Enyaliopsis iaculator, Naskrecki & Guta, 2019
|
publication ID |
https://doi.org/10.11646/zootaxa.4682.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:430B98EF-BFCB-4608-A562-DEFA9539C8B2 |
|
DOI |
https://doi.org/10.5281/zenodo.5629495 |
|
persistent identifier |
https://treatment.plazi.org/id/03D8878E-FC59-D916-CCFE-5076FE6431D3 |
|
treatment provided by |
Plazi |
|
scientific name |
Enyaliopsis iaculator |
| status |
sp. nov. |
Enyaliopsis iaculator View in CoL sp. n.
http://lsid.speciesfile.org/urn:lsid: Orthoptera .speciesfile.org:TaxonName:506811
( Figs. 3A View FIGURE 3 , 4E View FIGURE 4 , 5B View FIGURE 5 , 18 View FIGURE 18 A–F, 19A, D–G, K–P, 49I–L)
urn:lsid:zoobank.org:act:
Type locality. Mozambique: Sofala, GNP, Chitengo Camp ( -18.98194, 34.35122), 29 m, 11–25.ii.2014, coll. R. Guta & T. Castigo—male holotype ( EOWL) GoogleMaps
Differential diagnosis. Unmistakable among Gorongosa katydids, this large, flightless insect can be identified by the combination of its heavily armed, spiny pronotum and head, the lack of visible wings (males’ short yet overlapping tegmina are completely hidden under the pronotum, while those of females are reduced to minute, non-overlapping cuticular folds), and an extremely short ovipositor in the female. E. iaculator belongs to the petersi Group of species, as defined by Glenn (1991). From E. petersi ( Schaum, 1853) , a species described from an unknown location in Mozambique, it differs in the armature of the pronotum, with a significantly shorter, less extended anterior “rack of spines s1 and s2 on the anterior edge of the prozona; shorter spines s8 on the posterior corners of the metazoan; and the shape of the titillator (simple, undivided tip in E. petersi , divided in E. iaculator ) ( Figs. 18B, H View FIGURE 18 ). From E. nyala Glenn, 1991 from Malawi it differs in having shorter forward-facing spines s1 of the frontal “rack, spines s6 distinctly longer than s7, and shorter spines s8 on the posterior corners of the metazoan; the structure of the titillator (strongly divided and dilated apically in E. nyala ) ( Figs. 19C, I View FIGURE 19 ); and the pattern of male song (syllable length in E. nyala < 0.25 s, with 7–8 impulses; syllable length in E. iaculator ~ 0.4 s, with 11 impulses) ( Figs. 49 View FIGURE 49 I–L). From E. mulanje Glenn, 1991 , a species endemic to the Mulanje Massif in Malawi, it differs in the same aspects of the pronotal armature as mentioned above, the shape of the titillator ( Fig. 19J View FIGURE 19 ), and the pattern of the song (syllable length in E. mulanje ~ 0.75 s, with 13 impulses).
General. Body large, robust; male brachypterous, female squamipterous ( Figs. 18 View FIGURE 18 A–C).
Head. Antennae shorter than body; antennal scapus unarmed; eyes globular, strongly protruding; fastigium of vertex long, spur-shaped, 3–3.2 mm long; frons flat, vertical; ocelli absent.
Thorax. Pronotum surface rugose; pronotum armed with strong, sharp spines; metazona weakly convex. Anterior “rack with forward-facing spines s1 as long as or shorter than sideways-facing ones s2; spine s4 positioned distinctly in front of s5 and spine s6 almost twice as long as s7 ( Figs. 19D, E View FIGURE 19 ).
Legs. Legs short, robust; hind legs non-saltatorial; front tibia with 5 anterior and 5 posterior spines; mid tibia with 5 anterior and 4 posterior spines; hind tibia with 5 anterior and 19–21 posterior spines.
Wings. Tegmen reduced, completely hidden under pronotum, approximately rounded and about as long as wide, tegminal venation strongly reduced ( Figs. 19F, G View FIGURE 19 ); stridulatory file weakly sinuous, 2.9–3.1 mm long, 0.14–0.2 mm wide, with 53–60 teeth ( Fig. 19A View FIGURE 19 ).
Abdomen. Cercus strongly reduced, in a form of short, thick peg. Subgenital plate broadly rounded; styli absent ( Fig. 18E View FIGURE 18 ); titillator anchor-shaped, with long, straight middle arm, its apex thickened, weakly bilobed ( Figs. 19 View FIGURE 19 K–P). Ovipositor very short, dorsal and ventral valvulae divergent at apex, narrowly pointed ( Fig. 18F View FIGURE 18 ).
Coloration. Mottled brown, occasionally with green elements on pronotum, abdomen, and hind legs. Metazona of pronotum with narrowly triangular dark brown patches, lateral lobes of pronotum nearly black; legs often lighter brown or green, outer surfaces of hind femur often marked with darker spots ( Figs. 18 View FIGURE 18 A–C).
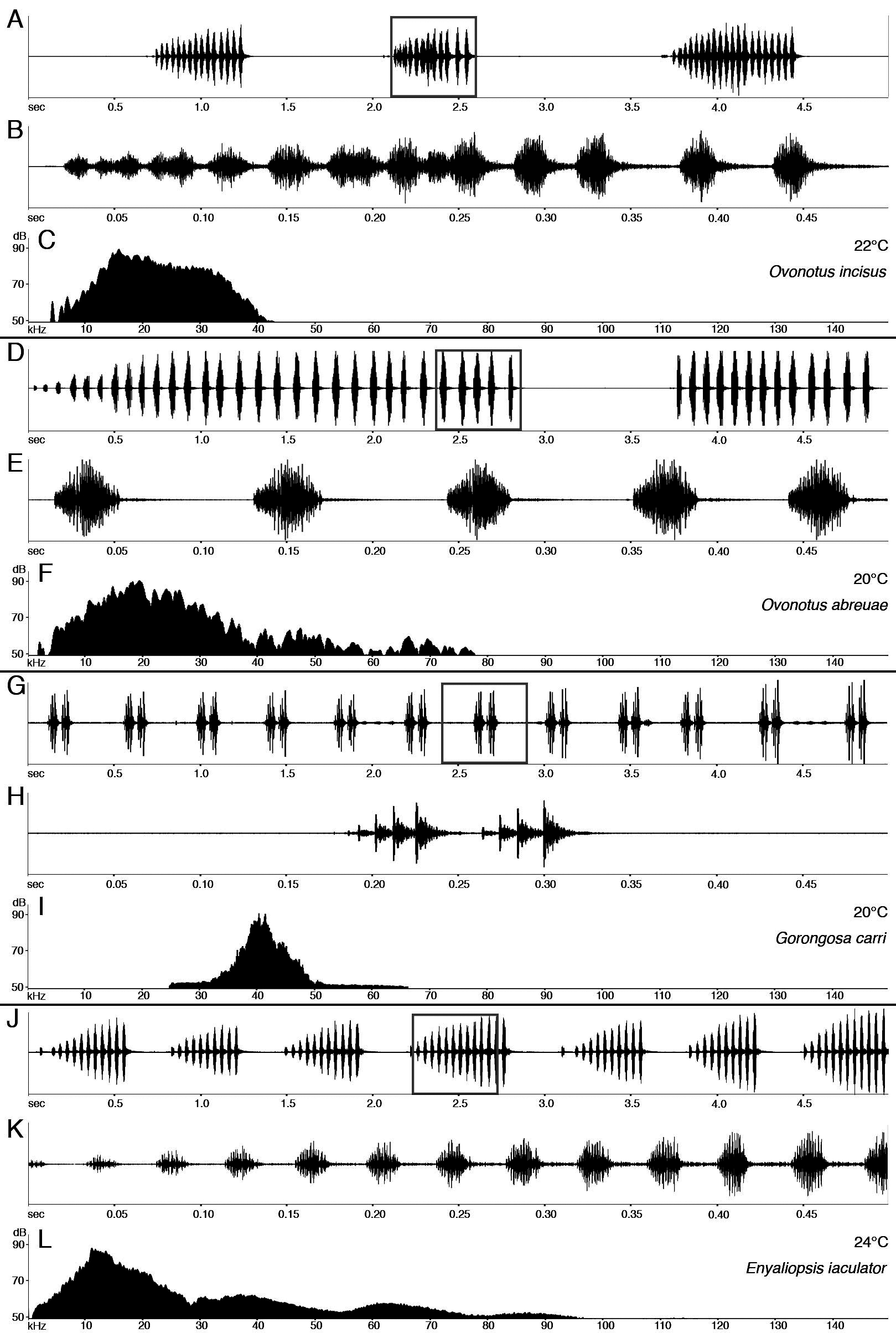
Bioacoustics. The call of E. iaculator is a continuous train of short but distinctly separate syllables, with syllables produced at the rate of only 2/sec (at 26°C); mean syllable duration is 0.04264 (SD=0.13340, n=790), with the frequency peak at 11.7–14.6 kHz ( Figs. 49 View FIGURE 49 I–L); each syllable consists on average of 11 impulses (SD=2.101, n=104). The call is loud and readily audible to the human ear from several meters.
Males usually start calling shortly after sunset from dense bushes, often very low to the ground (from a few centimeters to about 3 meters above the ground.) While calling, the male invariably hangs upside down on a thin branch and is relatively oblivious to disturbance. Calling males appear to be territorial and are spaced regularly by at least 10 m; the same tree or bush never has more than one calling male.
The population in the northeastern part of the Cheringoma Plateau is unusual in that the males often sing throughout the day, including noontime. This population, however, shows no statistically significant differences from the populations elsewhere in the Gorongosa region in either the parameters of the call or their morphological characters.
Distribution and natural history. This new species is currently known only from the Gorongosa region albeit it is likely to occur throughout suitable lowland miombo habitats in the coastal region of central Mozambique between rivers Save and Zambezi. The record of 2 female specimens of E. nyala from Beira, listed as being in the collection of the Iziko Museum in Cape Town by Glenn (1991) (but currently missing from the museum’s holdings), is highly doubtful and likely represents the new species.
E. iaculator is found primarily in the understory of Brachystegia miombo woodland, at elevations between 20 and 250 m. These katydids prefer shaded, dense bushes, where adults hide during the day by clinging to branches. In contrast, nymphs often spend the day under rocks and logs on the ground or in crevices of tree bark. They are primarily predaceous, capable of catching and devouring even relatively very fast prey, such as grasshoppers Zonocerus elegans Thunberg, 1815 . They also eat plant material, such as flowers and fruits.
In addition to the defense provided by the hard and spiny pronotum these katydids respond to threats by squirting a powerful jet of hemolymph from a small opening on the dorsal surface of the connective membrane between the coxa and trochanter of the mesothoracic leg ( Fig. 17D View FIGURE 17 ). The jet is powerful enough to reach the eyes of a person (and presumably other attackers) and it has a strong smell, similar to that produced by some leaf beetles ( Chrysomelidae ). The actual composition of the liquid and its chemical properties are still unknown, but it presumably contains compounds that predators find distasteful or toxic. In the experience of the authors the katydid hemolymph does not seem to have a discernible effect on the human skin albeit there are anecdotal cases of people having an allergic reaction following a contact with Enyaliopsis katydids. Strangely, despite having powerful mandibles, these katydids never attempt to bite the attacker.
Both nymphs and adults occur throughout the year, including the dry season, and males call virtually every day of the year. In captivity adults lived for more than two years. Mating takes place on branches and lasts for approximately an hour, during which time the male produces a large spermatophylax, approximately the size of 1/3 of his abdomen ( Fig. 3B View FIGURE 3 ); the female subsequently consumes the spermatophylax. Since the male cerci are non-functional, the male secures his position on the underside of the female by holding her with all his legs ( Fig. 3B View FIGURE 3 ). The male refraction period is unknown but males mate multiple times, most likely throughout the year. Females lay eggs in the soil ( Fig. 4E View FIGURE 4 ) by digging a hole with their short ovipositor and subsequently carefully cover the hole with soil. Eggs laid in captivity underwent a 2-year diapause before hatching. Nymphs of all stages resemble the adults in their general appearance and, unlike other Hetrodinae whose nymphs are often green, have brown, mottled coloration ( Fig. 5B View FIGURE 5 ).
Etymology. The specific epithet of this species, iaculator (“shooter in Latin), refers to the defensive behavior of this species, during which the insects spray the attacker with a jet of their own hemolymph.
Measurements ( 7 males, 6 females). body: male 40.6–51 (45.53.3), female 44–59.5 (52.35.2); pronotum: male 18.7–22 (20.11.4), female 18.2–20 (19.1.6); tegmen: male 6.9–7.8 (7.4.4); hind femur: male 16.8–20 (18.41.3), female 18.1–22.2 (19.81.4); ovipositor: 6–8.4 (7.2.8) mm.
Material examined ( 67 specimens). Mozambique: Sofala, Coutada 12, Inhamitanga Forest, Camp 1, elev. 210 m ( -18.23835, 35.33015), 9–14.iv.2018, coll. P. Naskrecki— 2 males (incl. 1 paratype) GoogleMaps ; same locality, 13.iv.2018, coll. P. Naskrecki— 1 female ( paratype) ( EOWL) GoogleMaps ; Coutada 12, Nyago hunting camp, elev. 70 m ( -18.660767, 35.455603), 8–16.iv.2016, coll. P. Naskrecki & J. Guyton— 1 male GoogleMaps ; Cheringoma, Coutada 12, Chironde camp, elev. 156 m ( -18.32780, 35.35799), 25.iii.–4.iv.2017, coll. P. Naskrecki, J. Guyton & M. Castene— 1 male GoogleMaps ; nr. Codzo ( Khodzue ), cave and nearby, elev. 216 m ( -18.564, 34.872222), 8–9.vi.2012, coll. P. Naskrecki— 1 male ( paratype) ( MCZ) GoogleMaps ; same locality, 14–25.iv.2017, coll. P. Naskrecki— 1 male, 1 nymph ( paratype) GoogleMaps ; Gorongosa , GNP, Chitengo , elev. 38 m ( -18.981067, 34.351983), 11–31.x.2014, coll. R. Guta—1 nymph male GoogleMaps ; Coutada 12, Camp 2, 7km north of Pawue Village , ( -18.43151, 035.35499), 13.iv.2018, coll. P. Naskrecki— 1 male ( paratype) GoogleMaps ; GNP, Bela Vista rang- er outpost, elev. 26 m ( -18.69470, 34.20853), 5–12.v.2015, coll. P. Naskrecki— 1 male ( paratype) GoogleMaps ; GNP, Chitengo , E.O. Wilson Laboratory, elev. 48 m ( -18.977722, 34.351333), 31.xii.2018, coll. P. Naskrecki— 2 males (incl. 2 paratypes) GoogleMaps ; GNP, Chitengo, nr. Pungue River , elev. 39 m ( -18.993972, 34.349694), 22.v.2016, coll. M. Castene—1 nymph GoogleMaps ; GNP, dense miombo between Bunga outpost and Chiuata , elev. 126 m ( -18.57054, 34.34510), 29.iv.2015, coll. P. Naskrecki— 1 female ( paratype) ( EOWL) GoogleMaps ; turn-off on path from Chitengo to Pungwe river , bush dinner spot, ( -18.985517, 34.353064), 25.vii.2015, coll. B. Woo— 1 male GoogleMaps ; Gorongosa Dist., Archway Gorge , campsite, elev. 63 m ( -18.95336, 34.61089), 22–29.iv.2013, coll. P. Naskrecki— 1 male, 1 nymph ( paratype) ( MCZ) GoogleMaps ; GNP, Chitengo , elev. 38 m ( -18.982867, 34.35185), 1–18.ix.2013, coll. P. Naskrecki— 1 female ( paratype) ( EOWL) GoogleMaps ; Claud’s Waterfall campsite, elev. 94 m ( -19.03011, 34.67592), 1–6.v.2013, coll. P. Naskrecki— 1 male ( paratype) GoogleMaps ; Wilson Laboratory, GNP, Chitengo , ( -18.97775, 34.351333), 21.vii.–8.viii.2015, coll. B. Woo—1 nymph GoogleMaps ; GNP, Chitengo , elev. 29 m ( -18.98194, 34.35122), 30.vii.2011, coll. Joel Sartore—1 nymph ( MCZ) GoogleMaps ; same locality, 9.v.–29.vi.2012, coll. P. Naskrecki—4 nymphs, 4 females, 7 males, 6 nymphs (incl. 11 paratypes) GoogleMaps ; same locality, 17.iii.–5.iv.2013, coll. P. Naskrecki— 2 females, 2 males, 1 nymph ( paratypes) GoogleMaps ; same locality, 17–31.iii.2013, coll. P. Naskrecki— 2 females, 2 males (incl. 4 paratypes) ( EOWL, MCZ) GoogleMaps ; same locality, 6–31.v.2013, coll. P. Naskrecki— 2 males, 1 nymph ( paratypes) ( MCZ) GoogleMaps ; same locality, 1–19.ix.2013, coll. P. Naskrecki— 1 female ( paratype) ( EOWL) GoogleMaps ; same locality, 4.ii.2014, coll. P. Naskrecki, R. Guta & F. Artur— 1 female ( paratype) ( EUMM) GoogleMaps ; same locality, 11–25.ii.2014, coll. R. Guta & T. Castigo— 1 female, 2 males (incl. holotype, 2 paratypes) ( EOWL, EUMM) GoogleMaps ; GNP, Explore Gorongosa camp, elev. 23 m ( -18.92092, 34.36575), 14.v.2012, coll. P. Naskrecki— 1 male, 2 nymphs (incl. 1 paratype) GoogleMaps ; GNP, humid woodland, elev. 30 m ( -18.96414, 34.38644), 12.v.2012, coll. P. Naskrecki— 2 males (incl. 2 paratypes); GNP, on Rd. 11, elev. 22 m ( -19.00469, 34.41064), 4.vi.-25.vii.2012, coll. P. Naskrecki— 5 males, 1 nymph ( paratypes) GoogleMaps ; GNP, on Rt. 2, elev. 18 m ( -18.95386, 34.42897), 20.v.2012, coll. P. Naskrecki— 1 male ( paratype) ( MCZ) GoogleMaps ; Nhambu , elev. 35 m ( -18.992778, 34.326944), 22.vii.2014, coll. M. Prager & R. Guta— 1 male ( paratype) GoogleMaps ; GNP, nr. Nhascuvo outpost, ( -19.062639, 34.232194), 24–28.vi.2014, coll. G. Daniel, I. Nganhane & R. Guta— 1 female, 1 male ( paratypes) ( EOWL) GoogleMaps .
| MCZ |
Museum of Comparative Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Hetrodinae |
|
Tribe |
Enyaliopsini |
|
Genus |