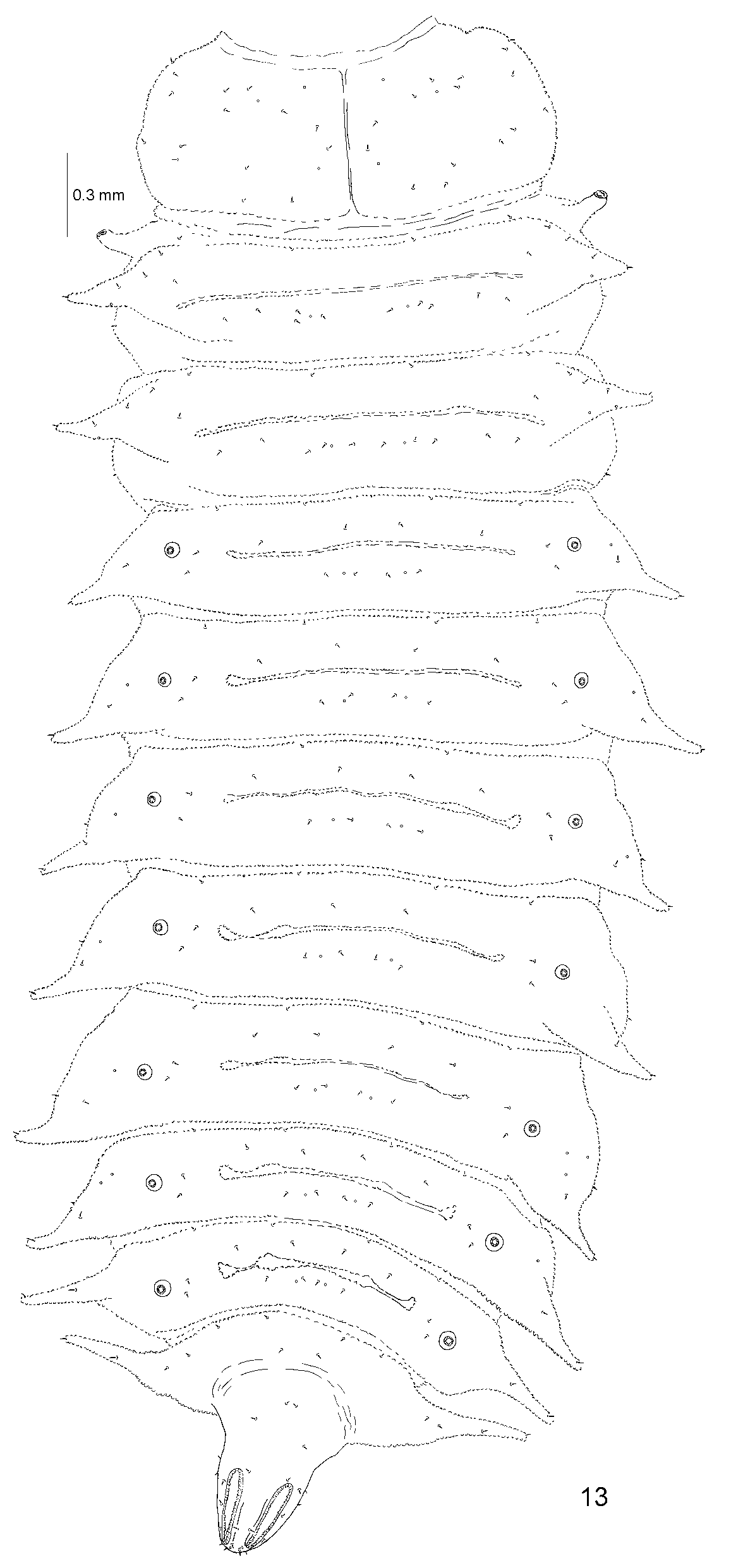
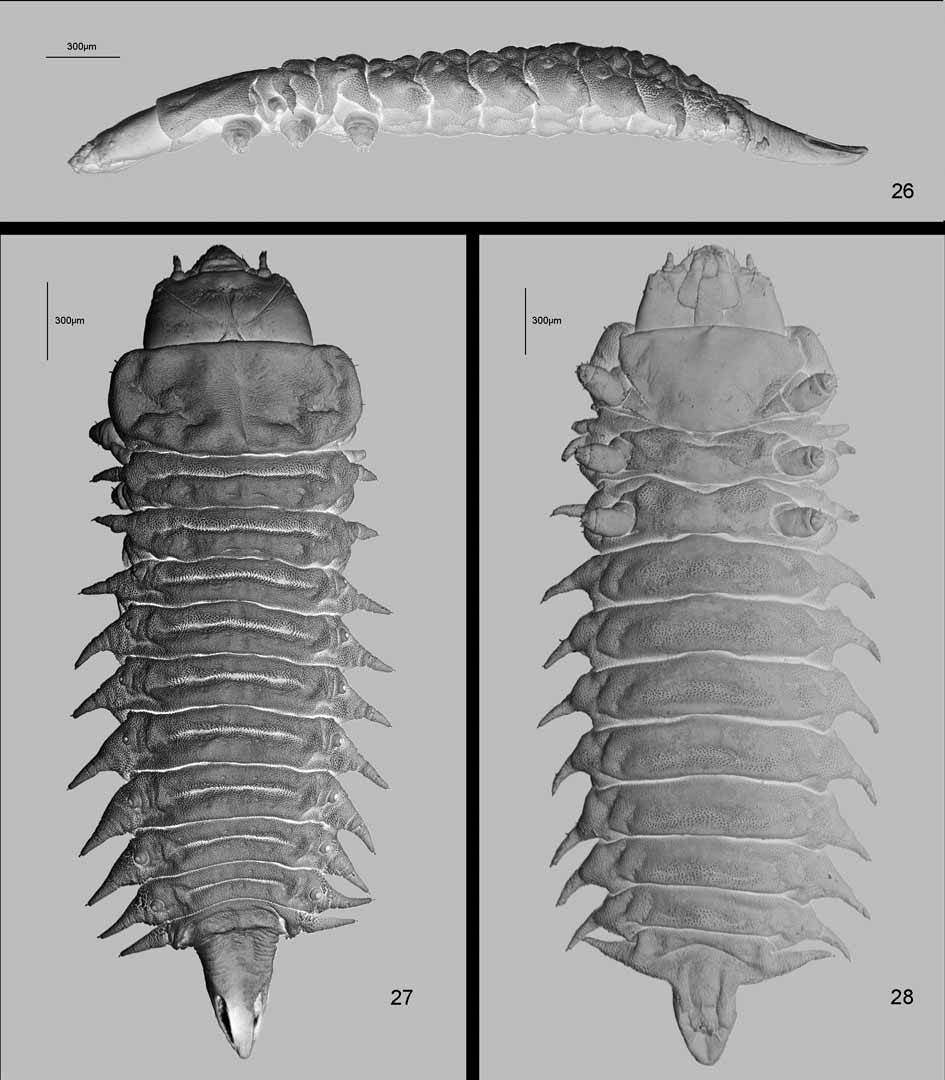
Body strongly flattened dorso-ventrally ( Fig. 26 View FIGURES 26 – 28 ), elongate, parallel-sided, narrowed posteriorly from abdominal segment VI, spiracles of eighth pair form posterior tip of body, triangular in shape (Figs 9, 10, 13, 14, 27, 28, 35, 36). Segments of thorax and abdomen are transverse. Pronotum is with broadly rounded lateral margin, without lateral scoli. Meso- and metanotum are approximately as wide as or slightly narrower than pronotum, each with a pair of lateral scoli. Abdominal segments are as wide as segments of thorax, gradually narrowed posteriorly from segment VI. Abdominal segments I–VIII on each side with one lateral scolus.
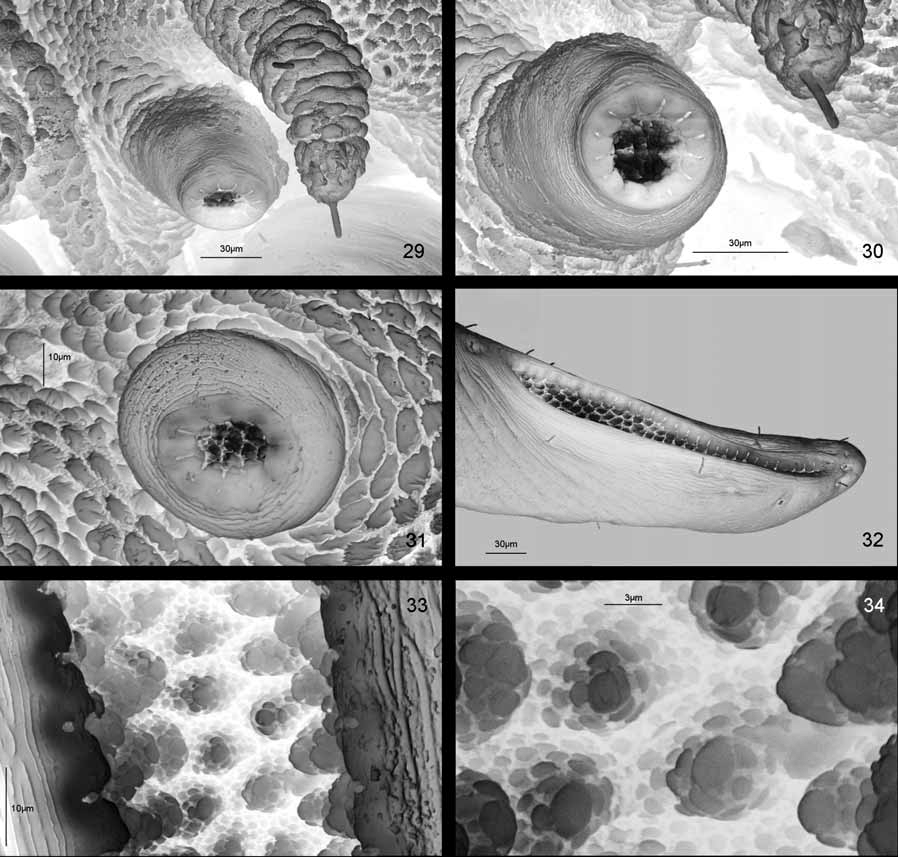
Nine pairs of spiracles: one pair on mesonotum and one pair on each abdominal segment I to VIII ( Figs 13 View FIGURE 13 , 27 View FIGURES 26 – 28 , 29 View FIGURES 29 – 34 , 35 View FIGURES 35 – 40 ). Spiracles of eighth abdominal segment elongate with elongate-oval outer spiracular opening placed dorsally ( Figs 13 View FIGURE 13 , 27 View FIGURES 26 – 28 , 32 View FIGURES 29 – 34 , 35 View FIGURES 35 – 40 ). Respiratory chambers of all spiracles covered with tubercles, each with numerous mushroom-like structures ( Figs 29–34 View FIGURES 29 – 34 ). Spiracles of eighth pair form triangular tip of body with anal slit placed ventrally ( Figs 14 View FIGURE 14 , 28 View FIGURES 26 – 28 , 36 View FIGURES 35 – 40 ).
Dorsal and ventral side of body with short pointed setae and slightly shorter minute setae placed at anterior margin of segments. Meso-, metanotum and first 7 abdominal segments possess a transverse fold (plical area) across the middle. Lateral scoli of thorax and abdomen armed apically with one pointed seta ( Fig. 37 View FIGURES 35 – 40 ).
Pronotum with 15 pointed setae and three campaniform sensilla on each side ( Fig. 13 View FIGURE 13 ). Anterior border of meso- and metanotum with two pairs of very minute setae. Moreover, meso- and metanotum with one seta on each side antero-laterally (close to plical area), two setae at the base of scoli, one seta on each postero-lateral side (seta visible from dorsal and ventral side), and row of 10 setae and two campaniform sensilla placed posteriorly. One seta on each thoracic scolus dorsally. Anterior border of I–VII abdominal segments with two pairs of minute setae. Dorsal side of abdominal segments I–VII with two pairs of setae close to anterior margin medially, row of four setae and two campaniform sensilla postero-medially, and two setae on each side close to spiracle. One seta on abdominal scoli 1–8 dorsally. The last abdominal segment different in shape from the remaining ones, with elongated spiracles placed very close to each other. The last segment with two pairs of minute setae at anterior border, a pair of setae anteriorly in the middle and two setae on each side at the base of lateral scolus. Spiracles of eighth pair not distinctly bordered from eighth abdominal segment, forming triangular posterior tip of body. Body tip with 16 setae placed dorsally and eight on lateral side (visible from dorsal and ventral side). Chaetotaxy of dorsal side of body as in Figure 13 View FIGURE 13 .
Prosternum with one minute seta at anterior margin and four pointed setae in the middle of sternite ( Fig. 14 View FIGURE 14 ). Meso-, and metasternum with two pairs of minute setae at anterior margin and four pointed setae in the middle of sternite. Three setae at the base of each coxa of the first pair of legs and one seta at the base of second and third pair. Abdominal segments I–VII with a pair of minute setae at the anterior border. Segments I, VI and VII with two groups of four setae antero-medially and two setae on each side postero-laterally. Segments II–V with two groups of five setae antero-medially and two setae on each side postero-laterally. One seta on abdominal scoli 1–7 ventrally. Last abdominal segment with a pair of minute setae at anterior border and six setae in row running across the sternite. Ventral side of the body tip with 10 setae. Chaetotaxy of the ventral side of body as in Figure 14 View FIGURE 14 .
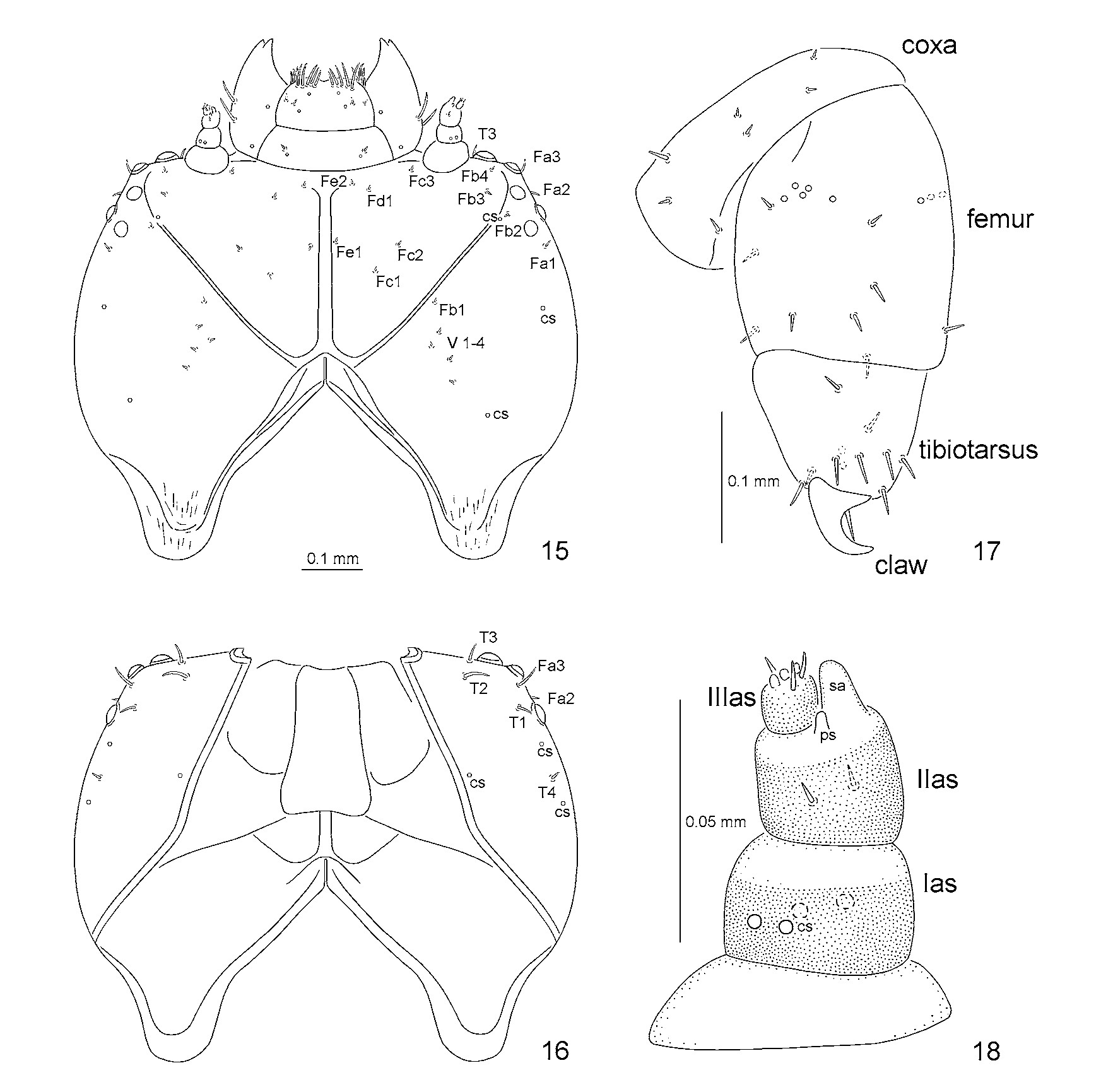
Head well sclerotized, prognathous, partially retracted into pronotum ( Figs 15, 16 View FIGURES 15 – 18 , 41 View FIGURES 41 – 45 ). Epicranial stem absent ( Fig. 15 View FIGURES 15 – 18 ). Median endocarina well developed, extending between frontal arms. Frontal arms V-shaped. Fronto-clypeal suture present. Clypeus with a pair of setae and a pair of campaniform sensilla. Frons on each side with one short seta (Fc3) close to antenna, and two short setae placed anteriorly (Fd1, Fe2); three short setae placed medially close to median endocarina and frontal arm (Fc1, Fc2, Fe1), and two short setae laterally close to frontal arm (Fb3, Fb4). Vertex (epicranium) on each side with five short setae (Fb1, V 1–4) and one or two campaniform sensilla (cs). On each side of head there are three longer pointed setae close to stemmata (Fa1, Fa2, Fa3), and one short seta (Fb2) and one campaniform sensillum close to frontal arm. Temporal side ( Fig. 16 View FIGURES 15 – 18 ) with three campaniform sensilla (cs) and four setae: three long (T1, T2, T3), and one slightly shorter than the first one (T4).
Stemmata black, five on each side of head.
Antennae 3- segmented ( Figs 18 View FIGURES 15 – 18 , 39, 40 View FIGURES 35 – 40 ). Basal segment (Ias) transverse, slightly wider than the second, with two pairs of campaniform sensilla (cs). Median segment (IIas) stout, slightly shorter than wide, with two short setae on sides, and short peg-like sensillum (ps) and sensory appendix (sa) at the top. Short peg-like sensillum is placed between sensory appendix and third antennal segment (IIIas). Sensory appendix as long as third antennal segment. Third antennal segment slightly longer than wide, with three small peg-like sensilla and three short setae at the apex.
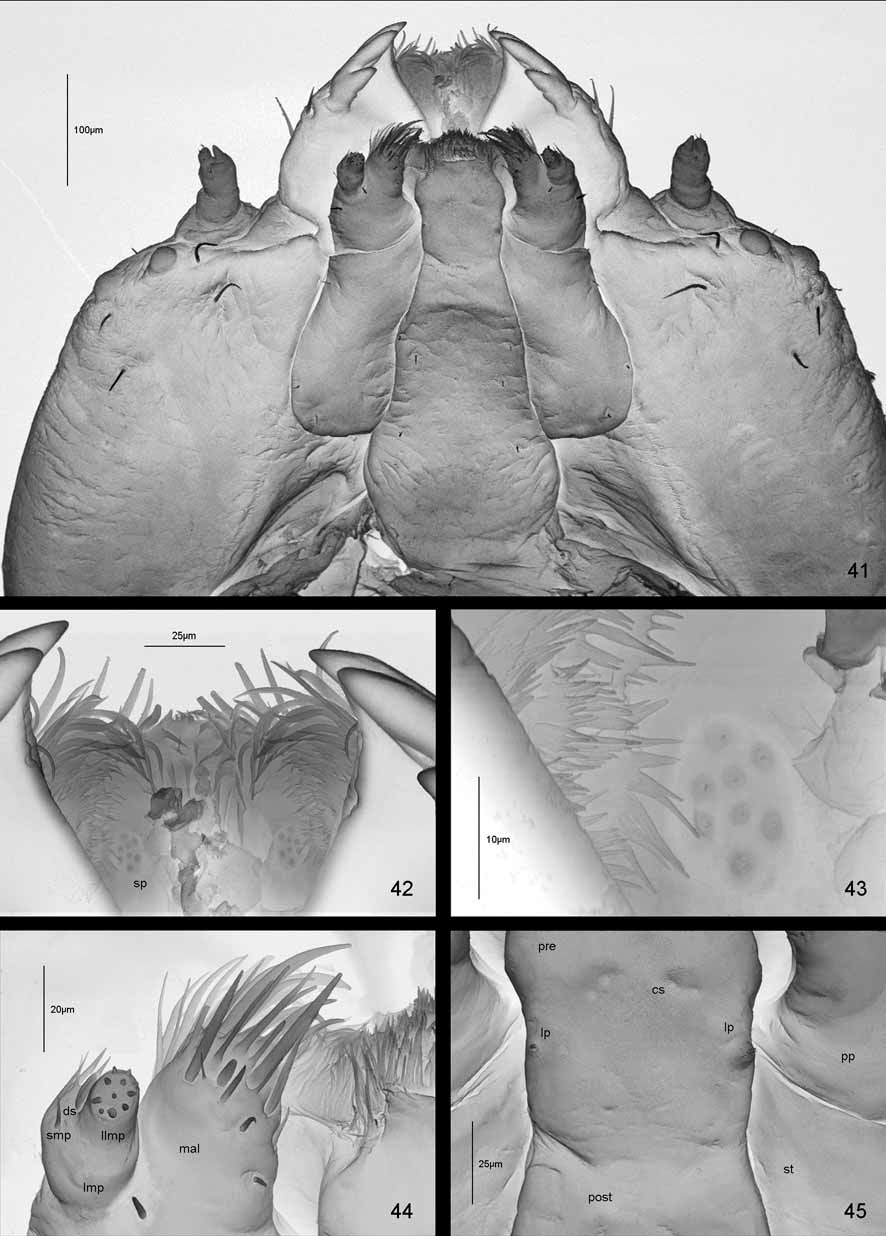
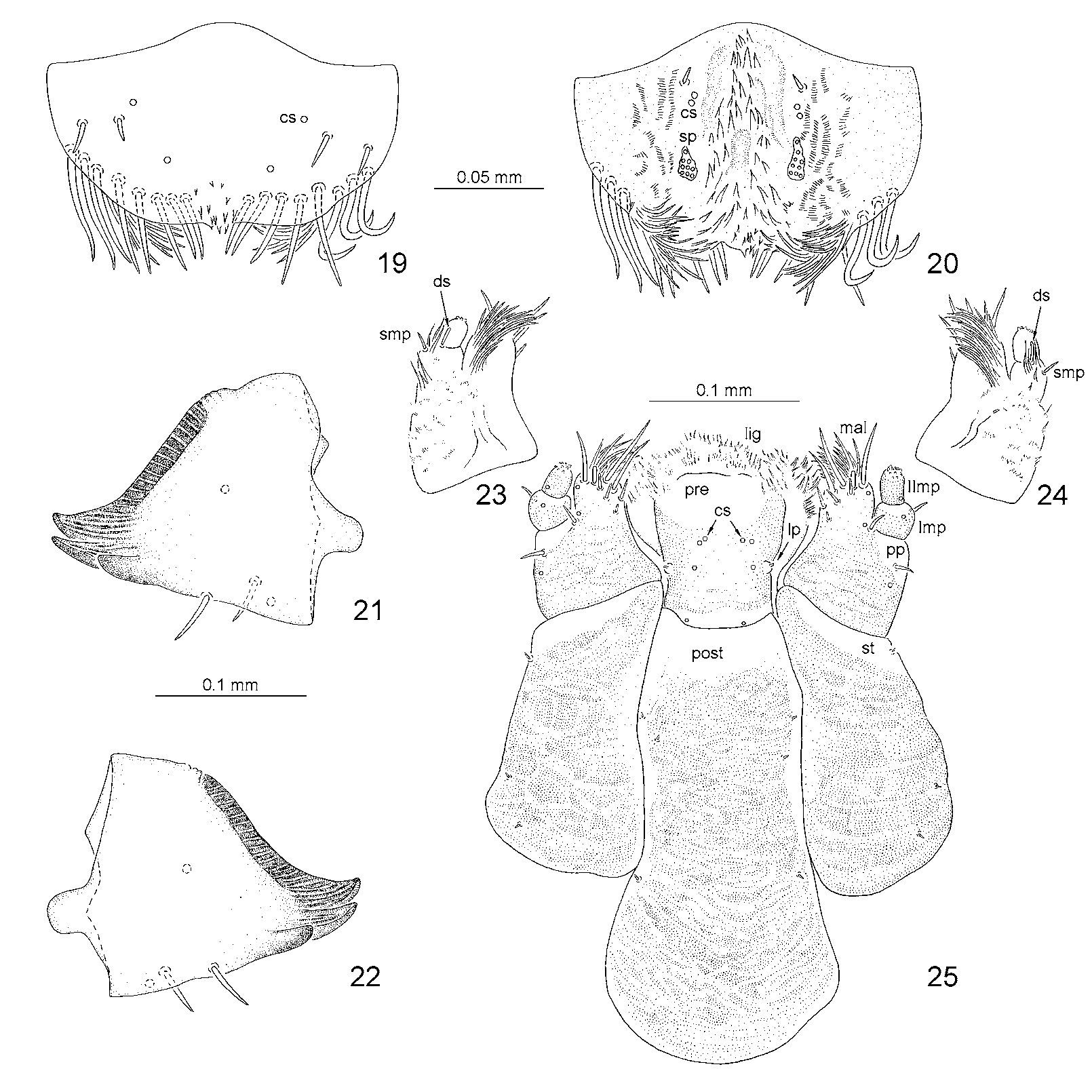
Labrum free, wider than long, without emargination at the anterior margin ( Figs 19, 20 View FIGURES 19 – 25 , 42, 43 View FIGURES 41 – 45 ). Anterior margin with eight stout, long, pointed setae on each side. Dorsal side of labrum with four setae and four campaniform sensilla (cs). Mid and anterior part of ventral surface (epipharyngeal area) with numerous stout spines, lateral parts with tiny spines. Ventromedian part with a pair of pointed setae, two pairs of campaniform sensilla (cs) and two groups of a few sensilla (sp - sensilla placodea?) ( Figs 42, 43 View FIGURES 41 – 45 ).
Mandibles are heavily sclerotized, 3-dentate, with distinctly sclerotised margin above teeth ( Figs 21, 22 View FIGURES 19 – 25 , 41 View FIGURES 41 – 45 ) with two setae and two campaniform sensilla on dorsal side.
Maxillae and labium connate ( Figs 23–25 View FIGURES 19 – 25 , 41, 44, 45 View FIGURES 41 – 45 ). Each stipes (st) with three short pointed setae. Palpiger (pp) distinct, heavily sclerotised in basal part, with two setae and two campaniform sensilla ventrally, and numerous short and long spines dorsally. Maxillary palp two-segmented. First segment of maxillary palp (Imp) with one campaniform sensillum ventrally, and one pointed seta (smp) and long spinules dorsally. Second segment (IImp) provided with a group of peg-like sensilla at apex, one campaniform sensillum below the apex, and one blunt digitiform sensillum (ds) at the base. Mala (mal) with one campaniform sensillum, 10 pointed setae at the top (7 placed ventrally, 3 dorsally), one blunt seta and numerous long spinules on dorsal side. Ligula (lig) covered with spines. Prementum (pre) with three pairs of campaniform sensilla (cs) medially and a pair at base. Besides, prementum shows two distinct tubercles (lp - labial palpi?), each with peg-like sensilla at the apex. Postmentum (post) with three pairs of setae.
Figures 9–12. Chaeridiona thailandica . (9) Dorsal side of mature larva; (10) ventral side of mature larva; (11) dorsal side of pupa; (12) ventral side of pupa
All legs stout, with similar chaetotaxy ( Figs 17 View FIGURES 15 – 18 , 38 View FIGURES 35 – 40 ), three-segmented: coxa, femur and tibiotarsus. Tibiotarsus apically with heavily sclerotized, short and curved, single and simple claw, armed basally with a pointed seta. Two campaniform sensilla above claw. Tibiotarsus with 9 long pointed setae: a complex of 7 setae surrounding claw and two setae in dorso-medial part. Femur with 7 long setae and one short seta close to the base dorsally. Basally, on internal side, a group of 5 campaniform sensilla and one short pointed seta, and at the base, ventrally, one campaniform sensillum. Also basally, but on external side, two campaniform sensilla. Coxa with setae arranged in three groups: first with three setae, second and third with a pair of setae.
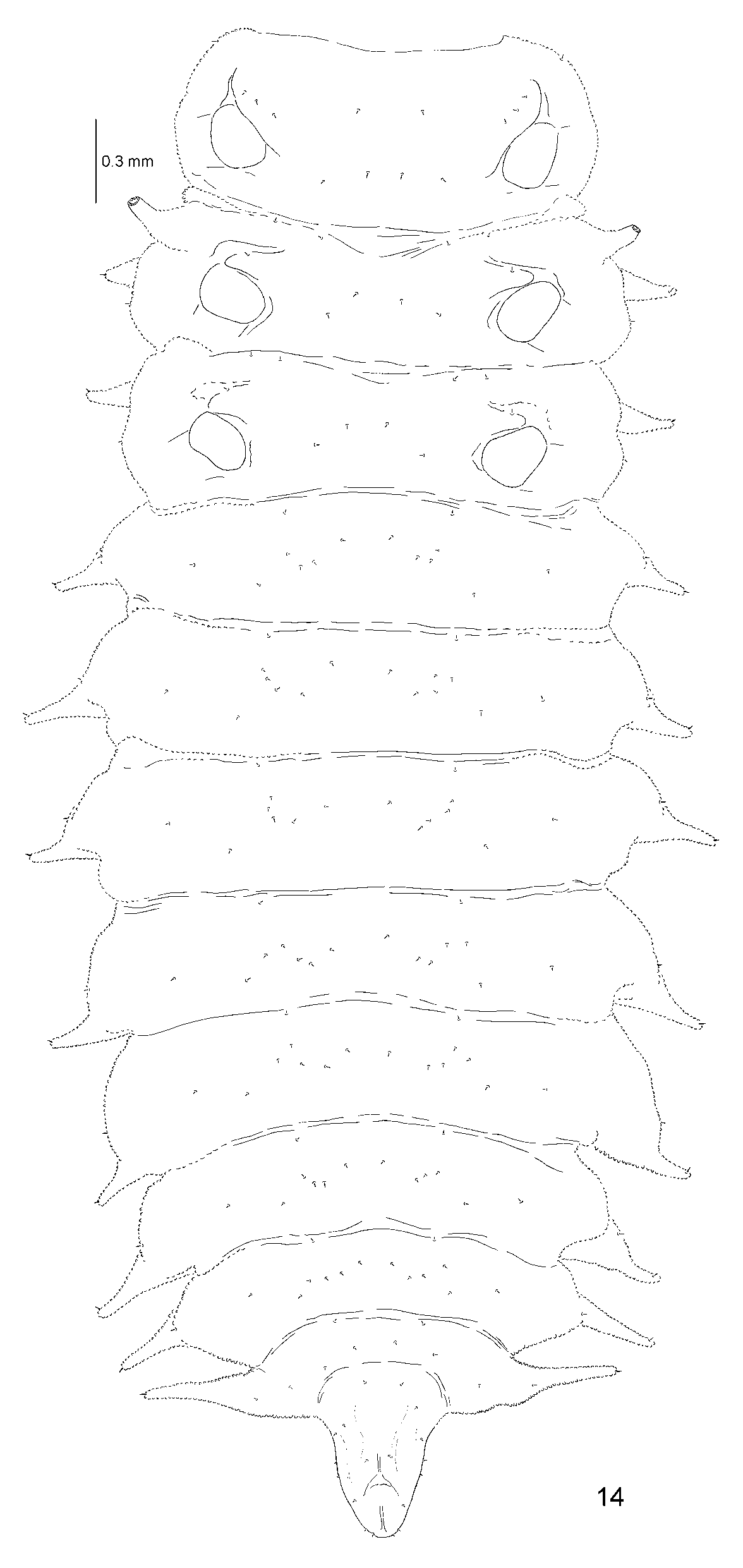
Pupa ( Figs 7, 8 View FIGURES 1 – 8 , 11, 12, 46–55). Length: 4.24–5.13 mm (n=10), width (across abdominal segment IV): 1.57–1.78 mm (n=10).
Body flattened dorso-ventrally, elongated, almost parallel-sided, widest across abdominal segment IV (Figs 11, 12, 46, 48). Colour of alcohol preserved pupa light yellow with brown spiracles of 5th abdominal pair (Figs 11, 12).
Head visible in dorsal view, dorsally with two small processes, each apically armed with seta ( Figs 50, 51 View FIGURES 50 – 55 ).
Prothorax with two small processes on each lateral margin. Each process apically armed with seta. Meso- and metathorax without lateral scoli.
Abdominal segments I–V without lateral scoli. Segments VI–VIII with one simple and one two-branched scolus on each side. Segment VIII additionally with two-branched scolus at posterior border ( Figs 47, 49 View FIGURES 46 – 49 , 53 View FIGURES 50 – 55 ). Each branch with two processes, each process apically armed with one seta.
Each abdominal segment with a pair of spiracles. Spiracles of first four segments of similar diameter. Spiracles of segments VI–VIII with slightly smaller diameter than those on segments I–IV. Spiracles of the segment V most prominent, elongated into cylindrical appendage (respiratory horns) with elongate-oval outer spiracular opening ( Figs 8 View FIGURES 1 – 8 , 11, 12, 46, 49, 54, 55).
Pronotum with few simple setae, meso-, metanotum and abdominal tergites with pair of minute setae at anterior border, two pairs of setae anteriorly and two pairs of setae posteriorly. Close to each abdominal spiracle there are two setae. Abdominal sternites IV–VII with row of 10 setae at posterior border and one to three setae on each antero-lateral side. Setae in row at posterior border placed on distinct tubercles ( Fig. 52 View FIGURES 50 – 55 ). Last segment with row of four setae anteriorly, and 8 on scolus at posterior border.
Comparative diagnosis. Within Chaeridiona only immatures of Ch. picea were described till now ( Świętojańska et al. 2006). We found several diagnostic characters distinguishing the immatures of Ch. thailandica from Ch. picea .
Ch. thailandica larva is slightly longer than Ch. picea (length 5.0 mm, n=1; to length 4.2, 4.4 mm, n=2 respectively). Body of mature larva of both species is elongate and strongly flattened dorso-ventrally but in Ch. picea it is slightly widened in abdominal part (the widest across abdominal segment IV) while in Ch. thailandica almost parallel-sided and narrowed posteriorly from abdominal segment VI.
Spiracles of 8th pair in both species form triangular tip of body but the tip of Ch. thailandica is distinctly broader than that of Ch. picea .
Segments of thorax of Ch. picea lack lateral processes while in Ch. thailandica meso- and metanotum possesses one pair of lateral scoli. Abdominal lateral scoli of Ch. picea are broader than those of Ch. thailandica .
Chaetotaxy of head and ventral side of body of both species is identical. Chaetotaxy of dorsal side of body is also very similar, however, Ch. thailandica has 15 setae on the pronotum, while Ch. picea has 17 setae. Differences are also visible in the number of setae on the tip of body (8th pair of spiracles): in Ch. picea there are four setae dorsally and two ventrally, in Ch. thailandica 16 setae dorsally, 10 ventrally, and 8 on sides.
The differences in mouth parts are visible in mandibles and the maxillo-labial complex. Mandibles of both species possess three teeth but Ch. thailandica has a distinctly sclerotised margin above teeth which is absent in Ch. picea . The mala of Ch. thailandica has more numerous long spinules on dorsal side than the mala of Ch. picea . The prementum of Ch. picea has no tubercles while Ch. thailandica has two distinct tubercles, each with a peg-like sensillum at the apex. The tubercles are placed in area where labial palpi occur in other species. This character is very interesting, because adults of species belonging to the genus Chaeridiona are distinguished from the genera Prionispa and Oncocephala by the absence of labial palpi.
Chaetotaxy of the legs of both species is the same except one additional seta on coxa in Ch. thailandica . Pupae of Ch. picea (n=2; length: 3.6, 4.3 mm; width: 2.0, 2.1 mm) are shorter but wider than those of Ch. thailandic a (n=10; length: 4.24–5.13 mm; width: 1.57–1.78 mm). Pupa of Ch. picea is elongate oval, widest across abdominal segment V, while in Ch. thailandica pupa is elongate, almost parallel-sided, widest across abdominal segment IV. In both species head is visible from dorsal side and has two processes, however, the processes of Ch. picea are much longer. Abdominal segments I–V of Ch. picea possess simple lateral scoli on each side, while in Ch. thailandica the scoli are lacking. At the posterior border of abdominal segment VIII of Ch. picea there is a 3-branched scolus, while in Ch. thailandica there are two 2-branched scoli.
Habitat and biological notes. The larvae of Ch. thailandica were found mining in the leaves of four species of Zingiberaceae growing on the forest floor. One species was Boesenbergia rotunda , the remaining three belonged to the genus Zingiber . There were no feeding marks on the leaves of other ginger species occurring in the same habitat, namely Globba bulbifera , G. pendula , Curcuma bicolor , C. roscoeana and C. rubrobracteata . Leaves of Hedychium coronarium , a ginger species cultivated in gardens in the study area also showed no traces of leaf-mining beetle larvae.
In ginger species forming tall stems the larvae fed in the lower, smaller leaves ( Figs 1, 3 View FIGURES 1 – 8 ). In ginger species with narrow leaves there was usually one larva per leaf, sometimes up to three. In the large, oval leaves of Boesenbergia rotunda one to five larvae were found in a single leaf. Each larva of Ch. thailandica mined in its own feeding area. The feeding areas were usually oblong, close to the mid-rib and located in the basal third of a leaf ( Fig. 3 View FIGURES 1 – 8 ).
The larvae were feeding with the back oriented towards the upper side of the leaf. They sometimes pushed their triangular apical plates upwards, piercing the plant tissue. Subsequently, they deposited their faeces on the upper surface of the leaf ( Fig. 4 View FIGURES 1 – 8 ) through the anal opening situated on the ventral side of the apical plate.
A similar behaviour was observed when water was poured down on the leaf. In this case, the apical plate was raised upwards apparently in order to take up fresh air through the two enlarged spiracles which form the apical plate ( Fig. 5 View FIGURES 1 – 8 ). When water was poured on pupae, they remained submerged (duration of experiment 2 hrs).
In pupae submerged in water the outer spiracular openings of the abdominal segment V were covered with an air sheath.
Mature larvae broke upwards through the leaf tissue and walked on the upper side of the leaf down to the mid-rib. Subsequently, they bore into the mid-rib with head oriented towards the stem of the plant ( Fig. 6 View FIGURES 1 – 8 ). In the mid-rib the larvae pupated after a few days. The pupae were usually hiding in their pupal chambers, but sometimes the apical part of the abdomen including the two spiracular horns could be seen at the entrance of the pupal chamber ( Figs 7, 8 View FIGURES 1 – 8 ).
Remarks. Most Oriental hispines feed on Monocots, especially on palms, grasses, bananas and gingers ( Kalshoven 1957). Feeding of Ch. thailandica on Zingiber and Boesenbergia (Zingiberaceae) is in line with previous observations on Oncocephalini. Until now Zingiberaceae [ Chaeridiona metallica Baly ; Oncocephala angulata Gestro ], Commelinaceae [ Prionispa fulvicollis Guérin ; Chaeridiona picea Baly ], Dioscoreaceae [ Oncocephala angulata ] and Convolvulaceae [ Oncocephala tuberculata Olivier ; O. quadrilobata (Guérin) ] ( Kalshoven 1957, Świętojańska et al. 2006) were recorded as a food plants of beetles belonging to three Oncocephalini genera. The observation of Oncocephala angulata on Orchidaceae needs to be confirmed ( Kalshoven 1957).
Immatures of Ch. thailandica possess some characters which seem to be adaptations for living inside leaves. The larvae of Ch. thailandica sometimes push their apical plates upwards, thus piercing the leaf tissue in order to deposit their faeces on the upper surface of the leaf. This behaviour has never been reported for any other hispine beetles.
Another conspicuous behaviour is the pupation in the mid-rib of a ginger leaf. A similar behaviour was mentioned by Kalshoven (1957) in Prionispa fulvicollis . The adaptive advantage of boring into the stem is possibly protection against predators and parasitoids. The pupal chamber is safer than the perforated leaf with the conspicuous feeding marks, particularly because the pupa can close the entrance of the pupal chamber with the broadened and flat caudal end of the abdomen.
A striking morphological feature of Ch. thailandica pupae are elongated spiracles of the 5th abdominal segment (respiratory horns). When a pupa is submerged in water a shiny, longitudinal band can be seen on the dorsal side of each respiratory horn. The shiny band consists of a thin film of air, which is held by the hydrofuge tubercles lining the spiracular chamber. The silvery sheen was still present after 2 hours of submersion. The size and structure of the hydrofuge layer and the persistence of the shiny band indicate that the gas film serves as a permanent physical gill (=plastron) that allows diffusion of O 2 from water into the spiracles.
A number of aquatic insects have a permanent physical gill or plastron, e.g., the water bug Aphelocheirus Westwood or the beetle Elmis Latreille ( Hinton 1976) . The plastron is usually held by minute hydrofuge hairs covering large areas of the body surface. Most plastron breathers are limited to fast-flowing, oxygen-rich streams. Plastron breathers living in standing water, like the chrysomelid Donacia Fabricius or various aquatic weevils, are slow-moving insects that can crawl to the water surface if oxygen levels become too low. In terrestrial environment plastrons mainly occur in the pupal or egg stages of insects living in habitats that are alternately dry and flooded., e. g. along streams ( Hinton 1961; 1968). The cuticular structures involved often consist of a dense cuticular meshwork covering the spiracular atrium and a small surrounding area (=spiracular gill) ( Hinton 1968). In many species the spiracular gills are located on appendages (spiracular horns) that are elevated above the body surface.
After heavy rain the larvae and pupae of Ch. thailandica are sometimes temporarily submerged in water. Thanks to their spiracular gills, the pupae can remain submerged for long periods without the need to access the water surface. If necessary, i.e. after prolonged submersion, the pupa can move upwards and place the respiratory horns above or closer to the oxygen-rich water surface. In contrast to pupae the larvae apparently need more oxygen when submerged. Soon after watering them they raise their apical plates formed by last pair of spiracles towards the water surface. Insects possessing spiracular gills lack both a regulatory apparatus of the spiracles and the appropriate muscles ( Hinton 1968). Nevertheless, the water loss is kept at low levels when exposed above water, because the plastron structures covering the spiracles are very dense, sometimes forming a canopy with tiny holes ( Hinton 1968). In Ch. thailandica the cuticular plastron structures are not as dense (the distance between the mushroom-shaped structures is larger), but the water loss can be prevented by closing the respiratory horn when exposed to the atmosphere ( Figs 54, 55 View FIGURES 50 – 55 ).
Spiracular horns have also been found on the fifth abdominal segments of pupae of other leaf-mining Cassidinae belonging to different tribes ( Świętojańska et al. 2006). They probably have the same function as the respiratory horns of Ch. thailandica .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Cassidinae |
|
Tribe |
Oncocephalini |
|
Genus |