Engelimyia Lopes, 1975
|
publication ID |
https://doi.org/10.5281/zenodo.173088 |
|
publication LSID |
lsid:zoobank.org:pub:315FC04E-2AE0-4CBE-B7EE-F7587668BC73 |
|
DOI |
https://doi.org/10.5281/zenodo.6260747 |
|
persistent identifier |
https://treatment.plazi.org/id/03DA87A8-FFB3-FF8C-8538-FBB89CAD225B |
|
treatment provided by |
Plazi |
|
scientific name |
Engelimyia Lopes, 1975 |
| status |
|
Genus Engelimyia Lopes, 1975 View in CoL View at ENA
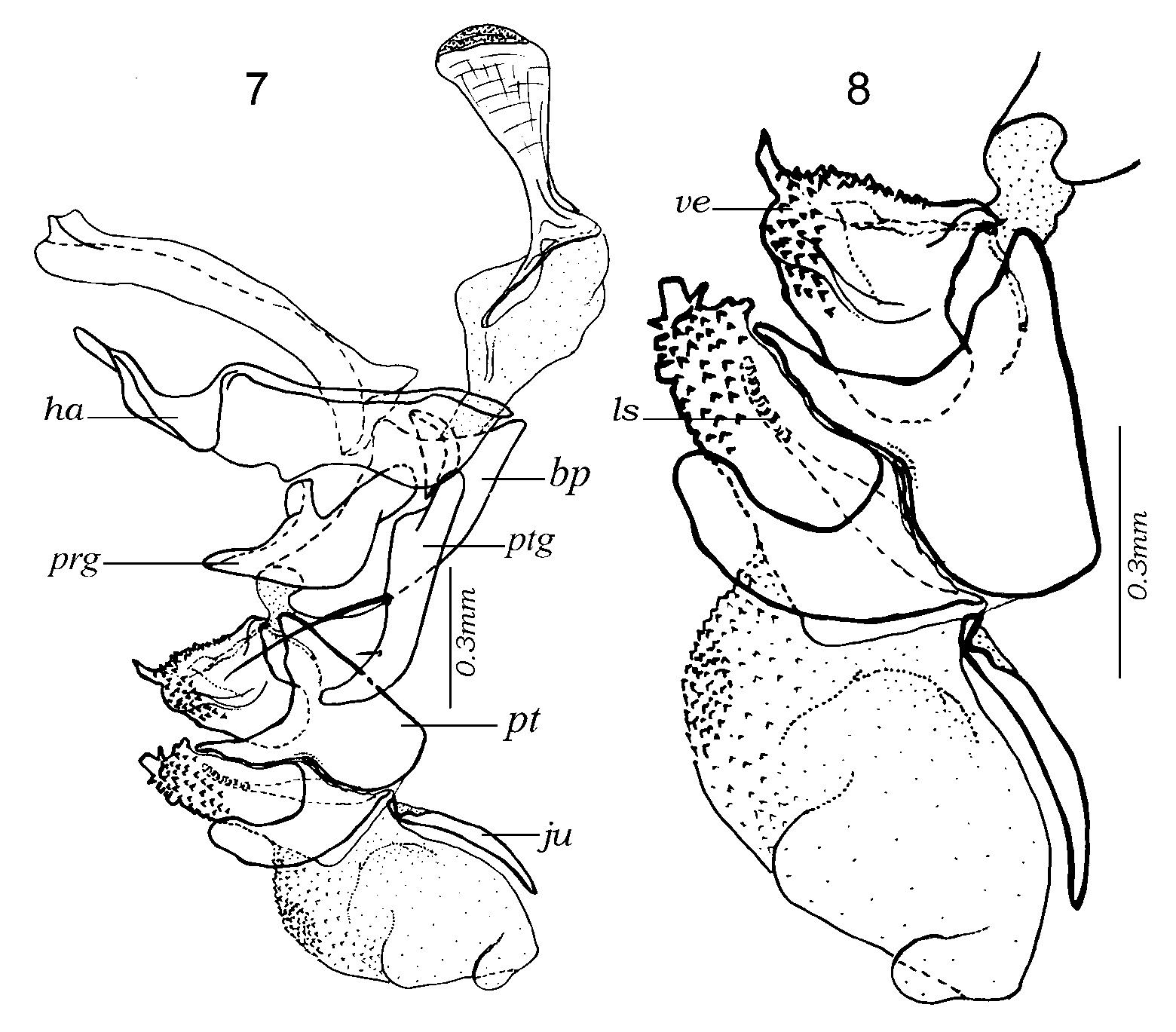
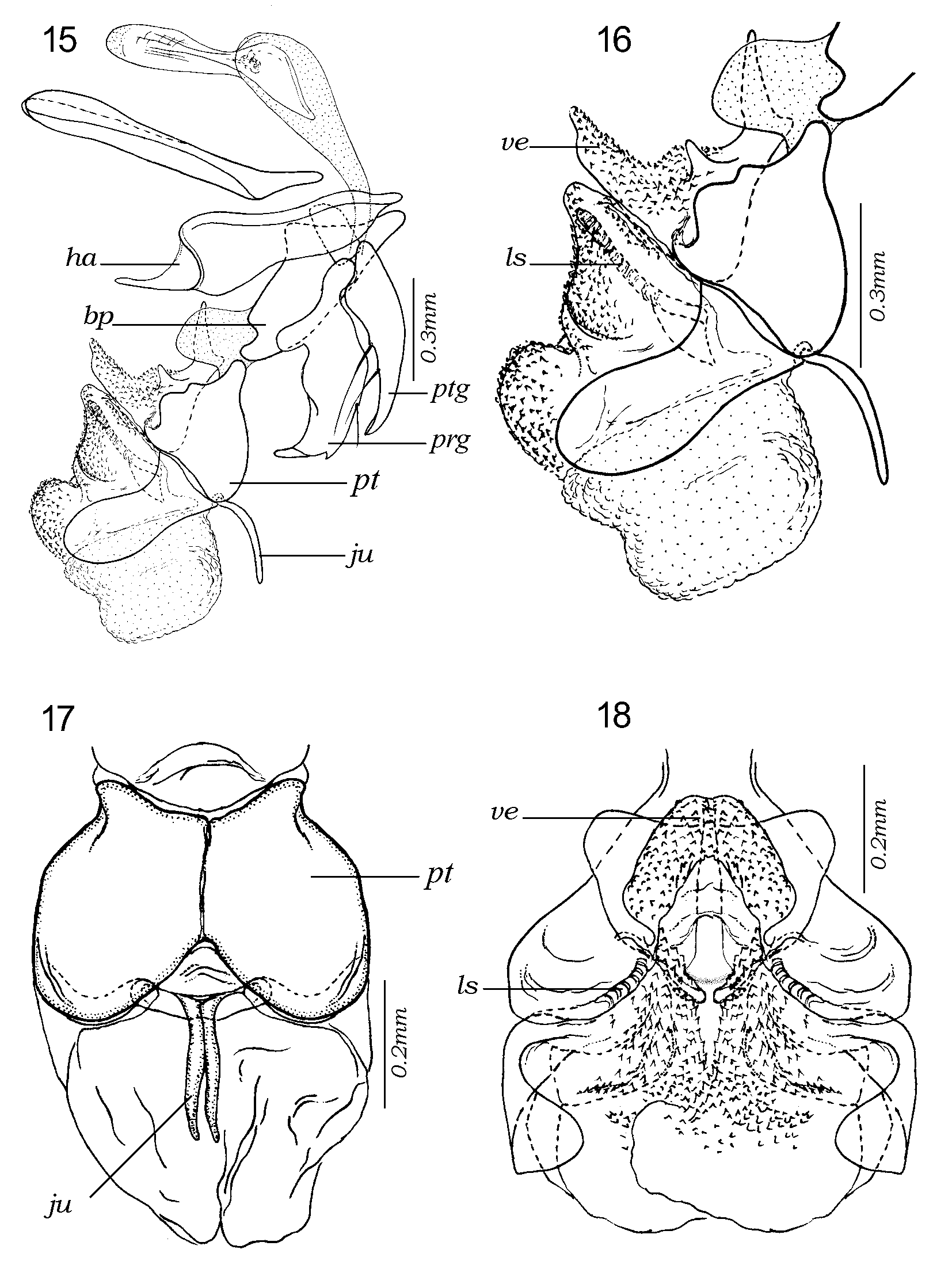
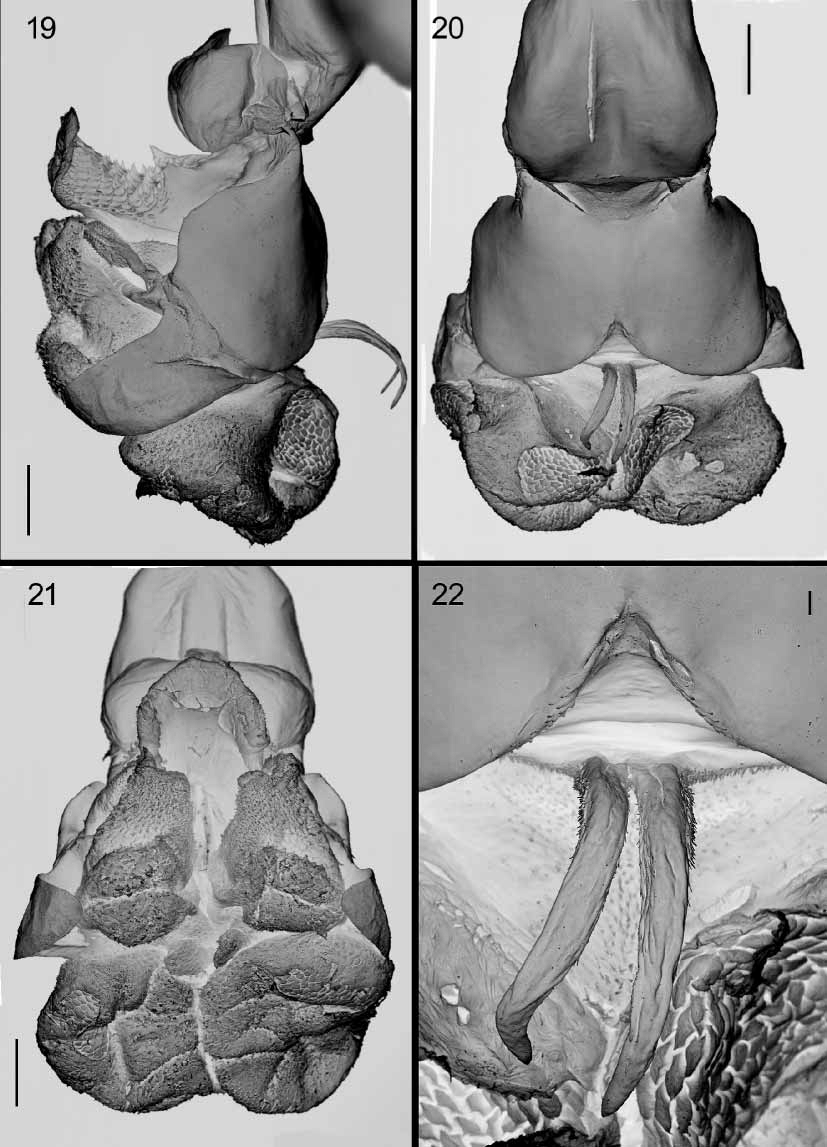
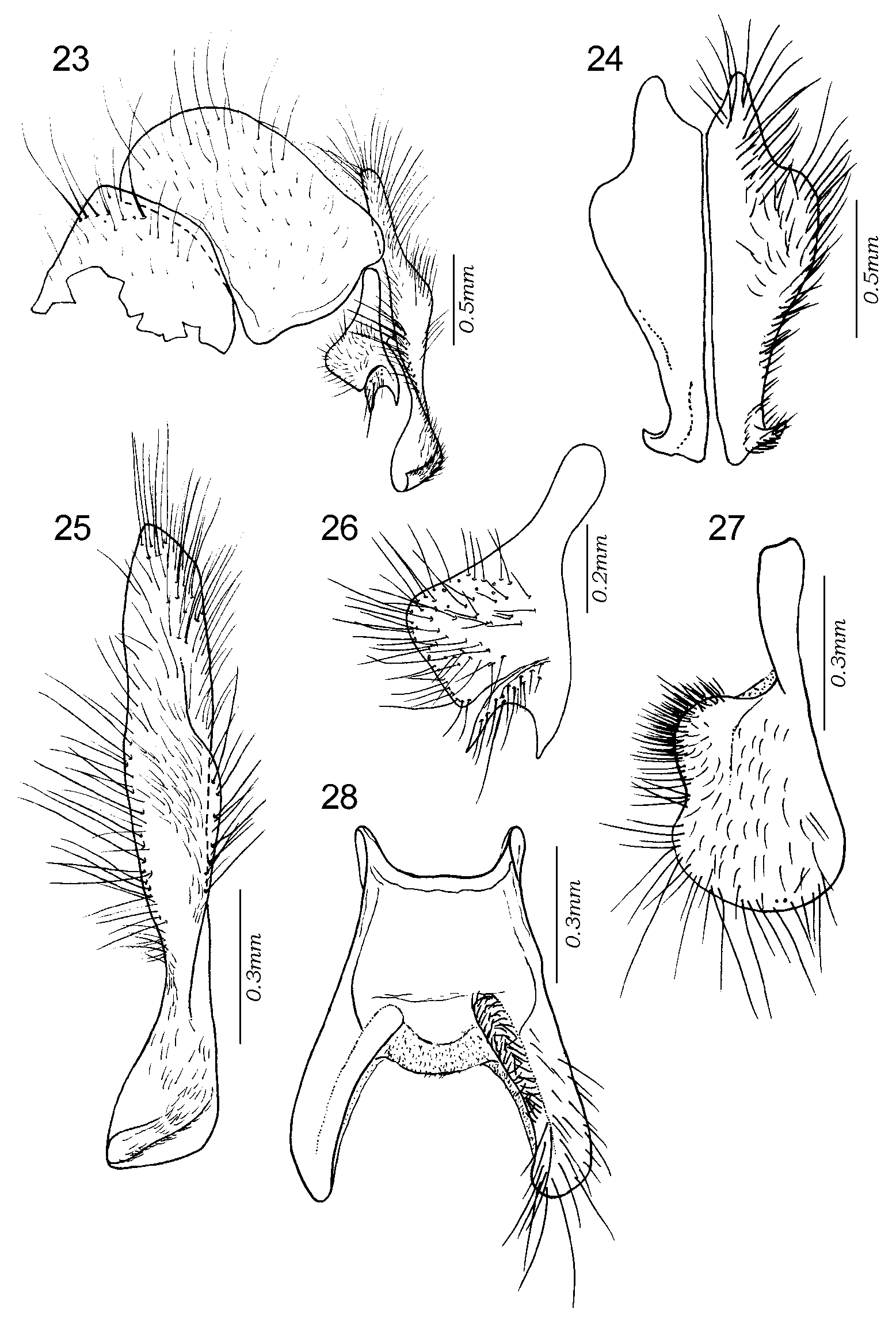
Figs 1–42 View FIGURES 1 – 6 View FIGURES 7 – 8 View FIGURES 9 – 14 View FIGURES 15 – 18 View FIGURES 19 – 22 View FIGURES 23 – 28 View FIGURES 29 – 30 View FIGURES 31 – 36 View FIGURES 37 – 38 View FIGURES 39 – 42
Engelimyia Lopes, 1973: 287 View in CoL . Nomen nudum.
Engelimyia Lopes, 1975: 425 View in CoL . Type species: Sarcophaga ( Paraphrissopoda) cassidifera Engel, 1931 View in CoL [= Sarcophaga inops Walker, 1849 View in CoL ], by original designation.
Generic diagnosis
Robust species of subfamily Sarcophaginae . Head with whitish setae on postgena, black setae on gena (i.e., no white setae anterior to genal sulcus). Frontal vitta posteriorly with scattered setulae between the two rows of frontals. A single presutural dorsocentral bristle present just anterior to the suture and as strong as or weaker than a subprimary notopleural bristle; two postsutural dorsocentrals in posterior part, and occasionally with two setae in front of these being slightly stronger than the adjacent clothing setae. Postalar wall distinctly setose. Fringe of long marginal trichiae of lower calypter extending half way to the outer hind corner. Thorax with the usual three strong black stripes, microtrichosity otherwise with a faint yellowish hue. Abdomen with no trace of yellowish microtrichosity and not particularly checkerboard patterned; pattern rather forming three very illdefined black stripes. When observed with the naked eye and without strong, incident light, the microtrichosity is a matt silver colour and the tessellation pattern changes less in response to a changing incidence of light as compared to most species of Sarcophaga (s.l.). With strong incident light (like fiber optics) the microtrichosity is silvery and strongly reflecting. Abdominal sternites 2–4 with dense, erect, black setae at least near apical margin. Sternite 5 cleft, with posterolateral lobes widely apart; setae of sternal lobes long and particularly dense on the median parts.
Male terminalia shining reddish or orangish, rarely with base of syntergosternite 7+8 brown. Tergite 6 present as a narrow dorsal strip with long marginal, bristly setae ( Fig. 1 View FIGURES 1 – 6 ). Syntergosternite 7+8 and epandrium uniformly covered with black hairlike setae, without bristles. Cercus apically swollen and more or less truncated or abruptly cut off, with apicolateral keels. Middle part of cercus with laterally or dorsolaterally directed bristles or long setae forming a more or less welldefined fringe. Surstylus with a broad base from which the distal part projects as a fingerlike prong or process (which may be secondarily modified). Surstylar base with black setae, surstylar tip with pale setae. Phallus with distinct basi and distiphallus linked by a narrow membrane; distiphallus ( Figs 39–42 View FIGURES 39 – 42 ) with a short and compact phallic tube (i.e., the tubeshaped part of the distiphallus proximal to juxta). Phallic vesica rather small and situated proximally on the distiphallus, shape triangular to almost quadratic in lateral view and beset with small spines. Tubular part of lateral styli set widely apart, each tube slender and slanting anteroproximally (often concealed by membrane). Basal part of lateral stylus forming an expanded, laterally recurving, flat structure (small in E. qibos but a distinct, armlike projection in remaining species, Figs 39–42 View FIGURES 39 – 42 ). Distiphallus with a large, membranous structure anteriorly and another such structure apically on the distiphallus. The anterior membranous structure forms a large cup or flaskshaped opening with the vesica ( Fig. 18 View FIGURES 15 – 18 ), that is directed anteriorly or even anterobasally (with phallus observed in vertical position). Juxta small and deeply bifid, shaped like a pair of short or long, fingerlike appendages and inserted subapically on the posterior (= dorsal) part of the phallus and therefore posterobasally to the large, apical, membranous structure.
Female terminalia show entire tergites 5 and 6, tergite 7 (or 8 in the terminology used by Shewell [1987]) divided into 2 plates without setae, spiracle 6 in membrane and 7 within the sclerite; epiproct as a single setose plate, hypoproct welldeveloped but not particularly sclerotized; sternites 6, 7 and 8 fused, sternite 8 represented by a membranous fold.
Monophyly
The shape of the male cercus, with the apex bluntly swollen or truncated and the cercal prong equipped with lateral keels, is unique in the Sarcophagidae and here considered autapomorphic. The highly modified phallus is considered strongly derived, but explicit autapomorphies are difficult to enumerate due to the uncertain homologies of some of the phallic components. The short and broad phallic tube, the large and membranous apical and ventral structure, the cupshaped structure on the ventromedian surface, the slender and deeply bifid juxta, the broadly separated and anterobasally slanting lateral styli, the armlike lateral projections from the base of the lateral stylus, and the small and microspinose vesica are particularly good candidates for generic autapomorphies. A proper evaluation of homologies would require a much more inclusive phylogenetic analysis that would fall beyond the aim of the present paper.
Recognition
There is no published generic key that serves to separate Engelimyia spp. from their congeners, and the most efficient method for sorting out specimens of Engelimyia from unidentified Sarcophaginae is to compare the male terminalia with the illustrations and SEMimages provided herein. The possible generic autapomorphies mentioned above, and particularly the male cercus with a truncated apex, the short and broad phallic tube with ample membranous material anteriorly and apically, and the slender and bifid juxta set distinctly subapically on the distiphallus should ensure an efficient separation.
Biology
Very little biological information is available. Lopes (1973) collected E. cassidifera “using human feces and rotten Coccus comosa as bait” as well as from “Lepidoptera traps with rotten bananas and brown sugar” (p. 287). Lopes (1973) also mentions that he reared E. cassidifera , but without indicating the medium. Engelimyia qibos has been collected from fish bait in Costa Rica (G.A. Dahlem, pers. comm.).
Distribution
The genus Engelimyia is currently endemic to the Neotropical Region, ranging from Buenos Aires ( Argentina) in the south to Veracruz ( Mexico) in the north [the latter record is based on a single female from Mexico, Veracruz, Sontecomapan, 20.vii.1969, W.R.M. Mason (CNC); not identified to species].
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Engelimyia Lopes, 1975
| Pape, Thomas & Mello-Patiu, Cátia Antunes De 2006 |
Engelimyia
| Lopes 1975: 425 |
Engelimyia
| Lopes 1973: 287 |