Ctenoneura Hanitsch, 1925
|
publication ID |
https://doi.org/10.11646/zootaxa.4237.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:30330D9E-BC76-449B-9C99-2B5EEDA0F8F5 |
|
DOI |
https://doi.org/10.5281/zenodo.6053077 |
|
persistent identifier |
https://treatment.plazi.org/id/03D2F661-FFC8-FF96-658E-FA45FE0E1DAD |
|
treatment provided by |
Plazi |
|
scientific name |
Ctenoneura Hanitsch, 1925 |
| status |
|
Ctenoneura Hanitsch, 1925 View in CoL
Ctenoneura Hanitsch, 1925: 100 View in CoL ; Princis 1950: 204; Princis 1953: 207; Princis 1963: 101 (Catalogue); Roth 1993: 83 (Revision); Roth 1995b: 117 (Rediagnosis); Roth 2003: 34.
Type Species: Ctenoneura major Hanitsch, 1925 (Designated by Princis 1950)
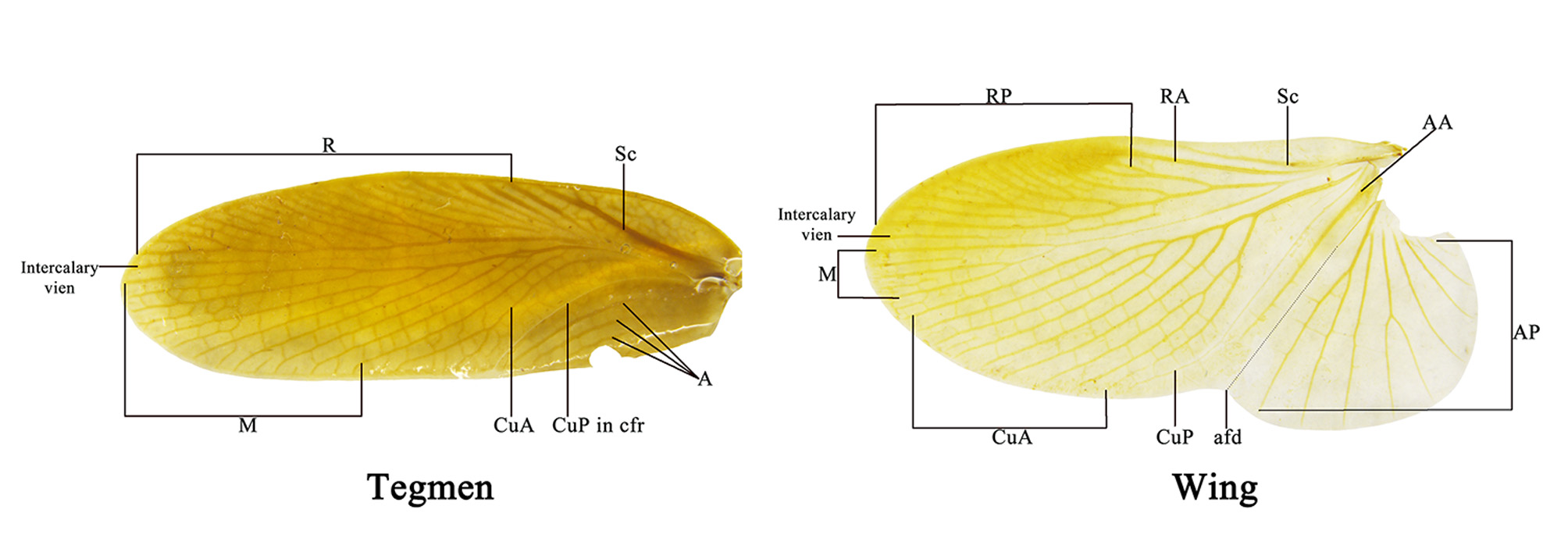
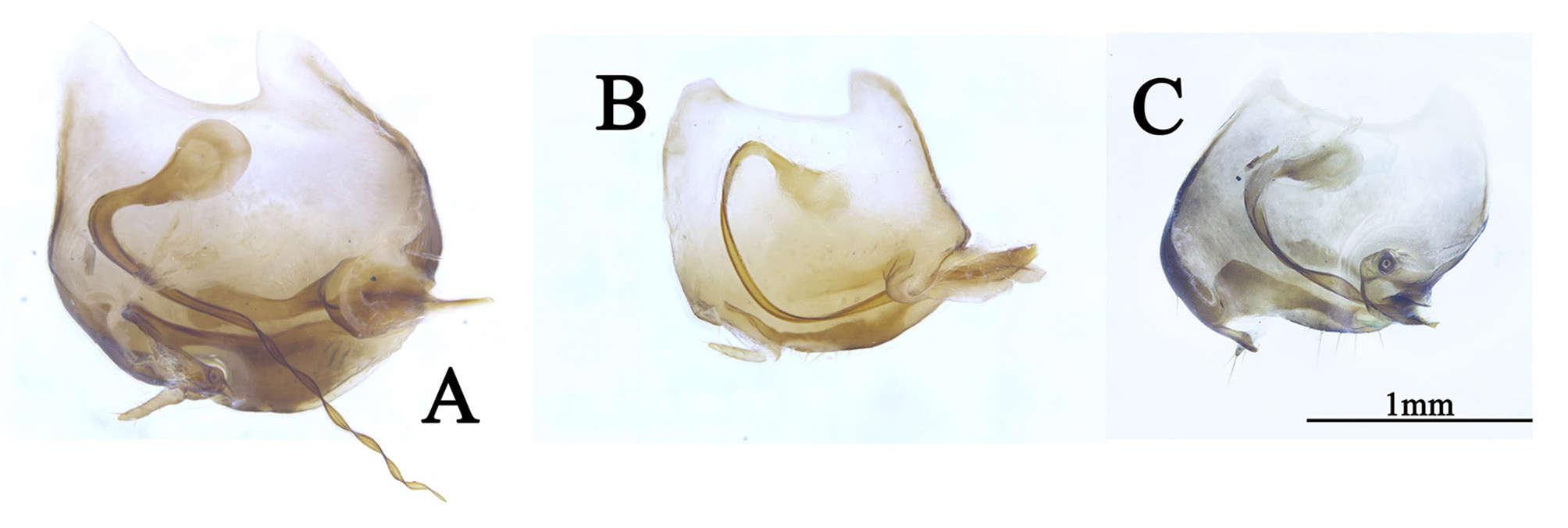
Diagnosis. This genus is peculiar among the Corydiidae . The small, narrow and smooth body distinctly differentiates it from many other Corydiidae genera whose body is pubescent and oval shaped. Roth (1995b) considered this genus most resembles genus Homopteroidea , a detailed generic comparison has been provided in Roth (1995b), we add the following comparison as supplement: 1) body smooth, not pubescent in Ctenoneura , while body smooth, with a few setae in Homopteroidea ; 2) tegmen with well-developed M, but simplified CuA in Ctenoneura ( Fig. 2 View FIGURE 2 ), while M simple, CuA developed into presutural vein in Homopteroidea ; 3) both tegmina and wings with an intercalary vein between R and M in Ctenoneura ( Fig. 2 View FIGURE 2 ), while without an intercalary vein in Homopteroidea ; 4) male genitalia simple, with reduced left phallomere, genital hook absent in Ctenoneura ( Fig. 1 D View FIGURE 1 ), while male genitalia extremely complex in Homopteroidea .
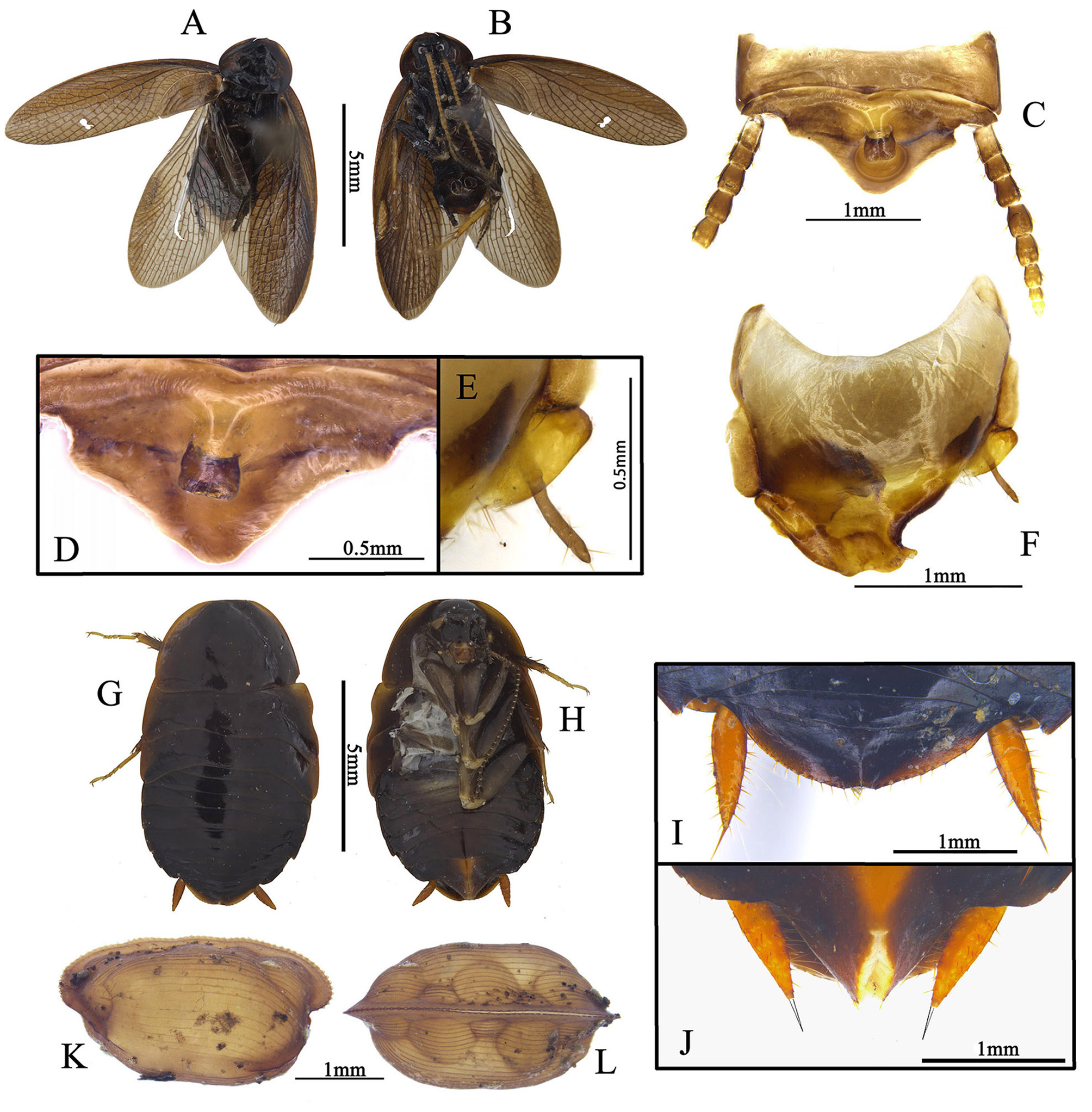
Redescription. Male. Small size, resembles small Ectobiidae cockroaches, body length (without wings) 5.0– 9.7 mm. Body smooth and shining, not pubescent, usually coloration ranging from brownish yellow to dark brown.
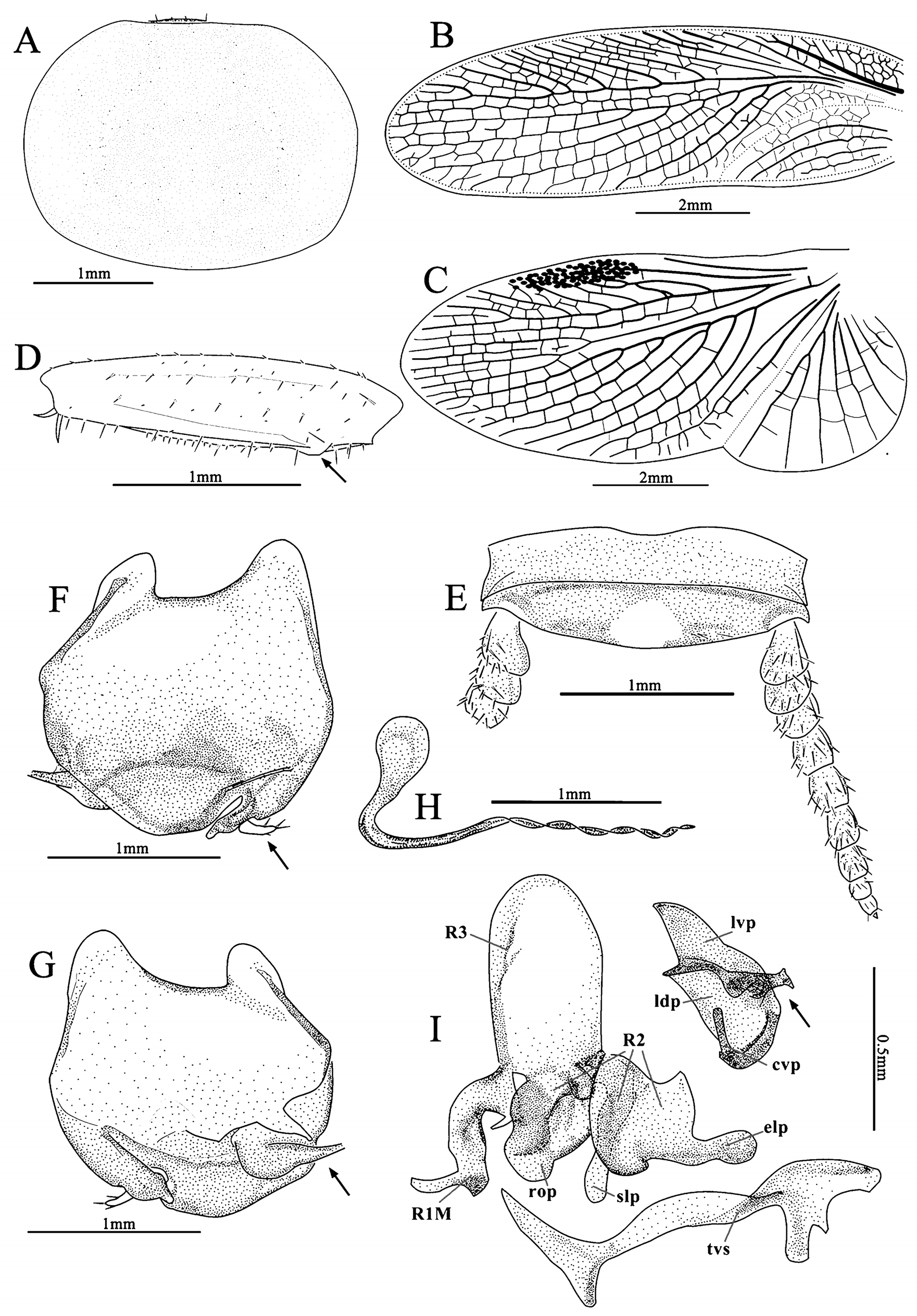
Head: hidden or not, vertex round, eyes wide apart, ocelli very small, nearly degenerate, antennal sockets round, large. Pronotum: generally subcircular or oval. Tegmina and wings: fully developed. Major veins often densely reticulate with numerous cross veins. Tegmina horny, Sc and branches of R usually oblique, M welldeveloped, usually with 3–6 branches, an intercalary vein present between R and M, sometimes incomplete, CuA usually simple, sometimes bifurcate; wings with a mass of spots between RA and RP, usually with an intercalary vein between R and M, sometimes incomplete, CuA usually with 3 to 8 branches that generally are parallel, and with numerous small cross veins connecting them, CuP usually slender, anal area folded over the rest of the wing in resting position as in other Corydiidae genera, AA thick, AP branches fan-like ( Fig. 2 View FIGURE 2 ). Legs: front femur Type C1, sometimes with small processes on hind margin basally and setae on the surface ( Figs. 23 View FIGURE 23 D; 24 D). Pulvilli absent, tarsal claws symmetrical, simple, arolia small or absent. Abdomen: abdominal terga unspecialized. Supra-anal plate transverse, with many different shapes. Cerci long, pubescent, sometimes the basal segments specialized (round or slightly protruded and directed medially, e.g. Fig. 20 View FIGURE 20 E). Subgenital plate asymmetrical, different from one species to another, usually with one stylus or none ( Roth (1993) stated some species without stylus). Some species with one slender and curved subgenital sclerite (sgs) present above the subgenital plate, below the genitalia, or integrated with subgenital plate, and an extending structure (eds) to hold the sgs ( Fig. 13 View FIGURE 13 ; 22 F–G).
Male genitalia: consists of three parts, viz. left phallomere, right phallomere and transverse sclerite (tvs). Left phallomere strongly reduced, two parts present, viz. left dorsal phallomere (ldp) and left ventral phallomere (lvp); lvp small, usually with irregular processes posteriorly, anterior usually acute, like a parrot peak; ldp connects lvp by the whole anterior margin, posterior with a curved part ( cvp), small, towards the anterior. Right phallomere consists of three parts, viz. R1M, R2 and R3, R1M irregular, R2 with three parts: a robust process (rop), a slender process (slp) and an elongate process (elp), R3 concave. Transverse sclerite (tvs) long, irregular ( Fig. 1 View FIGURE 1 ).
Female. Apterous, body smooth, eyes reduced, arolia absent, supra-anal plate transverse, suboval, apex slightly truncated, cerci robust, apex with a spine. Subgenital plate valvular ( Figs. 5 G–J View FIGURE 5 ).
Ootheca. Oval, with small serrations on the keel. The surface with many ribbed lines, eggs filled, can be seen in dorsal view ( Figs. 5 K–L View FIGURE 5 ).
Distribution. Oriental Region.
Natural history. Ctenoneura should be one kind of wood-feeding cockroach. For more details see “natural history” under C. heixuanfeng and C. delicata .
Discussion. The estimate of species abundance. Ctenoneura must be a genus rich in species in the Corydiidae . Members of this rarely-collected genus are mostly recorded from Southeast Asia. Former studies only found four species from China ( Bey-Bienko 1957, 1969), one species from Myanmar and one undescribed species from Vietnam ( Roth 1993).This paper adds three species from Yunnan, four species from Hainan Island and one unknown female from Guangxi. We suspect that there must be more new species in China, Myanmar and Vietnam .
Species groups suggestion. After dissecting the specimens, we found C. simulans , C. helicata sp. nov. and C. qiuae sp. nov. share some similar characters: 1) supra-anal plate simple, unspecialized; 2) subgenital plate with eds forming a groove, and separated sgs partly resting in the groove; 3) hind margin of subgenital plate usually with an incision or excavation, one stylus originating from the left of the incision or excavation. Here we build a simulans - group to contain C. simulans Bey-Bienko, 1969 , C. helicata sp. nov. and C. qiuae sp. nov. that all own the characters listed above. C. elongata sp. nov. is different from the typical simulans -group, it is distinct in owning an unseparated sgs and an extremely large eds; we include it in this group as an “aberrant” species. C. birmanica Princis , C. misera Bey-Bienko and C. yunnanea Bey-Bienko may belong to this group, but we lacked material to examine. The species from Hainan Island are quite distinguished from each other in the supra-anal plate and subgenital plate, but they all share some other identical characters: 1) supra-anal plate all specialized into different shapes; 2) subgenital plate protrudes toward right; and 3) body dark brown to brownish black, usually with small yellow areas in lateral fore borders of the pronotum. We suggest building a heixuanfeng -group for the Hainan species described in this article.
Discussion of females. The apterous female reported in this article is one important discovery on Ctenoneura . The previous records of the females ( Hanitsch 1925; Bey-Bienko 1957) indicates macropterous, but they are doubtful. Hanitsch (1925) described C. fulva based on six specimens, and C. major based on two specimens; within these were three macropterous females determined by Hanitsch himself. But there were two significant concerns about Hanitsch’s knowledge of Ctenoneura . One is the generic diagnosis of this genus having the male with two styli which later proved to have the male with one stylus or none. The other concern is about the type series of C.
fulva View in CoL . Roth (1993) has examined the type specimens of C. fulva View in CoL in the Museum of Oxford University. No females were found, but he discovered two new species within the type series of C. fulva View in CoL (one was described as C. murudensis View in CoL , the other was not described due to its bad condition). Such limited knowledge may lead to mistaken identification. At that time, the male subgenital plate of Ctenoneura View in CoL was poorly studied. Hanitsch probably mixed in other male species as the females of C. fulva View in CoL and C. major View in CoL . Bey-Bienko (1957) described C. acuticerca View in CoL based on two macropterous females, but this species may not belong to Ctenoneura View in CoL due to the absence of an intercalary vein which is an important character for Ctenoneura View in CoL . The discovery of the apteral female in this article may explain why the previous studies are absence of females; the sexual dimorphism may lead to overlooking the females.
Taxonomic status. So far the subfamily status of Ctenoneura View in CoL is still unclear. Princis (1950, 1953, 1963, 1967) put this genus in Latindiinae View in CoL / Latindiidae without giving any reasons, Roth (1993, 1995b, 2003) and Beccaloni (2014) did not place it in any subfamily. The many distinctive characters (the much reduced left phallomere, the absence of genital hook, specialized and asymmetrical subgenital plate, single stylus, smooth body, intercalary vein presenting in tegmina and wings, well developed M and simplified CuA in tegmen) make it unique in the Corydiidae View in CoL . It may belong to a new subfamily named Ctenoneuriinae. However, more Ctenoneura View in CoL examples need to studied, more comparison studies within Corydioidea genera need to be done, and molecular methods need to be used to solve this problem.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Corydioidea |
|
Family |
Ctenoneura Hanitsch, 1925
| Qiu, Lu, Che, Yan-Li & Wang, Zong-Qing 2017 |
Ctenoneura
| Roth 2003: 34 |
| Roth 1995: 117 |
| Roth 1993: 83 |
| Princis 1963: 101 |
| Princis 1953: 207 |
| Princis 1950: 204 |
| Hanitsch 1925: 100 |