Tyrannosaurus rex, Osborn, 1905
|
publication ID |
https://doi.org/ 10.5281/zenodo.3942813 |
|
DOI |
https://doi.org/10.5281/zenodo.4710609 |
|
persistent identifier |
https://treatment.plazi.org/id/03D56722-3624-FF79-84DE-FCDFF9A2F9D0 |
|
treatment provided by |
Jeremy |
|
scientific name |
Tyrannosaurus rex |
| status |
|
The large theropod Tyrannosaurus rex is the archetype carnivorous dinosaur ever since it was named in 1905 ( Osborn 1905).
Even its name, “tyrant-lizard king,” invoked it as a top predator. Recent challenges to its title as the largest terrestrial carnivore (e.g., Sereno et al. 1996; Calvo and Coria 1998) have not diminished its popularity. Osborn reasoned that Tyrannosaurus was a predator on the basis of its teeth. Coprolitic material, some containing fossilized soft tissue, supports a carnivorous diet (e.g., Chin et al. 2003), as does toothgrooved bones of prey ( Erickson and Olson 1996) and evidence of a failed attack on a hadrosaur ( Carpenter 1998) and ceratopsian (Happ this volume).
Although Osborn (1905, 1912) was clear that he considered Tyrannosaurus a predator, Lambe (1917) dissented, considering tyrannosaurids to be scavengers instead, primarily because of the apparent absence of tooth wear. Tyrannosaurids as obligatory scavengers never gained popularity until recency, when the hypothesis was reintroduced by Horner and Lessem (1993). By and large, though, Tyrannosaurus has been considered an active predator, although the mode of attack remains controversial and includes flank bite and run ( Paul 1987), opportunistic ( Farlow 1994), and neck or snout crushing ( Molnar 1998). The method of killing is assumed to have been the jaws, and the bite force has been variously calculated or estimated to have been 13,400 N ( Rayfield et al. 2001), 6400—13,400 N ( Erickson et al. 1996), or even 183,000-235,000 N ( Meers 2002). As Meers has noted, the high forces are consistent with either scavenging or predation.
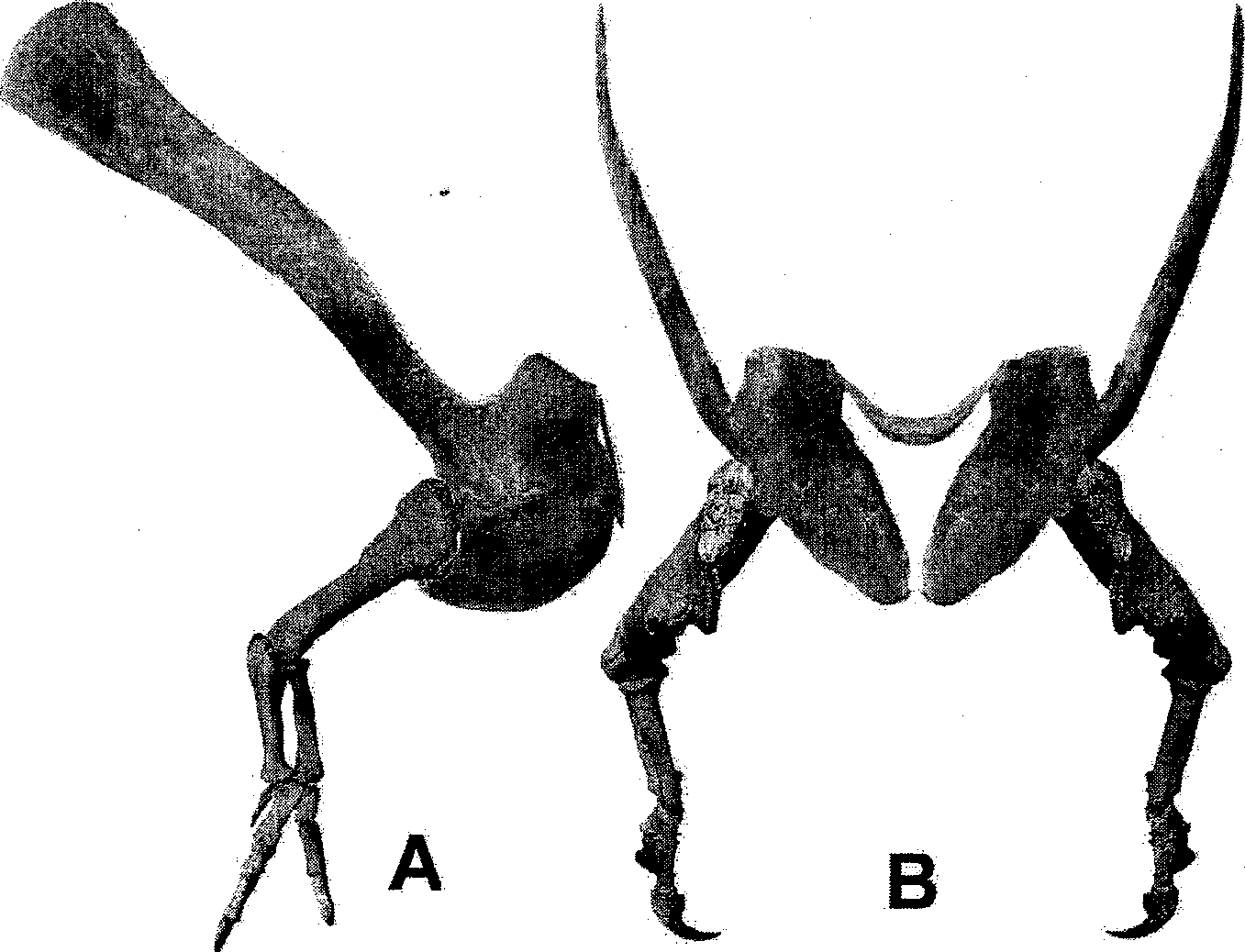
In arguing against predation, Horner and Lessem (1993) cite the shortness of the forelimbs as being useless for holding prey. In support of this position, Lingham-Soliar (1998) considered head shaking as a means of flesh removal from small prey and direct ripping of flesh from large prey. Carpenter (2002) has shown that without exception, no theropod could extend its forelimb beyond the snout, thus limiting its usefulness as a prey-grasping organ before the mouth was engaged ( Fig. 10.1 View Fig. 10.1 ). Does this mean, however, that the forelimbs of Tyrannosaurus were as useless as portrayed? Paul (1988, p. 320) considers such the question irrelevant: “the reduced size of the forelimb shows they were not important to their owners, so they should not be important to us.” This position, supported by Lockley et al. (this volume), is based on an unsubstantiated assumption (“were not important to their owners”), which is just as useless as the untestable speculations of Lockley et al. (this volume). In point of fact, Carpenter and Smith (2001) concluded that the forelimb was powerful, an interpretation previously made by Brown (1915, p. 271): “front limbs exceedingly small but set for a powerful clutch.”
New material has led to our reassessing the forelimb of Tyrannosaurus , and a stronger case is made for forelimb use during predation. Some of this new material displays pathologies, which is important because they are a reflection of lifestyle behaviors ( Rothschild and Martin 1993). As Paul has noted (this volume), Tyrannosaurus must have had a rough, active life.
The materials used in this study are as follows: scapula of BHI 3033 , DMNH 2827 , and MOR 555 ; furcula of FMNH PR2081 , MOR 980 , and TCM 2001.90.1 ; humerus of BHI 6230 , FMNH PR2081 , and MOR 555 ; ulna and radius of FMNH PR2081 , MOR 555 , and MOR 980 ; and manus of FMNH PR2081 , MOR 555 , MOR 980 , and BHI 6230 .
New Information on the Pectoral Girdle and Forelimb
The pectoral girdle and forelimb of Tyrannosaurus have been described by Carpenter and Smith (2001) and by Brochu (2002). Since then, the furcula has been described ( Larson and Rigby 2005), the third metacarpal and semilunate carpal have been found (described below), and new information is available for the scapula and coracoid. To date, no ossified sternal plates are known, but these are predicted to resemble those of Gorgosaurus as described by Lambe (1917). Brochu (2002) has discussed the possibility of ossified sternal plates in Tyrannosaurus but came to no conclusion.
Furcula
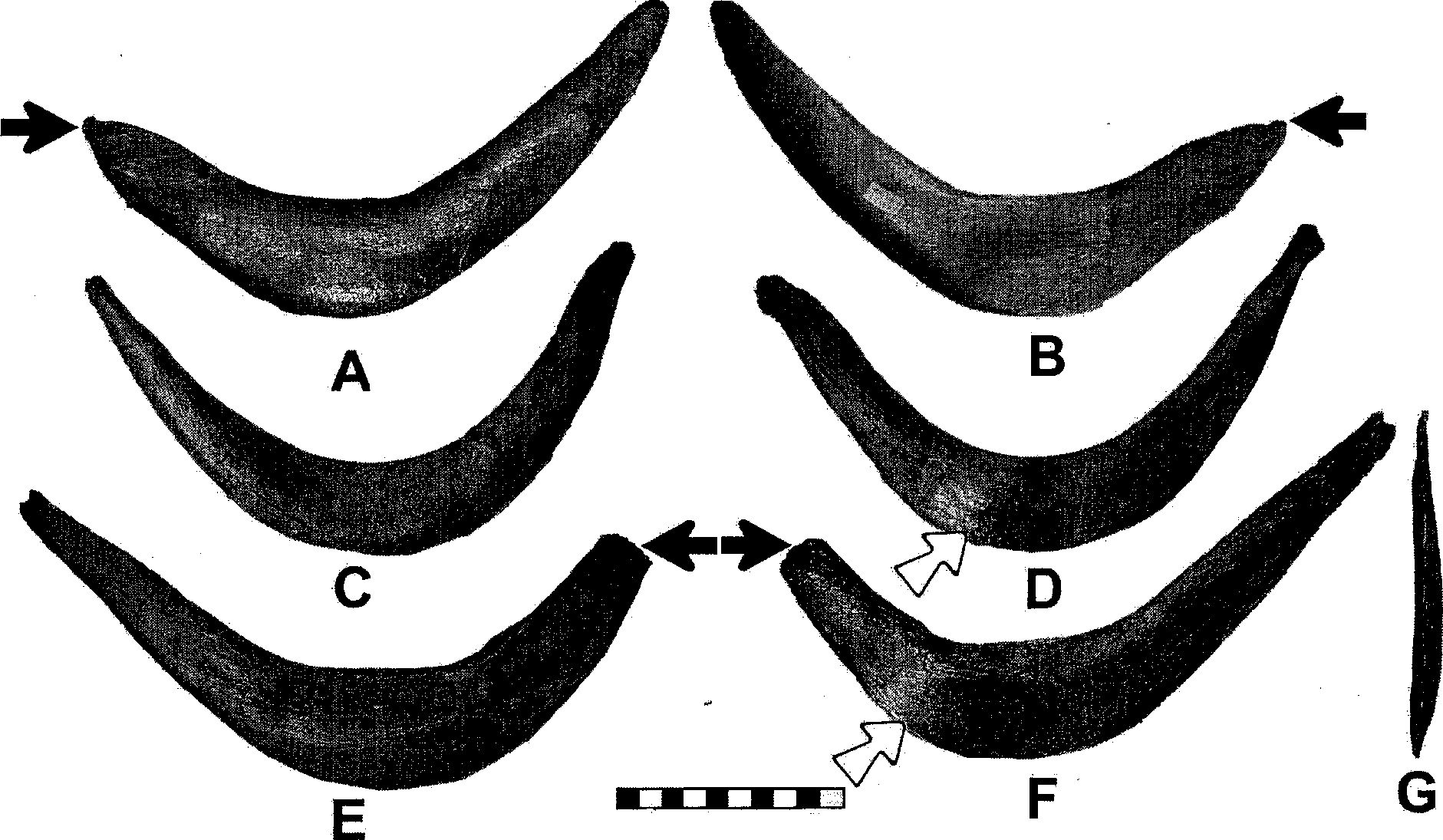
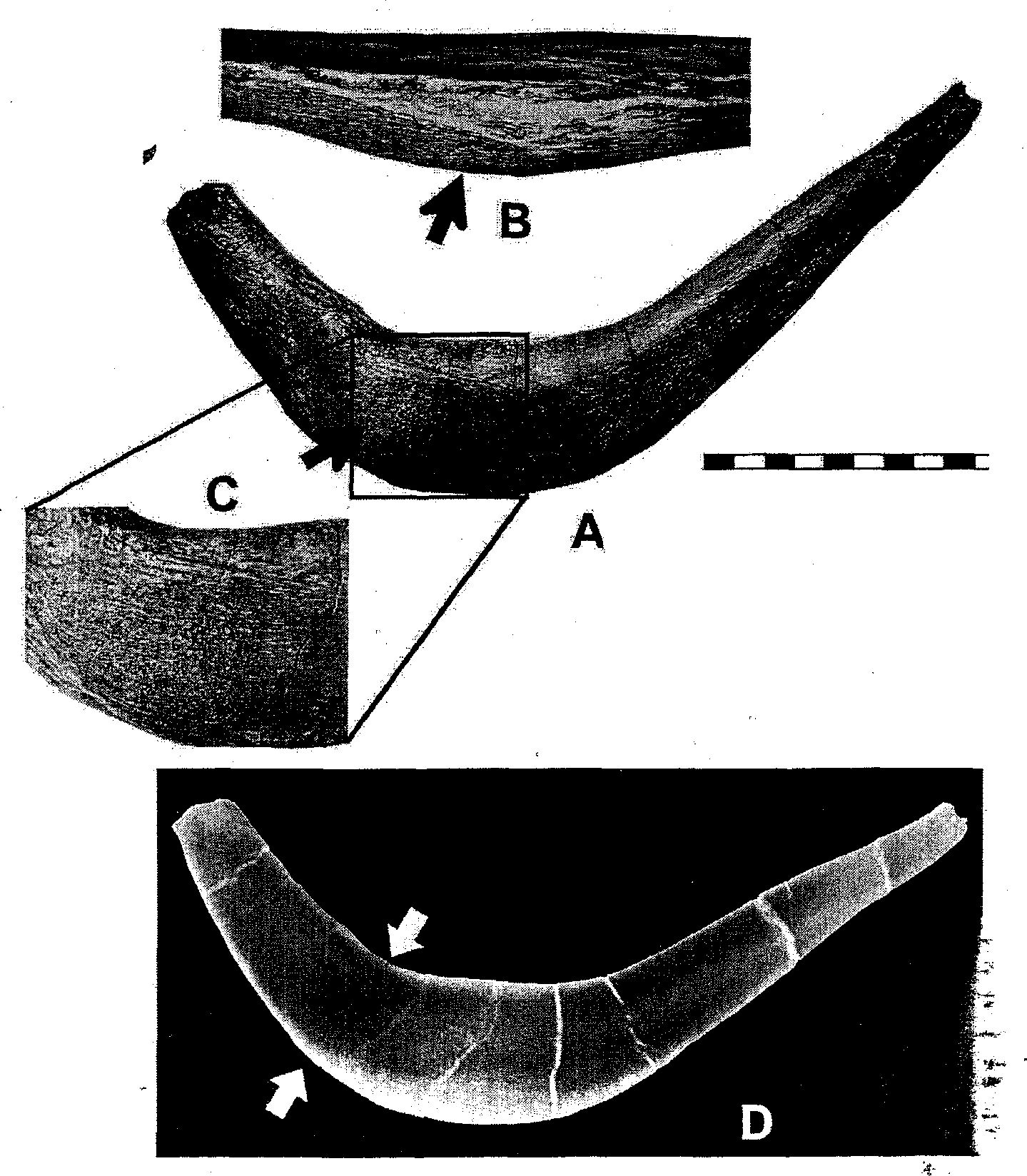
The furcula of Tyrannosaurus , the presence of which was predicted by Carpenter and Smith (2001), is now known for several specimens ( Larson and Rigby 2005). It is broadly U or boomerang shaped ( Fig. 10.2 View Fig. 10.2 ), with a roughened sutural scar (acrominal facet) on the epicleidium for a ligamentous attachment to the acromion of the scapula ( Fig. 10.3 View Fig. 10.3 ; see also below). In birds, the rami of the furcula have a nearly circular or laterally compressed cross-sectional geometry that allows them to act as a spring, with laterally directed tension added on the downstroke and medial directed recoil on the upstroke ( Jenkins et al. 1988; Boggs et al. 1997; Hui 2002). In Tyrannosaurus , however, the furcula is clearly designed to resist extreme lateral forces: (1) the ramfare anteroposteriorly flattened (cf. Fig. 10.2 View Fig. 10.2 F, G), thus prohibiting lateromedial springlike action; (2) the rami diverge, thus directing lateral stresses down the shaft ( Fig. 10.4 View Fig. 10.4 A; and (3) the rami deepen distally from the epicleidium and the furcula is deepest (thickest vertically in the anatomical position) near the midline to counter the stresses directed down the rami. In most theropods, the furcula is nearly uniform throughout its length (see Chure and Madsen 1996, fig. 2, 3; Makovicky and Currie 1998, fig. 1; Currie et al. 2005, fig. 16.8; Larson and Rigby 2005, fig. 12.3). Therefore, the great depth of the Tyrannosaurus furcula is unusual and is clearly adapted to resist stress.
Three of the 5 known Tyrannosaurus furculae are pathologic ( Fig. 10.2 View Fig. 10.2 ) and were examined by computed tomographic scanning and X-ray. Two of them are missing portions of a ramus ( FMNH PR2081 [ Fig. 10.2 View Fig. 10.2 A, B] and TCM 2001.90.1 [ Fig. 10.2 View Fig. 10.2 E, F]), and 2 show localized swelling of the cortical bone ( MOR 980 [ Fig. 10.2 View Fig. 10.2 D] and TCM 2001.90.1 [ Fig. 10.2 View Fig. 10.2 F]).
The missing sections of the rami were broken in life, with subsequent remodeling of the fracture surface. Surprisingly, none show a pseudoarthrosis joint at the site of the break, thus indicating significant displacement of the broken portion, possibly due to the contraction of the M. supracoracoideus brevis, which probably inserted along the ramus.
The amount of force needed to break a ramus was calculated from the largest furcula, TCM 2001.90.1 . The fracture is 2.5 cm wide; however, the bone is remodeled on the anterior and posterior sides so as to exaggerate the original thickness of the bone. Therefore, the anterior-posterior thickness is approximated from the undamaged ramus, where it is has the same width, which gives a thickness of 1.35 cm. The ramus can be modeled as an ellipse; therefore the area of the break is given by the following:
AB, (1)
or 2.6 cm2, where A is the radius ofthe ramus width and B is the radius of the thickness. Given that the shear strength of living cortical bone is conservatively ~102 N/cm2 ( Currey 2002), about 26,000 N (i.e., 2652 kg offorce) was required to break the furcula. As seen by computed tomography, trabecular bone occupies a small portion of the normal ramus, so it was not considered in the calculations because it would have reduced the fracturing force only slightly.
Two of the pathologic furculae also show a characteristic bony callus from a healed stress fracture ( Rothschild 1988) located on the posterior side ( Fig. 10.2 View Fig. 10.2 D, F; Fig. 10.5 View Fig. 10.5 ). Stress fractures occur as a result of repetitive loading on bone, which leads to mechanical failure and microfracturing ( Resnick 2002; Rothschild and Martin 1993; Rothschild 1988). R&thschild and Tanke (2005) and Rothschild and Molnar (this volume) note a high incidence of stress fractures in the manual elements of tyrannosaurids. They also note, “Active resistance of prey is required to overstress the manus,” which would also essentially include the rest of the forelimb and pectoral girdle. Because a stress fracture is a partial fracture, the amount of force must be less than the maximum required to completely break the bone. Again, the furcula of TCM 2001.90.1 was used to calculate the force. The normal portion of the furcula corresponding to the pathological portion measures 4.5 cm dorsoventrally and 1.7 cm anteroposteriorly. Assuming the regions of the stress fracture were approximately the same dimensions, then the force must have been less than 60,080 N (6128 kg of pressure), resulting from equation 1.
Scapula
The scapula or T. rex was mostly described by Carpenter and Smith (2001). New information is available based on DMNH 2827 . The acromion is a thin plate that is often damaged or lost in other specimens (e.g., MOR 555 ). Fortunately, this region is preserved in DMNH 2827 and shows a small facet for the epicleidium near the scapulocoracoid suture ( Fig. 10.3 View Fig. 10.3 ). This epicleidial facet measures 4.8 cm by 1.1 cm. Placement of the furcula connecting the epicleidial facets of the left and right scapula show how close together the coracoids really were in life ( Fig. 10.4 View Fig. 10.4 A, Fig. 10.6 View Fig. 10.6 ), a position supported by a nearly uncrushed Tyrannosaurus chest region found in situ that is currently under study ( Lipkin and Sereno 2004). A similar close placement is also known in hadrosaurs (e.g., Osborn 1912), suggesting that coracoids were closely placed in all dinosaurs (including sauropods), as has been discussed elsewhere (Carpenter 2002; Carpenter et al. 1994).
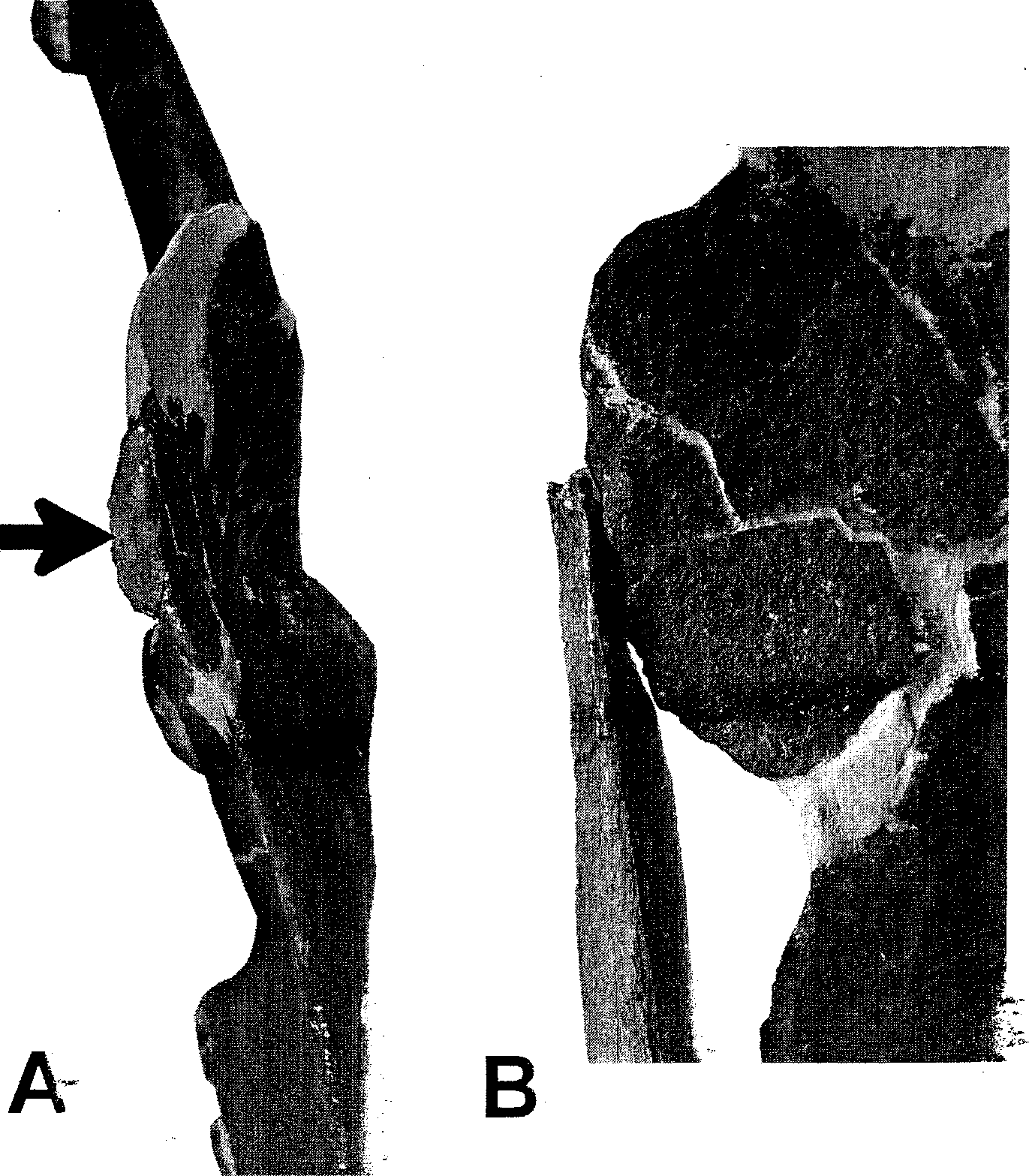
DMNH 2827 also shows an unusual pathology of the glenoid, which is partially collapsed as a result of ventroposterior rotation of the coracoid. Although the glenoid was partially damaged during preparation (the bone in the region was crumbly), it is clear that the 2 bones were initially damaged before co-ossification because the 2 bones are now firmly fused by remodeled bone ( Fig. 10.7 View Fig. 10.7 ). The rotation is less near the acromion and greater near the glenoid, suggesting that great rotational forces were applied to the coracoid in a posteroventral direction, thereby partially collapsing the glenoid. Although the damage may have resulted from a fall onto the chest, the direction of rotation also corresponds to the vector for the M. coracobrachialis brevis ventralis (although the terminology is retained for “ dorsal” versus “ventral” muscles, we are aware that the more vertical position of the humerus in Tyrannosaurus indicates a need for modified terminology). It is therefore possible that the damage occurred when the individual was young and the forelimbs were pulling struggling prey toward the chest. Unfortunately, so little of the skeleton was recovered (see N. L. Larson this volume) that the extent and location of damage to other bones is unknown (e.g., right scapula-coracoid, humerus, gastralia). The distribution of pathologies elsewhere on the skeleton might resolve between the 2 possibilities.
Humerus
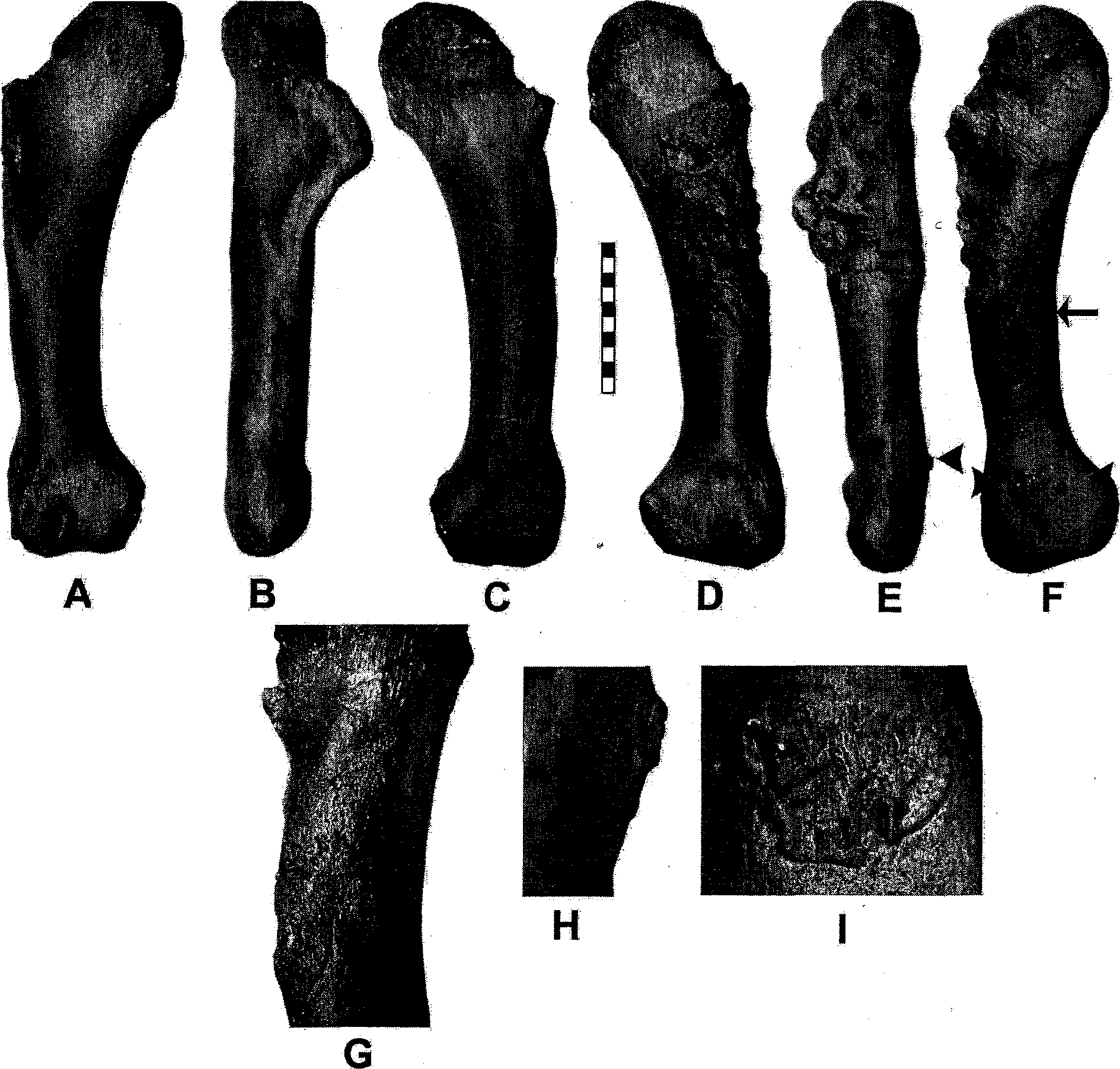
Several additional humeri are now known, including more with pathologies that reflect behavior. Overall, these specimens resemble those described by Carpenter and Smith (2001), differing only in minor detail. Two of the new specimens are of gracile morphs (e.g., MOR 980 and BHI6230 ), which are probably male (P. Larson this volume). The robust morph (e.g., FMNH PR2081 ) is broader proximally than the gracile morph (cf. Carpenter and Smith 2001, fig. 9.4, with Fig. 10.8 View Fig. 10.8 ).
As noted by Carpenter and Smith (2001), the humerus of FMNH PR2081 shows several pathologies, as does the new left humerus of MOR 980 (e.g., Fig. 10.8 View Fig. 10.8 ). This latter humerus shows an extensive juxtacortical pathology located proximally on the anterolateral, lateral, and posterolateral sides of the diaphysis, which has all but obliterated the deltopectoral crest ( Fig. 10.8 View Fig. 10.8 D, E). The pathology shows extreme disruption ofthe cortical bone and irregular patches of sclerotic bone that suggest uneven loss and regrowth of the periosteum. The resulting periostitis implies extensive inflammation of the region. The region of the pathology corresponds with the crocodile humerus to the insertions ofthe M. pectoralis, the common insertion for the M. supracoracoideus complex, M. deltoideus clavicularis, common insertion for the M. teres major and M. latisimus dorsi, and origins of the M. brachialis, M. humeroradialis, and part of the triceps brevis cranialis (cf. Figs. 10.9 View Fig. 10.9 B with 10.9 View Fig. 10.9 K, G with L). There is also a juxtacortical lesion on the posterior side that, in the crocodile, corresponds with the origin for the proximal part of the M. triceps brevis intermedius ( Fig. 10.8 View Fig. 10.8 F, G; compare 9G with L). More distally, there is a small spur on the posterior side as well ( Fig. 10.8 View Fig. 10.8 E, F, H, I). These 2 lesions blend with the diaphysis proximally but have sharp, overhanging margins distally that may be due to osteoblastic response to the extensor motion of the overlying M. triceps group.
The large pathology on the proximal end is irregular or knobby. Superfacially, it suggests a malignant neoplasm or extensive osteomyelitis, but as Donnelly et al. (1999) have noted, site location is important for differential diagnosis. The location and extent of the pathology is most probably due to avulsion of muscles in the vicinity of the deltopectoral crest, and the posterior pathologies due to periostitis from trauma to the periosteum associated with the event that caused the avulsion. The aggressive appearance of the proximal pathology, caused by bone resorption resulting in a lytic appearance of bone, is characteristic of a healing avulsion and can mimic the appearance of osteomyelitis or skeletal Ewing sarcoma ( Stevens et al. 1999). An avulsion is a failure of bone at a tendinous or aponeurotic insertion of muscle ( Tehranzadeh 1987; El-Khoury et al. 1997). Avulsions can either be acute (the result of extreme, abnormal muscle contractions) or chronic (the result of repeated microtrauma or overuse) ( Stevens et al. 1999), where reoccurrence of the injury is more frequent than the ability of the tissue to repair itself ( El-Khoury et al. 1997). The presence of a stress fracture in the furcula associated with this specimen suggests that repetitive overuse of the forelimb may have been a major factor leading to the avulsion. A secondary factor may have been a violent stress on the arm because so many different muscles were apparently affected. Such stresses would be generated in the sudden pull of the arm toward the chest at the same time prey was struggling in the opposite direction. It may not be incidental that MOR 980 is a young adult because Tehranzadeh (1987) noted that the incident ofavulsion is highest in younger human individuals. Regardless of the cause, these pathologies support the hypothesis of forelimb use in Tyrannosaurus .
Manus
The incomplete manus of Tyrannosaurus was described by Carpenter and Smith (2001). New specimens, MOR 980 and BHI 6230 , provide new information, including a carpal and metacarpal III ( Figs. 10.10 View Fig. 10.10 , 10.11 View Fig. 10.11 ). The carpal ( Fig. 10.10 View Fig. 10.10 A-F) is distal carpal I (“semilunate”) and shows a facet on the proximal surface for the missing radiale. This carpal shows that the damaged, incomplete one described by Carpenter and Smith (2001) is in fact a distal carpal I. Ventrally, the new carpal is faceted and fits snugly between the proximal ends of metacarpals I and II ( Fig. 10.11 View Fig. 10.11 C), rather than across them as in Deinonychus (Carpenter 2002). Metacarpal III is slender, posteriorly curving, and tapering, and lacks a distal condyle, so it thus had no phalanges. MOR 980 is from a larger individual than BHI 6230 , and the differences between them are probably ontogenetic. These metacarpals suggest that as the individual grew, the metacarpal became more robust and the proximal end formed a broader attachment to metacarpal II.
All of the new information on the forelimb was used to create a new view of the pectoral girdle and forelimb of Tyrannosaurus ( Fig. 10.12 View Fig. 10.12 ) and was used in following biomechanical study.
Reconstructing Forelimb Musculature
Mathematical models for scavengers not withstanding (e.g., Ruxton and Houston 2003), many of the same criteria and assumptions for scavenging theropods also hold for predatory theropods as well (see Holtz this volume). In reality, there are too many unknown variables (e.g., population densities, energentics, locomotion capabilities of both predator and prey) to ever have testable results. With so many assumptions built on assumptions, a house of cards results. Our approach is to minimize assumptions (including minimizing untested “common sense,” referred to by Ruxton and Houston 2003) and to rely on evidence and testable models.
Although we have attempted to minimize our assumptions, some are required as a result of the nature of fossilized remains. We assume the following: (1) The power output of muscles of extinct tetrapods is comparable to that of extant tetrapods—that is, muscles were not weaker or stronger than they are today. (2) Scars on fossilized bone that are clearly not pathological (e.g., lack of obvious remodeling; see Rothschild and Martin 1993) indicate insertion or origin for muscles or for ligaments (but see below). (3) Homologous origin-insertion scars, as determined by extant phylogenetic bracketing, identify each muscle (but see below). (4) The extent of the ioint surface can be determined from the smooth surfaces of the joints, which are separated from the diaphysis by a rim or abrupt textural transition, and denote the area capped by joint cartilage. (5) The movement of the joints was less than the area covered by the cartilage cap (based on dissections of birds) (Carpenter 2002). With these basic assumptions, we reanalyze the forelimb of Tyrannosaurus below.
!
s
A testable method of predicting musculature patterns for the forelimb elements of Tyrannosaurus is presented that uses extant phylogenetic bracketing, which is based on comparable elements of the crocodilian and bird. Previously, Carpenter and Smith (2001) had presented muscle maps for the forelimb of Tyrannosaurus , the results of which were partially criticized by Brochu (2002). The forelimb map was admittedly influenced too greatly by the phylogenetic placement of Tyrannosaurus as closer to modern birds than to modern crocodilians (e.g., Brochu 2002). It was believed that the muscle patterns should reflect this phylogenetic closeness, thus resulting in a loss of objectivity from the start (the loss of objectivity in theropod studies was subsequently criticized by Carpenter 2002, pp. 72-73).
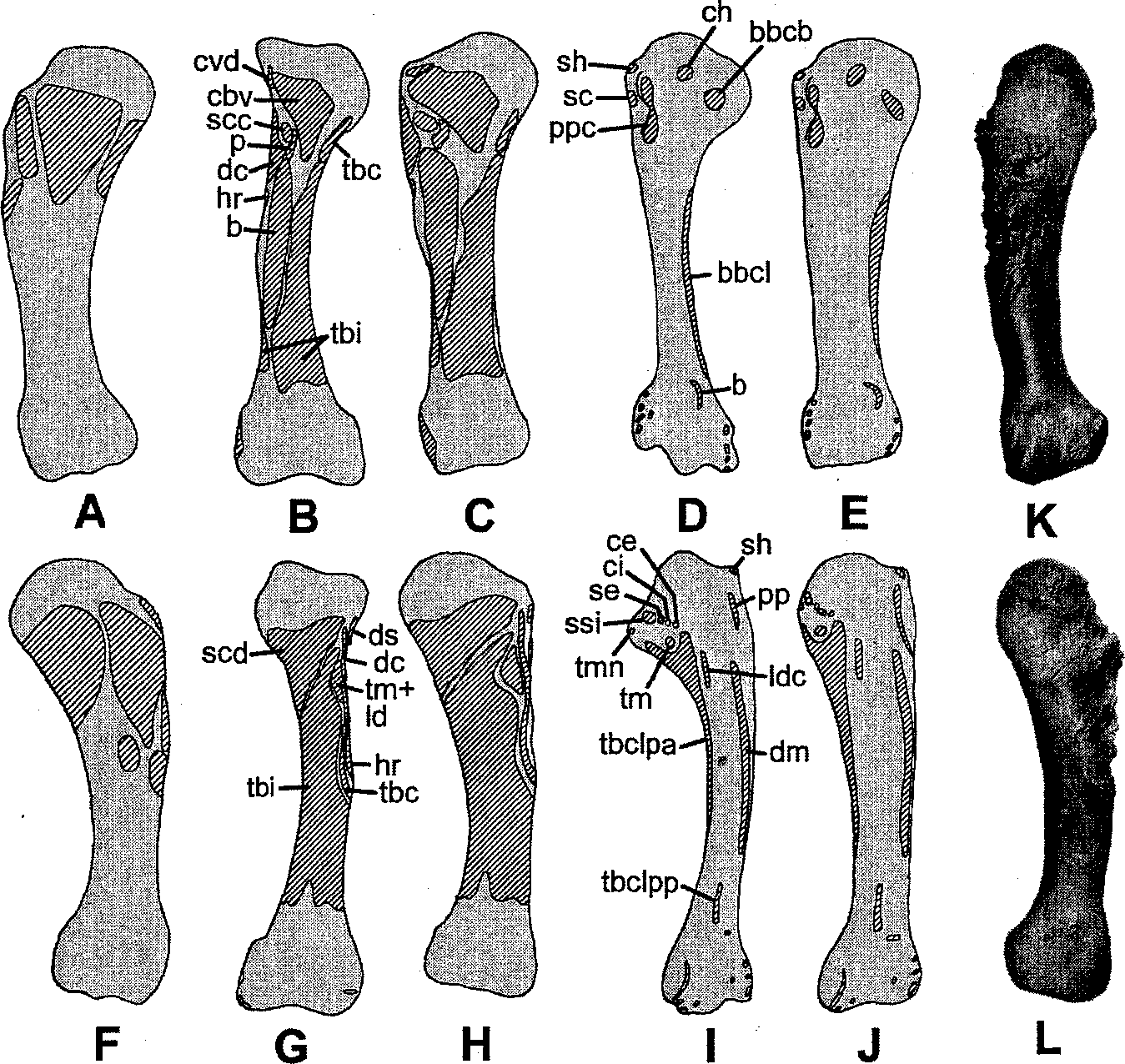
The scapula and coracoid of Tyrannosaurus (and dinosaurs in general) are the most difficult elements on which to map muscles because few muscle scars are present and the shape of the coracoid differs; these issues hamper the application of phylogenetic bracketing. Nevertheless, the scapula and coracoid are crucial in muscular reconstructions because the muscle origin patterns affect the muscle patterns for the rest of the forelimb. Although muscle scars remain the chief means for mapping muscles, supplemental information is needed in the case of the scapula and coracoid. This supplemental information, as a testable hypothesis, is obtained by deforming the scapulocoracoids of both a crocodile ( Alligator ) and bird ( Gallus ) to approximate that of Tyrannosaurus ( Fig. 10.13 View Fig. 10.13 ). The technique does not involve morphing of one scapulocoracoid to another because the scapulocoracoid of Tyrannosaurus is not used as one end point. Although the technique uses a Cartesian grid, it does not attempt to explain homologous points of 2 forms in the manner used by D’Arcy Thompson (1961). Instead, the technique attempts to predict the muscle origin and insertion patterns of the scapulocoracoid of Tyrannosaurus as it would be if the scapulocoracoid of the crocodile or bird were deformed into that of Tyrannosaurus . The results can then be used to determine which of the 2 muscle patterns, crocodilian or avian, is most like that seen on the bones of Tyrannosaurus .
The technique begins by scanning scapulocoracoid outlines on which the muscles have been mapped ( Fig. 10.13 View Fig. 10.13 B, E). Minimum thickness of the scapular neck was selected to standardize the scapulocoracoids of the Alligator , Gallus , and Tyrannosaurus ( Figs. 10.13 View Fig. 10.13 A, B, E) because of the peculiar shape of the avian coracoid precluded scapula-coracoid length as the standard. The Mesh Warp feature of Corel PhotoPaint 7 was used to deform the images using a 10 by 10 grid. By manually moving each intersect ofthe gridlines (node), a small area of the scapulocoracoid surrounding each node could be deformed. The deformation was smooth, meaning that no sharp angles and lines resulted, thus approximating changes in a biological structure. Nodes were moved until the outline of the scapulocoracoid closely approximated the that of Tyrannosaurus (cf. Fig. 10.13 View Fig. 10.13 A and 10.13 View Fig. 10.13 C, D, F). Because moving the nodes also moved the contents of each grid, the result is a prediction ofwhat the resultant muscle pattern would be like. As used, Mesh Warp is not mathematically as rigorous as the thin plate spline of Bookstein (1991) because measuring the change in landmark position is irrelevant. Two versions are presented for the deformed crocodile scapulocoracoid, with results differing in the acromion. Figure 10.13 View Fig. 10.13 D assumes the dorsal prominence just anterior to the scapular neck of the crocodile is homologous to the posterodorsal corner of the acromion in Tyrannosaurus , whereas Figure 10.13 View Fig. 10.13 C does not. This is tested below.
The muscle patterns of the deformed crocodile and avian scapulocoracoids were then compared against the few muscle scars on the scapulocoracoid of Tyrannosaurus ( DMNH 2827 ). As can be seen in Figure 10.13 View Fig. 10.13 G, several muscle scars on the scapulocoracoid of Tyrannosaurus seem to be homologous with the origins for the M. costocoracoideus, M. triceps longus lateralis, and M. supracoracoideus intermedius on the deformed crocodilian scapulocoracoid, than with any muscle origin on the scapulocoracoid of the bird. We may infer, then, that the other muscles, for which scars are not evident on the scapulocoracoid, were more homologous with those ofthe crocodile than ofthe bird. The large depression or fossa on the acromion of Tyrannosaurus seems to better match the pattern for the M. supracoracoideus intermedius in Figure 10.13 View Fig. 10.13 C than for the multiple muscles in this region, as seen in Figure 10.13 View Fig. 10.13 D. This suggests that the dorsal prominence of the crocodile is not homologous to the posterodorsal corner of the acromion in Tyrannosaurus .
Some independent support for the shoulder of Tyrannosaurus having the crocodilian muscle pattern rather than the avian pattern is seen in the scapular blade. In a random sample of various bird skeletons (DMNH avian collection, including Aechmophorus, Buteo, Cygnus, Corvus, Aquila, Gymnogyps , Gallus , and Struthio), a faint longitudinal trough is present in the distal half of the scapular blade. Interpreted another way, there is thickening ofthe scapular surface along the origins of the M. terres major and M. rhomboideus superficialis; the trough is the unthickened bone between these origins. In contrast, the crocodile has a single longitudinal thickening or ridge on the scapular surface that corresponds roughly to the common margin of the M. terres major and M. deltoideus scapularis. The scapula of Tyrannosaurus also has a similar ridge, not the trough seen in birds.
The deformation method outlined above was applied to the humerus ( Fig. 10.9 View Fig. 10.9 B-E, G-J) and tested against the muscle scars ( Fig. 10.9 View Fig. 10.9 A, F). Overall, the pattern most closely resembles that of the crocodile, with notable exceptions. Aside from differences in relative proportions of some muscles between the actual and the predicted (cf. Fig. 10.9 View Fig. 10.9 A and C, F and H), there are also some positional differences. For example, the common insertion for the M. terres major and M. latissimus dorsi is lower on the diaphysis and more centrally located in Tyrannosaurus (cf. Fig. 10.9 View Fig. 10.9 F, G, and H). Furthermore, there seems to be a distinct scar on all Tyrannosaurus humeri, suggesting that the M. supracoracoideus longus had a separate insertion slightly below the peak of the deltopectoral crest ( Fig. 10.10 View Fig. 10.10 )—either that or the pectoralis inserted more lateral to the deltopectoral crest than medial, but that seems highly unlikely. As noted above, the pathology on MOR 980 better matches the muscle pattern of the crocodile than the bird. With this information, it is possible to reconstruct the muscles of the pectoral girdle and arm of Tyrannosaurus ( Fig. 10.15 View Fig. 10.15 ).
Biomechanical Analysis of the Forelimb in Tyrannosaurus rex
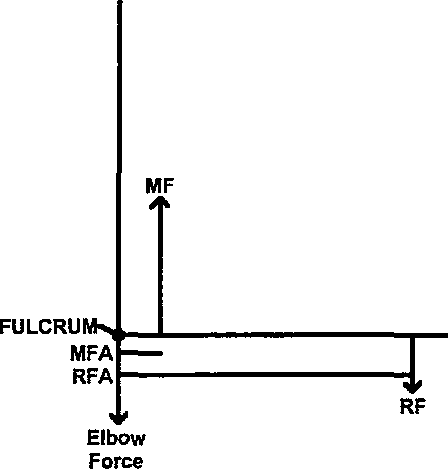
In this section, we do a reanalysis of Carpenter and Smith (2001) and an extension of the biomechanical properties of the forelimb in Tyrannosaurus rex based mostly on FMNH PR2081 . In order to work out the forces acting on the forelimb, we start by modeling the forelimb as a third-class lever ( Fig. 10.16 View Fig. 10.16 ). In a third-class lever, the effort force (M. biceps muscle) is applied between the fulcrum (elbow joint) and the resistance force. The elbow joint is a hinge where the humerus, ulna, and radius articulate. Of all of the muscles coordinating and controlling the movement of the elbow, the M. biceps is the most powerful flexor of the elbow joint ( Ozkaya and Nordin 1999). Our model assumes that the M. biceps is the major flexor and that the line of action (the tension) at the biceps is vertical.
Anatomical measurements were used to derive the motive force arm (MFA) and the resistive force arm (RFA) ( Table 10.1). For the ulna, the MFA was measured from the sigmoid notch to the midscar of the insertion point for the M. biceps (motive force, MF), and the RFA was derived from measuring the ulna from the sigmoid notch to the distal end ( Fig. 10.16 View Fig. 10.16 ). To obtain the MFA of the radius, we measured from the radial head to the midscar of the insertion point for the M. biceps and from the radial head to the distal end to determine the RFA.
A tendon tensile strength of 100 MPa, the global mean across all species, was used to estimate the tendon tensile strength in Tyrannosaurus ( Nigg and Herzog 1999). The safety factors in the values of bird tendons range from 1.19 to 4.10 ( Van Snik et al. 1994; Alexander 1981). A safety factor of 3 will be used in this study.
The normal working range (NWR) is one-third the safety factor ( Carpenter and Smith 2001). Although the size, shape, and the biomechanical behavior of each tendon differs, the basic structure of tendons and their mechanical properties are similar (Jozsa and Kannus 1997). The size of the cross-sectional area of a tendon is directly related to the size of the load that can be carried before failure ( Butler et al. 1978).
The surface area of the scar for the insertion of the M. biceps is 122.11 mm2 on the radius and 192 mm2 on the ulna. The conversion for the tendon strength, expressed as MPa, is 1 MPa = 1,000,000 Pa, with 1 Pa = 1 N/m2. Therefore, 1 MPa = 1 N/mm2. The maximum working range (MWR) and the NWR are calculated from the tensile strength of the tendon. The formula for estimated tendon tensile strength is as follows:
tendon tensile strength/area2 x surface area of the scar for insertion of the M. biceps = estimated tendon tensile strength (1)
Tendon tensile strength for the radius is
100 N/mm2 x 122.11 mm2 = 12,211 N
where MWR is 12,211 N/3 = 4070 N, and NWR is 4070 N/3 = 1357 N
Tendon tensile strength for the ulna is:
100 N/mm2 X 192 mm2 = 19,200 N
where MWR is 19,200 N/3 = 6400 N, and NWR is 6400 N/3 = 2133 N.
The values for the MWR and NWR represent the estimated strength of the tendon at the insertion of the M. biceps and are used as the MF in the analysis of the power of the Tyrannosaurus forelimbs. The following equations are used to estimate the amount of force the arm of Tyrannosaurus can resist (resistive force, or RF):
MFxMFA = T (2)
RF x RFA = T (3)
Measurement of the manus (177.6 mm) was taken from a cast of FMNH PR 2081 , from the proximal end of the wrist to the proximal end of the claws. It was then added to the RFA (166.2 mm).
MWR for the radius of T. rex is as follows:
4, 070 N x 0.0152 m = 61.86 Nm
RF x 03438 m = 61.86 Nm
RF = 179.93 N (or 18.36 kg)
NWR for the radius of T. rex is as follows:
1,357 N x 0.0152 m = 20.63 Nm
RF x 0.3438 m = 20.63 Nm
RF = 60.01 N (or 6.12 kg)
MWR for the ulna of T. rex is as follows:
6,400 N x 0.0458 m = 293.12 Nm
RF x 0.3642 m = 293.12 Nm
F = 804.83 N (or 82.13 kg)
NWR for the ulna of T. rex is as follows:
2,133 N x 0.0458 m = 97.69 Nm
RF x 0.3642 m = 97.69 Nm
RF = 268.23 N (or 27.37 kg)
Adding the resistive forces of the radius and ulna results in 984.76 N (100.49 kg or 221.10 pounds) for the MWR (no safety factor) and 328.24 N (33.49 kg or 73.70 pounds) for the NWR (with safety factor). The conversion factor for kilograms to newtons is 1 kg = 9.8 N. These results are summarized in Table 10.2.
An average strength of 5 kg/cm2 per cross-sectional area of muscle was used to determine the cross-sectional area of the M. biceps in Tyrannosaurus ( Carpenter and Smith 2001). The NWR ofthe tendon tensile strength for the radius and ulna were added together to get the MF: 1357 N + 2133 N = 4490 N (356.12 kg). The formula used to determine the cross-sectional area of muscle is MF (kg)/strength (kg x cm-2) = cross-sectional area (cm2). Thus, the estimated cross section of the Tyrannosaurus M. biceps is 356.12 kg/5 kg x cm2 = 71.224 cm2. This translates into a diameter of9.52 cm. Of course the M. biceps is not the only arm protractor. In fact, by using half the estimated cross-sectional area of the upper arm (based on a diameter of 25 cm), the amount offorce generated is estimated to have been around 1150 kg, or 11,270 N. Of this, the biceps generated about 40%, and thus was a major muscle.
Mechanical Analysis of the Forelimb in Tyrannosaurus rex
A small lever arm requires greater muscle tension to balance a load. Therefore, while resisting prey or holding prey, it is disadvantageous to have a muscle attachment close to the elbow joint. The advantage to having the muscle attachment close to elbow joint is that it will have a larger range of motion of the elbow flexion-extension, and therefore the hand can move faster toward the upper arm or shoulder ( Ozkaya and Nordin 1999).
The mechanical advantage is the amount of force a given effort can produce. It can be expressed as a ratio of the resistive force to the MF, or as a ratio of the MFA to the RFA ( Kreighbaum and Barthels 1985). Both of the equations produce the same result.
The Tyrannosaurus forelimb is found to have a mechanical advantage of the 0.09 (RFA measurement including the hand) and 0.18 (RFA measurement excluding the hand). The mechanical advantage of a human forearm is 0.07 (RFA measurement including the hand) and 0.13 (RFA measurement excluding the hand).
Next we evaluate the force at the elbow joint. The sum of the MFs (NWR + MWR) at the radius (138.3 kg) and the ulna (217.5 kg) minus the RF at the manus (33.5 kg) must equal the force at the elbow for a static configuration. Therefore, Tyrannosaurus has a force of 138.3 + 217.5 - 33.5 = 322.3 kg at the elbow joint for the NWR. This compares with a force of about 128.25 kg at the elbow joint of an average adult male human for the NWR.
Acceleration
From the torque ot the Forearm, the force that could be applied at the manus and the resultant Force at the elbow joint were determined. We now estimate the acceleration that could be generated at the claws using the moment of inertia. The fleshed-out version ot the arm of Tyrannosaurus ( Figs. 10.15- 10.17 View Fig. 10.15 View Fig. 10.16 View Fig. 10.17 was converted to a closely packed series of elliptical cylinders. The cross sections of each elliptical cylinder were determined from Figure 10.15 View Fig. 10.15 . Assuming a density of 1000 kg/m2 for tissue, the data from these cylinders result in a mass of 1.8 kg for the forearm plus manus. The integral of the density times the perpendicular distance to the pivot point results in a moment of inertia of 0.06 kg m2. Because the NWR torque of the forearm and hand to be 118.3 Nm, the angular acceleration (torque divided by the moment of inertia) is 1983 s-2. The angular acceleration can be converted to a linear acceleration by multiplying it by the distance (0.354 m) from the pivot point to the claws, which results in a linear acceleration of 702 ms-2. This is likely an overestimate because the skin and the claw sheath are not factored in. Also, this only gives the initial acceleration. The force from muscles is known to rapidly reduce at high speeds ( Hill 1938).
Conclusions
As we have shown, the forearm of Tyrannosaurus was capable of resisting large forces and moving at high accelerations. These results strengthen the hypothesis that the forelimbs were used during predation. However, because of the small size of the forelimb relative to the body size, it is unlikely that the Tyrannosaurus would use the manus for striking prey, as discussed in Carpenter (2002). Rather, the forelimbs may have been used to cling to prey. Our results of finding large forces at the elbow joint and possible signs of injury at the furcula further support this hypothesis.
Finally, in contrast to the belief of Lockley et al. (this volume) that “no useful function is plausible” to explain the forelimb of Tyrannosaurus , our results support the previous assertion that the forelimb played a functional role in predation. By implication, the short forelimbs of other tyrannosaurids had a similar function. In support of this, we note that a progressive reduction in the forelimb does not occur in the Tyrannosauridae ( Fig. 10.18 View Fig. 10.18 ), contrary to Paul (1988) and Lockley et al. (this volume). In point of fact, once the shortened forelimb of the tyrannosaurids was established, it remained proportionally stable relative to hindlimb length.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |