Stenochrus portoricensis, Chamberlin, 1922
|
publication ID |
https://doi.org/10.1093/isd/ixab032 |
|
publication LSID |
lsid:zoobank.org:pub:ADF09C2F-8537-42E6-AD72-B405432CE01E |
|
persistent identifier |
https://treatment.plazi.org/id/03D32B04-FFBC-FF95-FF36-7BA8FE15FD0B |
|
treatment provided by |
Felipe |
|
scientific name |
Stenochrus portoricensis |
| status |
|
Phylogeography of S. portoricensis View in CoL View at ENA
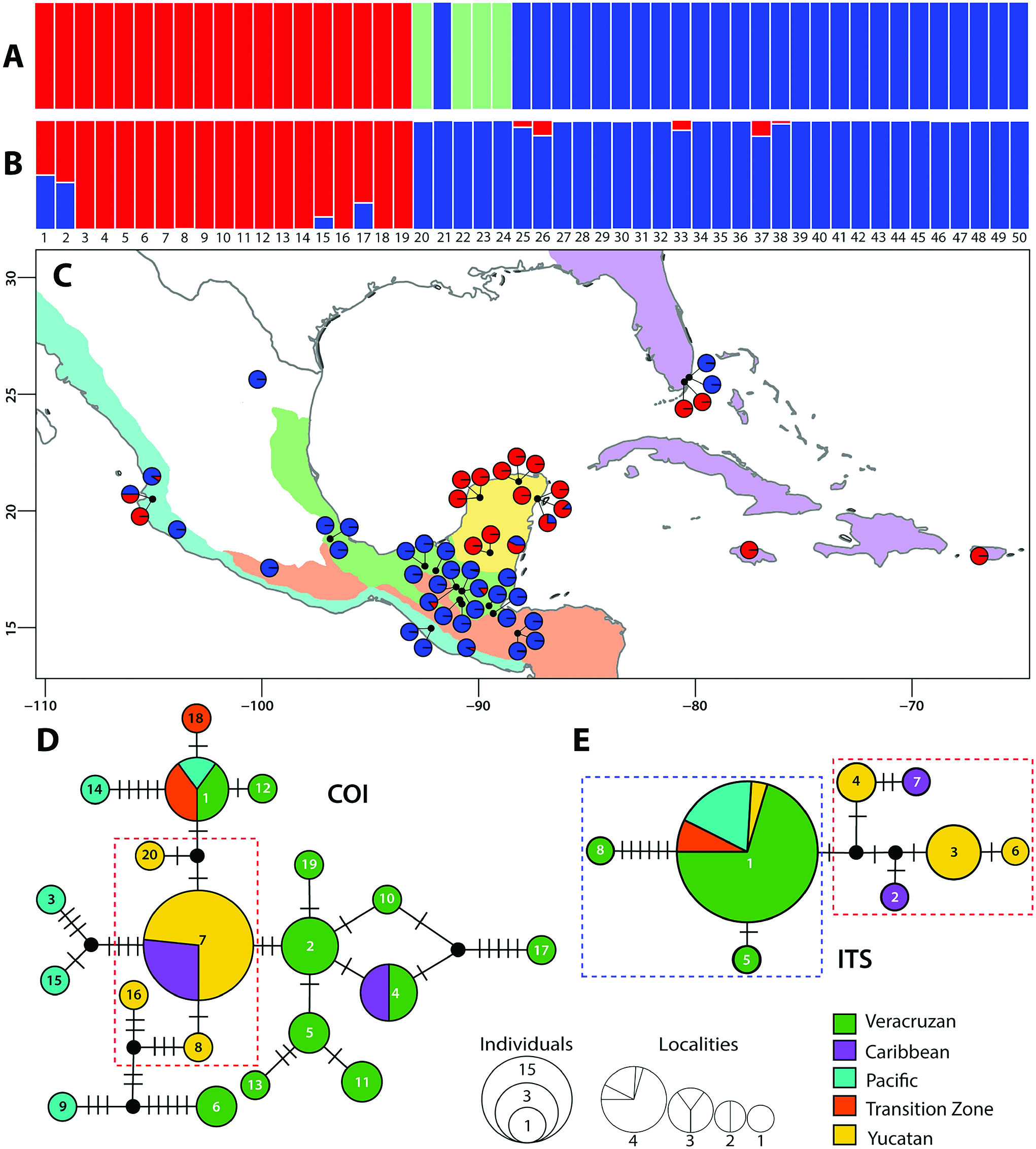
Phylogenetic studies confirm that many schizomids are short-range endemics ( Edward and Harvey 2008), species restricted to single localities or habitats (e.g., a cave system or biome with no apparent barriers). As such, unique haplotypes per population are expected. However, ITS and COI did not recover unique haplotypes for a single locality in the present study. Although surprising for a group of organisms with low vagility, it is impossible to compare with other taxa endemic to specific localities, as this is the first study addressing haplotype diversity in schizomids.
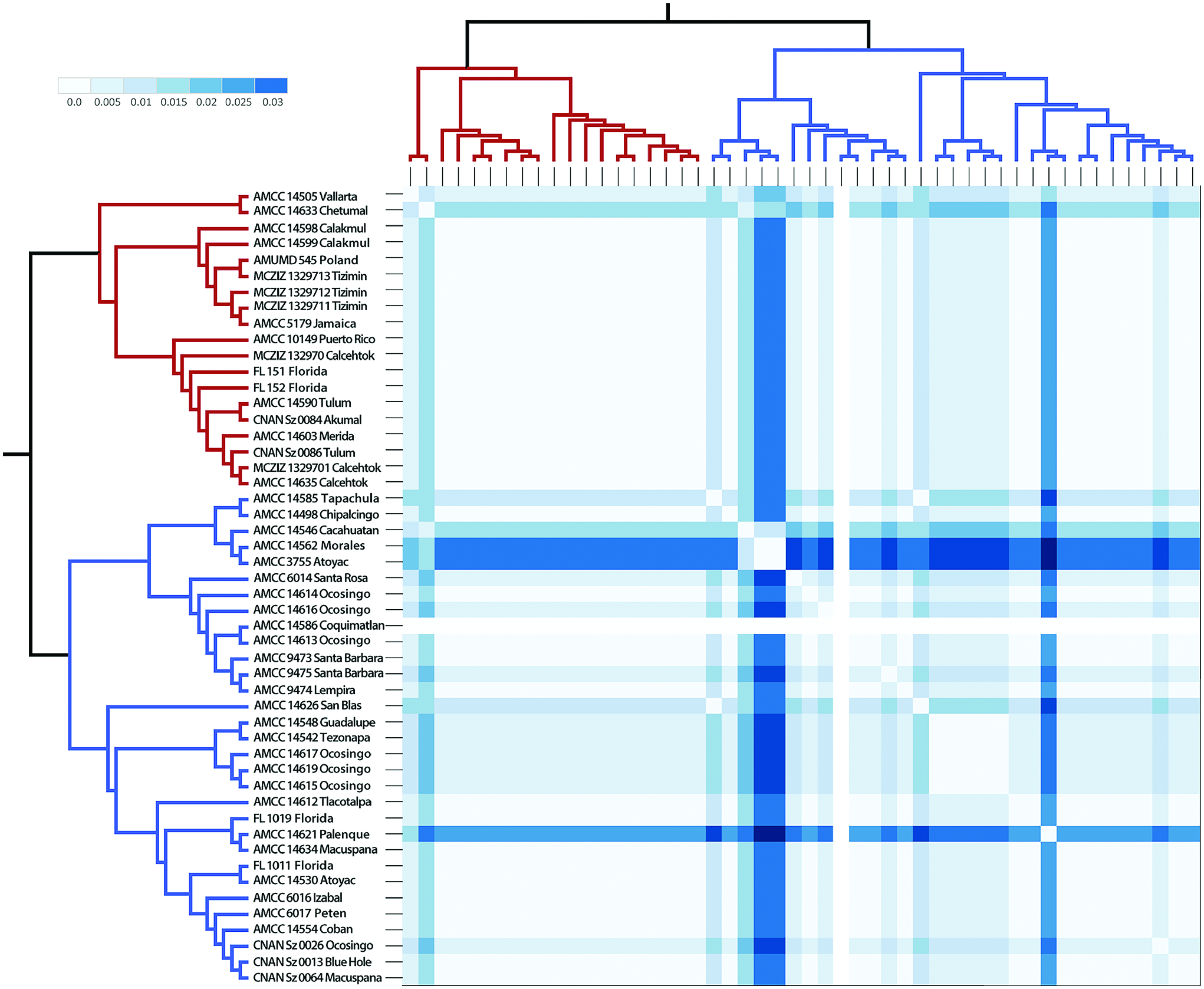
The population structure analysis revealed low genetic structure in S. portoricensis , consistent with the haplotype networks ( Fig. 2D and E View Fig ) and phylogenetic analyses, as well as with results of the AMOVA, in which most of the genetic variation is within and not among populations ( Table 3). Invasive or introduced species tend to possess less genetic diversity due to bottlenecks and/or founder events resulting from the colonization of new habitats by a few, or single individuals. However, cases of introduced species with high genetic diversity were the result of multiple re-introductions from the original source population ( Morim et al. 2019, Urquía et al. 2019). When comparing the subclades of S. portoricensis , less genetic diversity is evident in Subclade 1 than Subclade 2 ( Table 2). Additionally, Subclade 1 is mostly restricted to the Yucatán Peninsula and the Caribbean ( Fig. 2C View Fig ), suggesting several introductions may have occurred from the Caribbean to the Yucatán or vice versa, and from either source to other, more distant localities such as Jalisco and Europe. Additional evidence for multiple introductions of S. portoricensis is the percentage of admixture in both clusters (Subclades 1 and 2) suggested by the STRUCTURE analyses for samples 1, 2, 15, 17, 25, 26, 33, 37, and 38 ( Fig. 2B View Fig ) which implies hybridization between individuals of the two different clusters introduced from two different localities. The p-distances of the samples from Poland ( Fig. 4 View Fig ), although not included in the haplotype network, are identical to those from the samples of Subclade 1 ( Fig. 4 View Fig ). Several introductions of Subclade 1 to different localities are also supported by the ITS haplotype network, which recovered higher genetic diversity (Subclade 1, Hd = 0.83) than the COI (Subclade 1, Hd = 0.49), with five different haplotypes restricted to the Yucatán Peninsula and the Caribbean ( Table 2; Fig. 2 View Fig ).
Subclade 2 has a different evolutionary history which is nevertheless concordant with that of Subclade 1. Subclade 2 contains more genetic diversity in the COI, but less in the ITS, suggesting it is independent from Subclade 1 and restricted to southeastern Mexico, Guatemala, and Honduras. Although the haplotype networks reveal introductions, there have been fewer in comparison with Subclade 1, and these introductions occurred in continental areas, as well as in Florida.A common pattern observed among samples from Florida and Jalisco is the presence of haplotypes from both subclades, suggesting two different introductions from at least two different subclades.
Genetic and nucleotide diversity are also a measure of divergence time, as ancient lineages have had more time to accumulate diversity than recent lineages which are still evolving and adapting to new habitats ( Nei and Tajima 1981). Subclade 2 contains more genetic and nucleotide diversity in the COI dataset ( Table 2), greater genetic distances ( Fig. 4 View Fig ), and a more complex phylogenetic structure, as detected by the BAPS analyses and COI haplotype networks, than Subclade 1. This is consistent with the phylogenetic hypothesis of Stenochrus and the ancestral area reconstruction, which suggest that S. portoricensis originated in Mesoamerica and was subsequently introduced to the Caribbean and elsewhere in the New World and Europe. Therefore, S. portoricensis is probably not closely related to the schizomid fauna endemic to the Caribbean, although a comprehensive analysis including more Caribbean taxa is needed to test this hypothesis more rigorously.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.