Trichoferus campestris (Faldermann)
|
publication ID |
https://doi.org/10.1649/0010-065X-64.1.13 |
|
persistent identifier |
https://treatment.plazi.org/id/03CD87DB-9678-FF96-FD0C-FE03422AFC46 |
|
treatment provided by |
Carolina |
|
scientific name |
Trichoferus campestris (Faldermann) |
| status |
|
Trichoferus campestris (Faldermann) View in CoL
campestris Faldermann, 1835: 435 ( Callidium) View in CoL . Type locality: “ China borealis”. Type series: single female ( holotype), in Zoological Institute , St. Petersburg, Russia (not examined).
= turkestanicum Heyden, 1886: 193 ( Stromatium View in CoL )
= rusticus Ganglbauer, 1886: 133 ( Hesperophanes)
= flavopubescens Kolbe, 1886: 219 ( Hesperophanes)
Material Examined. Adults: ♂, CANADA: Quebec, Repentigny, 05–VIII–2002, P. Chagnon, CVCCOLVG00000051; ♀, same data as ♂, except 10–VII–2006, CVCCOLVG00000052. Numerous adults, larvae and a single pupa intercepted by CFIA inspectors mainly at the Port of Vancouver from wooden dunnage in containers arriving from P. R. China (including Hong Kong) .
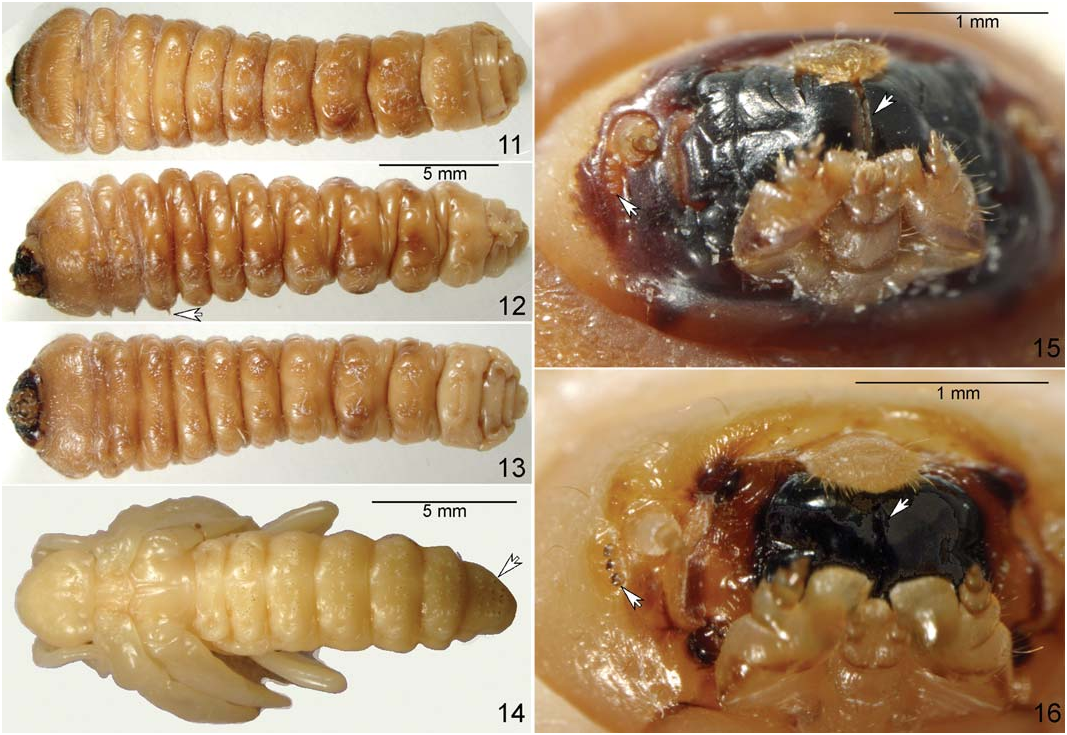
Morphological Description. A d u l t. L e n g t h 16 mm (n=2) and parallel-sided, uniformly brownblack ( Figs. 1, 2 View Figs ), not shiny; antenna about 90% and 70% of body length in male and female, respectively; body dorsally with short, uniform pubescence and with sparsely distributed, long, erect setae exceeding pilosity height by 3–4 times; pronotum with fine uniform sculpture; male genitalia as in Figs. 7 and 8 View Figs ; female genitalia as in Figs. 9 and 10 View Figs . Adult sexual dimorphism. Females with pronotum narrower in relation to elytra ( Figs. 2, 5 View Figs ) than in males ( Figs. 1, 4 View Figs ); posterior margin of abdominal sternum VI (visible ventrite 4) straight in females and widely notched at middle in males. Pupa. Body length 18 mm (n=1); whitebeige ( Fig. 14 View Figs ); with sclerotized dark spines on terga, most noticeable on tergum VI. Mature larva. Length 15–30 mm (n=10); white when live and white-beige when blanched and stored in alcohol; with six short thoracic legs ( Figs. 11–13 View Figs ); abdominal apex without urogomphi or other projections or sclerotized structures; labrum much narrower than clypeus; mandibles with the spoon-shaped apices meeting at the medial line; each side of head with three stemmata arranged in vertical row ( Fig. 15 View Figs , left arrow); anterior part of head capsule dark.
Morphological Diagnosis. An attempt to identify adults of T. campestris using available keys for the North American Cerambycidae ( Linsley 1962; Yanega 1996; Turnbow and Thomas 2002; Lingafelter 2007) lead to the native H. pubescens , the only species of the Trichoferus-Hesperophanes complex native to the Western Hemisphere ( Figs. 3, 6 View Figs ). Hesperophanes pubescens is distributed throughout eastern North America from Quebec and Ontario south to Georgia and Alabama and west to Minnesota and Iowa ( Linsley 1962), is rarely encountered, and its host plant, an undetermined oak species ( Quercus sp. ), was only recently discovered (Vlasak and Vlasakova 2002). Both species are known to occur near Montreal, Quebec (McNamara 1991). Adults of T. campestris are somewhat darker ( Figs. 1, 2 View Figs as compared to Fig. 3 View Figs ) and can be distinguished from H. pubescens by the presence of a few sparse, long, erect setae protruding above the pubescence ( Fig. 5 View Figs ; best visible on lateral side of pronotum just dorsal to the profemora). Cerambycidae pupae are too inadequately known to allow pupal diagnosis of T. campestris ( Fig. 14 View Figs ), although the latter has unusual sharp, sclerotized dark projections on abdominal terga I– VI, most developed on tergum VI. Mature larvae of the genus Trichoferus , having all characteristic features of the subfamily Cerambycinae (such as short legs, mandibles with spoon-shaped apices meeting at the medial line ( Figs. 15, 16 View Figs , arrow), labrum much narrower than clypeus; see more in Švácha and Danilevsky 1988), differ from most others by having three stemmata (instead of two, one, or none) arranged in a vertical row ( Fig. 15 View Figs ). This rare feature is known elsewhere in Cerambycinae only from Hesperophanes, Hylotrupes Serville, 1834 , and a few others, most notably, although not exclusively, members of the tribe Cerambycini that are exotic to North America. Larvae of Hylotrupes bajulus (Linnaeus, 1758) , a Palaearctic species established in the USA since at least the last century ( Linsley 1964), is only doubtfully known from Canada, based on a single Quebec specimen collected in the 1800s ( Laplante 1989; McNamara 1991). Larvae of that species are most reliably distinguished from those of Trichoferus by their lightercolored head capsules ( Fig. 16 View Figs ), especially by the lack of pigmentation behind the stemmata. Švácha and Danilevsky (1988) used the presence of “large rugose transverse protuberance immediately behind stemmata” to distinguish larvae of Hesperophanes from those of Trichoferus , although they did not have larvae of the North American H. pubescens , which are still unknown.
Molecular Identification. A GenBank Blast search indicated that the sequence most similar to that of the single sequenced specimen (see above) was that of T. campestris , accession number DQ224241 View Materials , 454 nucleotides long, with Identities= 407/417 (97%), uploaded by An et al., Jiangsu Entry-Exit Inspection and Quarantine Bureau, Laboratory of Plant Quarantine, 99 Zhonghua Road, Nanjing, Jiangsu 210001, China. This is the only orthologous sequence of a Hesperophanini beetle currently available at GenBank (accessed on February 4, 2009). The second most similar sequence was EU877950 View Materials , corresponding to Shaperius Waltl sp. ( Sphaeriusidae ), a myxophagan beetle, with Identities=83%. A Barcode of Life search indicated that the three most similar sequences were those of a species named “ campestris ” (either under the generic name Trichoferus or Hesperophanes), with the following associated data: “ H. campestris, CERPA 289-08, Canada, British Columbia ” (two sequences, similarity 98.27 and 98.14%), and T. campestris , Russia, Primorskiy Kray, similarity 98.25%. The origin of these two “ H. campestris ” specimens marked “ British Columbia ” is unclear, because the species has never been reported to be found in western Canada, except for specimens intercepted in imported goods at Canadian ports of entry. Specimens of H. pubescens ranked in similarity number four and five with the values of 84.81% and 84.42%, respectively. These five sequences were, apparently, all of the currently sequenced representatives of Hesperophanini uploaded at the Barcode of Life site (accessed on February 04, 2009).
Taxonomic Identity and Position. The identity of the species commonly referred to as “ T. campestris ” is far from certain. As currently accepted, this species has a relatively widespread distribution (see below) extending throughout half of the Palaearctic region. Numerous narrowly distributed xerophilous Trichoferus species have been described from the eastern part of the Mediterranean region ( Sama 1994; Kadlec and Rejzek 2001; Sama and Makris 2001; Kadlec 2005; Sama et al. 2005) and sometimes it is difficult to distinguish them from the sympatrically distributed T. campestris . This species is well-known partly because it is widespread and locally abundant in the eastern part of its native range. As a result, the majority of Trichoferus interceptions from P. R. China and surrounding territories were attributed to this species, although there is no certainty whether they might or might not be conspecific with the true T. campestris . Both Trichoferus and Hesperophanes require careful taxonomic revision to delimit species concepts and to solve the longstanding uncertainty on the taxonomic status of Trichoferus , which is considered as either a valid genus (Plavil’ shchikov 1940; Gressitt 1951; Švácha and Danilevsky 1988), a subgenus ( Villiers 1978; Turnbow and Thomas 2002; Niisato 2007), or a junior synonym ( Linsley 1962; Monné and Hovore 2006) of Hesperophanes. This issue, however, is outside the scope of the present paper.
Native Distribution. Trichoferus campestris is native to the southeastern part of the Palaearctic region from Japan ( Niisato 2007), the Russian Far East, Korean peninsula, Mongolia and most of P. R. China ( Gressitt 1951; Cherepanov 1981), continuing westwards through the southern Ural Mountains in Russia ( Shapovalov et al. 2006) and Central Asia eastwards up to Armenia and the southeastern part of European Russia (Danilevsky and Miroshnikov 1985). It is not known to be native to Central and Western Europe.
Biology. Iwata and Yamada (1990) indicated about 40 genera of woody spermatophyte plants, both conifers and angiosperms, as host plants for T. campestris and concluded that this species can potentially attack most woody plants. Švácha and Danilevsky (1988) reported that the larvae live under bark and in dry dead wood and complete their development in two or more years. Adults emerge from July to August and readily fly.
Pathways, Quarantine Significance, and Likelihood of Establishment in North America. Like most other alien Cerambycidae ( Cocquempot 2006) , the Montreal specimens of T. campestris most likely arrived in North America via international solid wood-packaging material (=dunnage). Dunnage is normally made from low quality wood that is often infested by live wood-dwelling insects, predominantly beetles. The genus Trichoferus (51 records under the names Hesperophanes and two records under the name Trichoferus ) was the fourth most commonly intercepted cerambycid genus at the US ports of entry for the period between 1985 and 2000 ( Haack 2006), following the genera Monochamus Dejean, 1821 with 432 records, Xylotrechus Chevrolat, 1860 with 126 records and Ceresium Newman, 1842 with 114 records. Characteristic larvae of Trichoferus are often found in the dunnage of containers from P.R. China at Canadian ports of entry (Cavey 1998; Grebennikov 2005). Trichoferus campestris has been reported to emerge in European quarantine facilities from wood imported from P.R. China ( Cocquempot 2006). In 1997, a small, localized infestation of this species occurred in a storage site in New Brunswick, New Jersey (not New Brunswick, Canada), but was later eradicated ( Cocquempot 2006). The species is considered a quarantine pest in Europe and is included by the European and Mediterranean Plant Protection Organization (EPPO) in the EPPO A2 List of pests recommended for regulation ( Anonymous 2007, 2008).
Has T. campestris become established in North America? Available material does not permit a definitive answer. The discovery of two adult specimens near Montreal, collected four years apart in a residential area, distant from an international port or a commercial facility, indicates that the species was present, at least temporarily, in Canada. It seems unlikely that such a residential neighborhood would have been the original point of outbreak for an introduced population. The lack of additional records might be a result of (A) collected specimens were single individuals that failed to establish a population, or (B) our collecting efforts were inadequate to detect an infestation due to, for example, being too late in the adult flight season for this species. Considering, however, that this species is often intercepted in North America from wood-packaging material of Asian origin, as well as its previous introductions into Europe and North America, it is plausible to assume that T. campestris has a high likelihood of becoming established in the temperate regions of the New World.
Overview of Recent Introductions of Alien Cerambycidae to North America. Besides T. campestris , seven other alien cerambycid species were introduced into North America during the last two or three decades ( Haack 2006); these events are summarized in Table 1. Among them, Tetropium fuscum (Fabricius, 1787) is currently under regulatory action in Atlantic Canada; its introduction is feared to cause large economic losses. Anoplophora glabripennis (Motschulsky, 1853) is, arguably, the most publicized alien beetle species, which attracted significant attention to the problem of invasive alien species. The recent record of Heterachthes rugosicollis Martins, 1970 ( Swift 2008) most likely represents a natural range expansion of this species native to Mexico.
| R |
Departamento de Geologia, Universidad de Chile |
| VI |
Mykotektet, National Veterinary Institute |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Trichoferus campestris (Faldermann)
| Grebennikov, Vasily V., Gill, Bruce D. & Vigneault, Robert 2010 |
campestris
| Faldermann 1835: 435 |