Microsciurus flaviventer ( Gray, 1867 )
|
publication ID |
https://doi.org/10.1093/mspecies/sew006 |
|
publication LSID |
lsid:zoobank.org:pub:2CC091BB-538C-4575-AB90-D5C2A7CADC88 |
|
persistent identifier |
https://treatment.plazi.org/id/03D187D7-FF87-7E3E-FF01-FAFE6E80FB94 |
|
treatment provided by |
Felipe |
|
scientific name |
Microsciurus flaviventer ( Gray, 1867 ) |
| status |
|
Microsciurus flaviventer ( Gray, 1867) View in CoL
Amazon Dwarf Squirrel
Macroxus flaviventer Gray, 1867:432 . Type locality “ Brazil;” restricted to “the area of Pebas,” by Cabrera (1961) based on information published by Thomas (1928).
Sciurus chrysurus: Thomas, 1893:337 . Part, not chrysurus Pucheran (1845:337) .
Sciurus peruanus Allen, 1897:115 . Type locality “Guayabamba [= Cajabamba, Cajamarca province], alt. 4000 feet [ 1219.2 m].”
Sciurus similis Nelson, 1899:78 . Type locality “Cali, Cauca Valley, Colombia (alt. 6000 ft. [ 1828.8 m]).”
© 2016 by American Society of Mammalogists.
Sciurus simonsi Thomas, 1900:294 . Type locality “Porvenir, near Zaparal, Province of Bolivar, Ecuador. Altitude 1500 m.”
Sciurus ( Microsciurus) peruanus napi Thomas 1900:295 . Type locality “Mouth of Coca River, upper Rio Napo.”
Sciurus otinus Thomas 1901:193 . Type locality “Medellin, Colombia.”
Microsciurus otinus: Allen, 1914:156 . Name combination.
Microsciurus simonsi: Allen, 1914:161 . Name combination.
Microsciurus peruanus: Allen, 1914:161 . Name combination.
Microsciurus napi: Allen, 1914:163 . Name combination.
Microsciurus rubrirostris Allen, 1914:163 . Type locality “Chanchamayo, central Peru; altitude 2000 m.”
Microsciurus florenciae Allen, 1914:164 . Type locality “Florencia (altitude 1000 feet [ 304.8 m]), Caquetá district, Colombia.” Microsciurus avunculus Thomas, 1914: 574 . Type locality “Oriente of Ecuador. Type from Gualaquiza; alt. 2500’ [ 762 m]”.
Microsciurus rubricollis Thomas, 1914: 574 . No type locality given; incorrect subsequent spelling of Microsciurus rubrirostris Allen, 1914 .
Microsciurus manarius Thomas, 1920:275 . Type locality “Acajutuba, Rio Negro, near its mouth.”
Microsciurus sabanillae Anthony, 1922:2 . Type locality “Sabanilla, Prov. de Loja, Ecuador; altitude, 5700 feet [ 1737.36 m].”
Microsciurus flaviventer: Thomas, 1928:290 View in CoL . First use of current name combination.
CONTEXT AND CONTENT. Order Rodentia View in CoL , suborder Sciuromorpha View in CoL , superfamily Sciuroidea , family Sciuridae View in CoL , subfamily Sciurinae View in CoL , tribe Sciurini View in CoL . Moore (1959) placed Microsciurus View in CoL in the tribe Microsciurini .
Eight subspecies of Microsciurus flaviventer are currently recognized (Thorington and Hoffmann 2005):
M. f. flaviventer ( Gray, 1867:432) . See above; manarius Thomas is a synonym.
M. f. napi ( Thomas, 1900:295) . See above; avunculus Thomas and florenciae Allen are synonyms.
M. f. otinus ( Thomas, 1901:193) . See above.
M. f. peruanus ( Allen, 1897:115) . See above.
M. f. rubrirostris Allen, 1914:163 . See above; rubicollis Thomas is a synonym.
M. f. sabanillae Anthony, 1922:2 . See above.
M. f. similis ( Nelson, 1899:78) . See above.
M. f. simonsi ( Thomas, 1900: 294) . See above.
NOMENCLATURAL NOTES. The Spanish common name for Microsicurus flaviventer is la ardilla enana amazónica (BuitronJurado and Tobar 2007). Ecuadorian common names for M. flaviventer are ardilla chica, ardilla chiquita, and ardillita ( Tirira 2004). The Oriente names for M. flaviventer are ardilla negra, ardilla negrita, ardilla de bolsillo, ardilla de la pequeña, ardilla matapalera, ardilla matapalo, and ardilla voladora ( Tirira 2004). The Achuar-shuar name for M. flaviventer is wíchi n k and kunamp-rush ( Tirira 2004). The Cofán name for M. flaviventer is tiriri ( Tirira 2004). The Huaorani name for M. flaviventer is kemo ( Tirira 2004). The Quichua del oriente name for M. flaviventer is shitipu ( Tirira 2004). The Shiwiar name for M. flaviventer is konam and wíching ( Tirira 2004). The Siona-se-coyas name for M. flaviventer is kwiri sisi ( Tirira 2004). The Matses name for M. flaviventer is kapa kudu (Fleck and Voss 2006). The Andoke name for M. flaviventer is I’x’siko (Vasco-Palacios et al. 2008). The Muinane name for M. flaviventer is Tyityio (VascoPalacios et al. 2008). The Uitoto name for M. flaviventer is Nopi (Vasco-Palacios et al. 2008).
Recent evidence suggests that Microsciurus is polyphyletic (Mercer and Roth 2003; Steppan et al. 2004; Pečnerová and Martínková 2012) as M. flaviventer and M. alfari occurred on separate nodes (Pečnerová and Martínková 2012). There is no consensus on the location of M. flaviventer within the phylogenetic tree and support for positions is weak, perhaps due to markers used in analyses, mtcyb, irbp, 12S ribosomal, 16S ribosomal, and c-myc exon 3, which are better predictors of deep nodes than recent nodes (Mercer and Roth 2003; Steppan et al. 2004; Pečnerová and Martínková 2012). Additionally, the congener M. alfari is positioned more distant from M. flaviventer than expected (Pečnerová and Martínková 2012). Further work is required to elaborate on the phylogenetic relationship of Microsciurus and sister taxa (Mercer and Roth 2003). A detailed analysis on the evolutionary relations of M. flaviventer subspecies is also recommended (Pečnerová and Martínková 2012).
DIAGNOSIS
The only sympatric congeners of Microsciurus flaviventer are M. mimulus (western dwarf squirrel— Eisenberg 1989; Emmons and Feer 1997; Eisenberg and Redford 1999) and M. santanderensis ( Santander dwarf squirrel— Thorington et al. 2012). In western Colombia, M. flaviventer differs from M. mimulus in that M. flaviventer is larger ( M. mimulus is about 81% the body size of M. flaviventer ). In M. flaviventer , length of head and body is 120–160 mm with a mass of 80–132 g ( Eisenberg 1989; Eisenberg and Redford 1999; Hayssen 2008; Thorington et al. 2012), compared to 128–150 mm in body length and mass of 120 g in M. mimulus (Emmons and Feer 1997; Hayssen 2008). M. flaviventer has an agouti brown dorsal pelage and a banded tail ( Fig. 1 View Fig ), whereas M. mimulus has a grizzled black dorsum with a pale orange buff on hair tips and no rings on its tail (Eisenberg and Redford 1999). M. santanderensis possesses a faint black spot on the top of the head with a cinnamon tinge, a faint middorsal black line and a pinkish buff venter (Borrero-H and Hernandez-Camacho 1957) but overlaps with M. flaviventer in all external measures (length of head and body [mm], 133.5–156.0— Eisenberg 1989; Hayssen 2008).
Subspecies differ slightly in pelage color. M. f. flaviventer can be distinguished from other subspecies by a darkish olive pelage and the middle of the dorsum is darker than other subspecies ( Thorington et al. 2012). M. f. peruanus and M. f. napi have large white postauricular patches; however, M. f. napi can be distinguished by a yellowish rufous venter ( Thomas 1900; Allen 1914; Thorington et al. 2012). M. f. rubrirostris has a yellow frosted tail and orange ochraceous venter ( Allen 1914; Thorington et al. 2012). Similar to M. f. rubrirostris , M. f. sabanillae has an ochraceous venter; however, M. f. sabanillae lacks postauricular patches ( Anthony 1922; Thorington et al. 2012). M. f. simonsi is an unspotted form with a yellow eye ring and fulvous venter ( Thomas 1900; Thorington et al. 2012). M. f. otinus is an isolated subpopulation in central northern Colombia and has white tipped ears and white frosted tail ( Thomas 1901; Thorington et al. 2012). Another isolated subspecies is M. f. similis in southern Colombia, and lacks auricular and postauricula patches and has an orange rufous venter ( Nelson 1899; Allen 1914; Thorington et al. 2012).
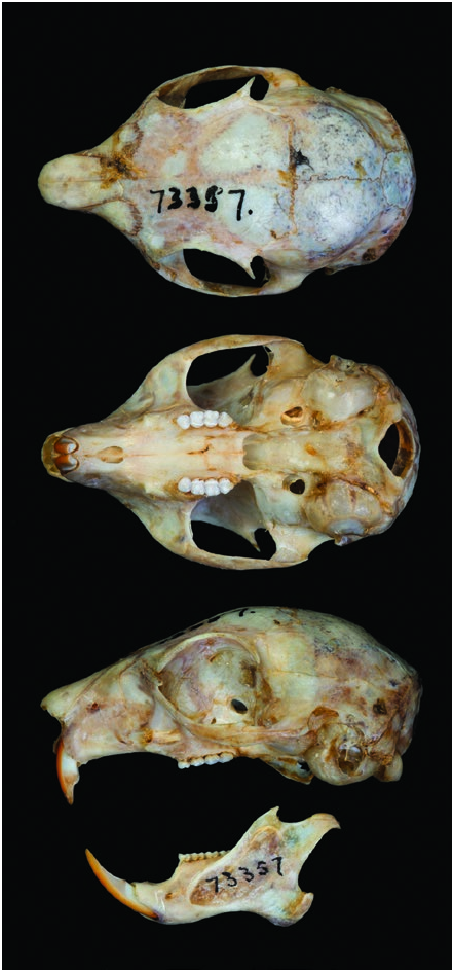
Other small squirrels sympatric with M. flaviventer are the Neotropical pygmy squirrel ( Sciurillus pusillus ), Sanborn’s squirrel ( Sciurus sanborni ), Guianan squirrel ( Sciurus aestuans ), and Bolivian squirrel ( Sciurus ignitus —Emmons and Feer 1997). S. pusillus is considerably smaller (length: 89–115 mm; mass 33–45 g) than M. flaviventer ( S. pusillus is about 35% the body mass of M. flaviventer ) and has a pale gray pelage, compared to the agouti brown pelage of M. flaviventer (Emmons and Feer 1997) . S. sanborni (length: 152–175 mm; mass not available), S. aestuans (length: 160–202 mm; mass 159–218 g), and S. ignitus (length: 180–195 mm; mass 225–240 g) are distinguishable from M. flaviventer by long ears that protrude above the crown of the head (Emmons and Feer 1997). Furthermore, M. flaviventer is distinguishable from other Sciurus species by several cranial features. In M. flaviventer , the postorbital process is almost directly over the base of the posterior root of the zygomatic arch, the postglenoid foramen pierces the squamosal bone, the upper 3rd premolars are present and the upper incisors are pro-odont, and there is 1 pair of transbullar septae ( Fig. 2 View Fig ; Salazar-Bravo et al. 2002).
GENERAL CHARACTERS
Microsciurus flaviventer has a reddish brown to olivaceous dorsum with a small, pale yellow postauricular patch ( Gray 1867; Patton et al. 2000). Venter ranges in color from yellow, pale orange, or grayish. The venter is brightest in the chest area and not sharply defined from the dorsal pelage (Emmons and Feer 1997). The ears are very short (below the crown of the head) and are covered in short, pale hairs. The tail is shorter than the body, slender and tapered toward the tip, and is faintly banded with pale yellow to orange and frosted with white hair (Emmons and Feer 1997; Eisenberg and Redford 1999; Patton et al. 2000; Fig. 1 View Fig ).
Length of head and body in M. flaviventer ranges between 120 and 160 mm; length of tail is 80–155 mm; hind foot length is 39–43 mm; and ear length is 16–17 mm ( Eisenberg 1989; Eisenberg and Redford 1999). M. flaviventer body mass ranges from 86.2 to 132 g (Eisenberg and Redford 1999; Hayssen 2008). Measurements taken from confirmed male and female specimens suggest that females are slightly smaller than males in head and body length (males = 136.3 mm, females = 133.5 mm), tail length (males = 110 mm, females 109.9 mm), and mass (males = 98.0 g, females = 86.2 g — Hayssen 2008).
The coronoid process in M. flaviventer is reduced and anteriorly displaced relative to arboreal dwarf squirrels (flying squirrels, Petaurillus and Petinomys ) and the condylar process is reduced to low prominence and is recurved to form a hook ( Hautier et al. 2009). The lower mandible in M. flaviventer is distinguishable from phylogentically related species of Sciurus by slight differences, including a shortened coronoid, an elongated condyloid, and a smaller diastema (Swiderski and Zelditch 2010). Ranges (mm) of cranial measurements from 22 M. flaviventer specimens were: greatest length of skull, 35.3–40.3; greatest zygomatic breadth, 20.5–24; interorbital breadth, 12.4–14.3; breadth of braincase, 17.6–19; and length of nasals, 9–11 ( Allen 1914). M. flaviventer can be distinguished from S. pusillus by a smaller skull (greatest length of skull, 28.1 mm; greatest zygomatic breadth, 19.6; interorbital breadth, 11.6; breadth of braincase, 15.0; and length of nasals, 8.0— Jessen et al. 2013).
DISTRIBUTION
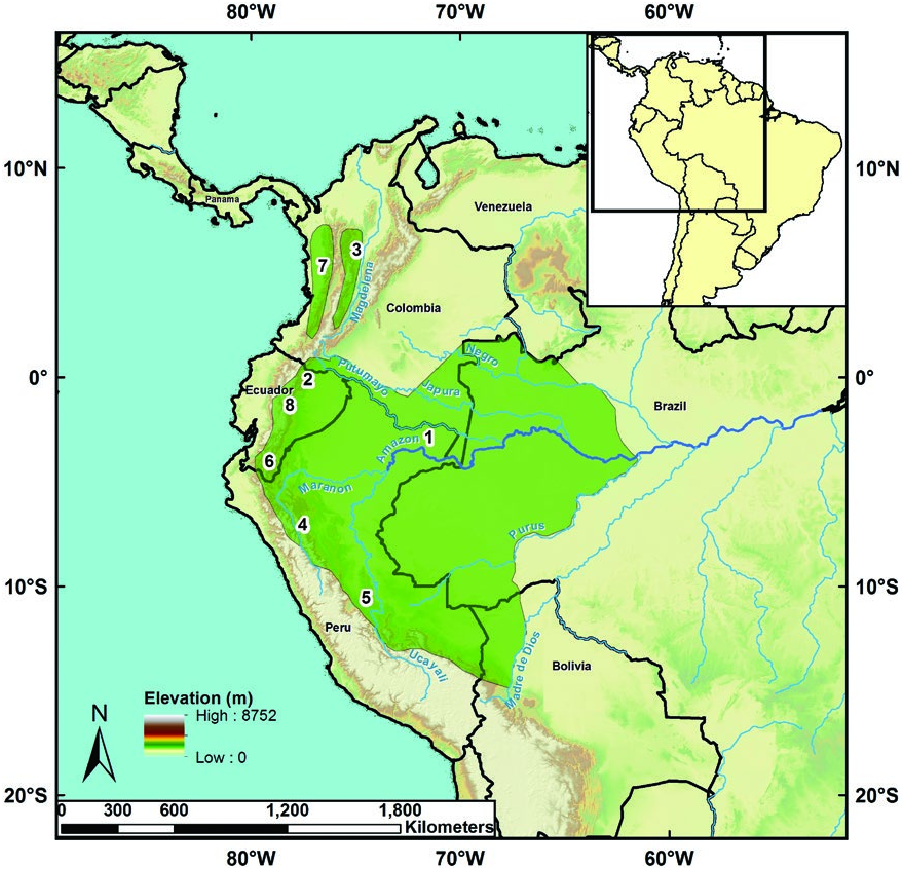
Microsciurus flaviventer is found in the Amazon basin of South America throughout western and southeastern Colombia, Ecuador, southern Perú, Brazil west of Río Negro, and Madeira (Emmons and Feer 1997; Eisenberg and Redford 1999; Patton et al. 2000). Recent observations in Santa Rosa, Bolivia (Salazar-Bravo et al. 2002) represent the southernmost recorded distribution ( Fig. 3 View Fig ). M. flaviventer occurs primarily in lowland and foothill regions of the Andes up to 2,000 m in elevation (Emmons and Feer 1997; Eisenberg and Redford 1999).
There are 2 groups of subspecies: 1 group of 2 subspecies occurs in western Colombia, whereas a 2nd group, containing the remaining 6 subspecies, occurs in the Amazon basin. The distribution of the 8 recognized subspecies of M. flaviventer cannot be fully ascertained beyond where type specimens were collected, and accurate geographical boundaries that separate each of these subspecies remain unclear ( Fig. 3 View Fig ). No fossils of M. flaviventer are known.
FORM AND FUNCTION
In the genus Microsciurus , normal vertebral numbers are 7 C, 12 T, 7 L, and 3 S, total 29. Nevertheless, some specimens of M. flaviventer have been documented to have 4 sacral vertebrae (Thorington and Thorington 1989). The dental formula for M. flaviventer is i 1/1, c 0/0, p 2/1, m 3/3, total 22 ( Allen 1914).
The foreleg limb bones of M. flaviventer are unique in their proportions, with the humerus equal in length to the radius. Longer forelimbs in the Amazon dwarf squirrel increase the squirrel’s ability to climb larger diameter trees and to allow the squirrel to spend considerable time in a vertical position on the tree while foraging (Thorington and Thorington 1989). In tree squirrels, forelimb muscles from the trunk attach at midshaft on the humerus, decreasing the mobility of the shoulder. Longer forelimbs can compensate for this lack of mobility and allow the squirrel to climb larger trees (Thorington and Thorington 1989). Additionally, longer hind limbs of M. flaviventer (86–92% of the body length versus 71–72% in larger squirrel species) provide extra force during leaping and bounding (Thorington and Thorington 1989; Youlatos 1999). M. flaviventer , a smaller squirrel, needs more force to leap over the same gap as a larger squirrel, and M. flaviventer compensates with longer hind limbs (Thorington and Thorington 1989).
The mandible demonstrates a strong trend toward dwarfism with an abbreviated coronoid process anteriorly displaced and a condylar process that is reduced to a low prominence and is curved backward like a hook ( Fig. 2 View Fig ; Hautier et al. 2009); however, the coracoid process is poorly developed relative to dwarf flying squirrels ( Petaurillus — Michaux et al. 2008; Hautier et al. 2009). It is likely that dwarf squirrels use their coronoid process for grasping and removing fragments of bark from trees ( Hautier et al. 2009).
ONTOGENY AND REPRODUCTION
There is almost no information describing the ontogeny and reproduction of Microsciurus flaviventer . Documented litter size is small, with 1 female specimen carrying 2 embryos (Eisenberg and Redford 1999). A lactating female Microsciurus flaviventer was recorded in November by Hice (2003). Six mammae occur in females ( Allen 1914; Emmons and Feer 1997; Eisenberg and Redford 1999). At the headwaters and central Rio Juruá, 4 male specimens with scrotal testes were collected in October, the dry season, and February, the wet season ( Patton et al. 2000).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Microsciurus flaviventer ( Gray, 1867 )
| Jessen, Timothy G., Kilanowski, Allyssa L., Gwinn, R. Nathan, Merrick, Melissa J. & Koprowski, John L. 2016 |
Microsciurus flaviventer : Thomas, 1928:290
| THOMAS, O. 1928: 290 |
Microsciurus sabanillae
| ANTHONY, H. E. 1922: 2 |
Microsciurus manarius
| THOMAS, O. 1920: 275 |
Microsciurus otinus :
| ALLEN, J. A. 1914: 156 |
Microsciurus simonsi :
| ALLEN, J. A. 1914: 161 |
Microsciurus peruanus :
| ALLEN, J. A. 1914: 161 |
Microsciurus napi :
| ALLEN, J. A. 1914: 163 |
Microsciurus rubrirostris
| ALLEN, J. A. 1914: 163 |
Microsciurus florenciae
| ALLEN, J. A. 1914: 164 |
| THOMAS, O. 1914: 574 |
Microsciurus rubricollis
| THOMAS, O. 1914: 574 |
Sciurus otinus
| THOMAS, O. 1901: 193 |
Sciurus simonsi
| THOMAS, O. 1900: 294 |
Sciurus ( Microsciurus ) peruanus napi
| THOMAS, O. 1900: 295 |
Sciurus similis
| NELSON, E. W. 1899: 78 |
Sciurus peruanus
| ALLEN, J. A. 1897: 115 |
Sciurus chrysurus :
| THOMAS, O. 1893: 337 |
| PUCHERAN, J. 1845: ) |
Macroxus flaviventer
| GRAY, J. E. 1867: 432 |