Caenorhabditis elegans (Albertson & Thomson, 1976)
|
publication ID |
https://doi.org/10.1111/j.1096-3642.2009.00632.x |
|
persistent identifier |
https://treatment.plazi.org/id/03CB6C6C-FFA3-FFAE-FE92-D485017DF9C4 |
|
treatment provided by |
Valdenar |
|
scientific name |
Caenorhabditis elegans |
| status |
|
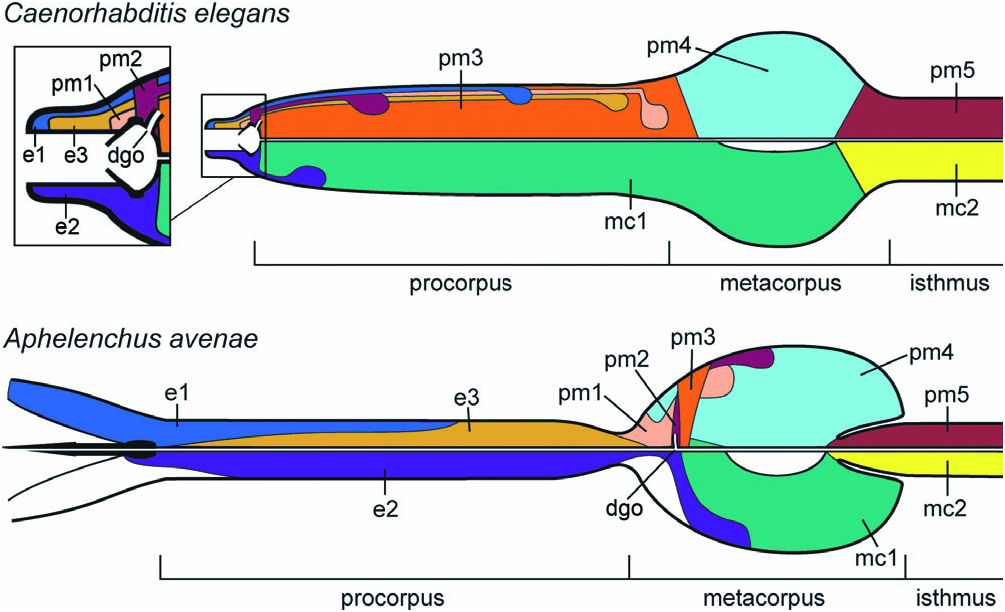
Complete reconstruction of the pharynx of C. elegans ( Albertson & Thomson, 1976) offers an extensive point of reference for assessing homologies of individual pharynx cells of A. avenae ( Fig. 7 View Figure 7 ). Hypotheses of homology can be proposed based on their position within the series lining the luminal cuticle; relative positions of stomatal and pharyngeal cells are highly conserved ( Bumbarger et al., 2006; Ragsdale et al., 2008). Numbers of cells or nuclei within each class, connectivity to other cells including pharyngeal neurones, relationships to distinct cuticular features, positions of nuclei, and cellular morphology serve as evidence to test and often support homology hypotheses. Equal numbers of cells, or at least nuclei in the case of cell fusion, allows necessary tests of conjunction for putative homologues between taxa ( Patterson, 1982). To limit the set of hypotheses required, especially between putative homologues that show some divergence in expression, the corpus as an entity is defined by boundaries that are most clearly homologous across taxa.
Definition of the corpus
Crucial for delimiting the corpus as an arena for more specific tests of homology is establishing homology for the isthmus cells. Previous identification of the homologous arcade syncytia surrounding the stylet ( Ragsdale et al., 2008) has already distinguished the anterior boundary to the corpus. The isthmus radial cells therefore provide a landmark enabling predictions of homology for all cells lining the luminal cuticle anteriad, to the border of the corpus with stomatal tissue. Evidence for the homologies of isthmus radial cells is supported by their similar morphology across all taxa examined. Details of the junction between metacorpus and isthmus cells are also conserved: immediately posterior to the pump chamber or central metacorporal lumen are the subventral gland orifices, the anterior tips of the isthmus cells, and the pharyngeal commissure. Similarity criteria thus support homology of the isthmus radial cells with pharyngeal muscle (pm) ‘pm5’ of C. elegans as well as other taxa for which this cell has been identified, including the tylenchid sensu stricto Basiria gracilis ( Baldwin et al., 2001) and other outgroups in the class Chromadorea ( Zhang & Baldwin, 1999, 2000, 2001). A posterior set of marginal cells that associates only with the isthmus radial cells anteriorly in A. avenae is consistent with marginal cell mc 2 in C. elegans , further supporting the isthmus as a homologous landmark across taxa.
The corpus of A. avenae comprises six sets of radial cells and two sets of marginal cells line the luminal cuticle. This is exactly the number of cell classes between the arcade syncytia and pm5 of C. elegans ( Table 1; Fig. 7 View Figure 7 ), providing a putative cell-for-cell correspondence in the pharyngeal corpus that can then be tested by other criteria.
Procorpus
Equating the series of corpus cells across examined taxa suggests that the stylet protractors and procorpus radial cells in A. avenae are the homologues of epithelial cells (e) e1 and e3, respectively, in C. elegans . Numbers of cells and nuclei, three for each class, are equal in both taxa. Positions of the nuclei of these cells within the procorpus are highly similar between A. avenae and C. elegans , with those of the anterior set of cells (e1) being located in the middle of the procorpus and those of the posterior set (e3) being at the posterior end of the procorpus. However, the margin of the lining of cells e1 and e3 along the lumen, as well as the volumes of the cells, differs greatly between the two nematodes; in A. avenae , these cells comprise the entire procorpus. Another obvious difference is the expression of ‘e1’ as a muscle cell as opposed to an epithelial cell in A. avenae , which challenges similarity based on cell function. This difference in cell expression, in addition to developmental studies of the pharynx, was previously interpreted to reject this homology statement, originally proposed by De Ley et al. (1995), in favour of considering the most anterior muscle cells of Rhabditida (‘ ma’ and pm1) to be homologous and excluding e1 and e3 ( Dolinski et al., 1998). Although the expression of e1 as epithelial appears to be unique to Rhabditomorpha and Diplogasteromorpha, the present hypothesis for homologies of the anterior two classes of cells in the pharynx is consistent with that of De Ley et al. (1995). The expression of e3 as epithelial is present in both A. avenae and C. elegans , although this differs from examined cephalob taxa where the putative homologue (‘ mb’) is muscular ( Van de Velde et al., 1994; Baldwin & Eddleman, 1995; De Ley et al., 1995).
The marginal cells of the procorpus and stomatostylet apparatus in A. avenae are hypothesized to be the homologues of the e2 epithelial cells of C. elegans . Similarly to cells e1 and e 3 in A. avenae , e2 lines the pharynx for a much greater length than in C. elegans , persisting throughout the procorpus and for the anterior part of the metacorpus. Given the margin of contact of e1 and e3, this finding is consistent with C. elegans in that e2 cells are interspersed between individuals of both sets e1 and e3. The position of the nuclei of e2 is posterior in A. avenae as compared to C. elegans , where they are in the anterior part of the metacorpus. In A. avenae , putative e2 cells form gap junctions with two additional classes of radial cells posterior to e3, thus being associated with the most anterior four layers of pharyngeal cells; this is consistent with the putative homologues of e 2 in freeliving outgroups, including C. elegans ( De Ley et al., 1995) and several Cephalobomorpha ( De Ley et al., 1995; Dolinski et al., 1998). A consequence of this hypothesis is that the stoma or buccal cavity of C. elegans and other free-living Rhabditida is equivalent to the basal knobs of the stylet, all of the procorpus, and the anterior part of the metacorpus of A. avenae .
Metacorpus
Posterior to the e3 cells, the metacorpus comprises the proposed homologues of pharynx muscles (pm) 1–4. Although numbers of individual cells vary between A. avenae and C. elegans for each class, the total number of nuclei in every class is six for both taxa ( Albertson & Thomson, 1976). Putative homologues of at least pm1 and pm2 also have six processes or nuclei per class in representatives of Cephalobomorpha ( De Ley et al., 1995; Dolinski et al., 1998) and Panagrolaimorpha ( De Ley et al., 1995) and in the possibly more distant outgroup Myolaimus byersi ( Giblin-Davis et al., 2010) .
The homologues of the most anterior set of metacorpus radial cells in A. avenae , the constraining muscles, are proposed to be pm1 of C. elegans . In C. elegans , this class of cells has been described as a single syncytial ring in the stoma ( Albertson & Thomson, 1976), although the presence of only a single syncytium has been challenged ( De Ley et al., 1995; Dolinski & Baldwin, 2003). The pm1 cells of C. elegans have posterior processes extending to cell bodies containing six nuclei. Despite the difference in where these cells line the luminal cuticle between the two taxa, in both cases they have their nuclei in processes posterior to the contractile part of the cell(s).
The anterior valve muscles of A. avenae are hypothesized to be homologous with pm2 cells of C. elegans . The pm2 cells of C. elegans are a set of three radial syncytia that line the posterior region of the stoma and have posterior cell bodies within the procorpus. The positions of putative pm2 nuclei relative to those of other radial cells are highly divergent between the two taxa: they are the most anterior of the pm cells in C. elegans but in A. avenae they are the second most posterior pm cells. However, as with pm1, putative pm 2 in both taxa are characterized by posterior processes extending to cell bodies. Corroborating the hypothesis of the anterior valve muscles as homologous to pm2 is the position of the DGO between these cells in A. avenae . The DGO is consistently located in the fourth layer of cells of free-living Rhabditida outgroups, including several Cephalobomorpha and Panagrolaimorpha ( Van de Velde et al., 1994; Baldwin & Eddleman, 1995; De Ley et al., 1995), the *Not described in text of original study ( Shepherd et al., 1980).
e1–3, ‘epithelial’ cells 1–3; DGO, dorsal gland orifice; mc1–2, marginal cells 1–2; pm1–5, pharyngeal muscle cells 1–5.
diplogasterid Aduncospiculum halicti ( Baldwin et al., 1997) , C. elegans ( Wright & Thomson, 1981) , and the possibly more distant outgroup Myolaimus byersi ( Giblin-Davis et al., 2010) . Thus, whereas the position of the DGO in the corpus is dramatically different between A. avenae and C. elegans , it is essentially unchanged in its relationship to surrounding cells ( Fig. 7 View Figure 7 ).
The hypothesized homologues of the posterior valve muscles in A. avenae are the pm3 of C. elegans , which in the latter nematode constitute the entire procorpus. Yet despite the difference in cell length along the pharynx, the nuclei of the cells in both taxa are located within the contractile part of the cell and not in posterior cell bodies.
The metacorpus pump muscles of A. avenae are most likely to be the homologues of pm 4 in C. elegans . Aside from this class of cells being a set of three syncytia in C. elegans , morphology of these cells is highly similar in both taxa. These muscles constitute most of the swelling characteristic of the metacorpus, insert on thickenings in the luminal cuticle, and have the most posterior nuclei of all corpus radial and marginal cells.
The metacorpus marginal cells (mc) of A. avenae are the putative homologues of marginal cell mc 1 in C. elegans . In both taxa these three morphologically similar cells contain nuclei near their posterior ends, near the level of the metacorpus pump chamber. The homology of these cells also supports the hypotheses of homology for pm3 and pm4, which are radially interspersed with exactly these two sets of cells in both taxa. Unique to A. avenae is that the putative mc1 marginal cells surround the luminal cuticle exclusively between the insertion points of putative pm3 and pm4.
A discrepancy in equating the series of metacorpus radial cells to pm1–4 of C. elegans is in the relative positions of nuclei within the metacorpus. In particular, the position of the pm2 nuclei, which are the most anterior set of pm nuclei in C. elegans , is in the anterior half of the procorpus; in A. avenae , these are at a position much further posterior, between the nuclei of putative pm2 and pm4. Aside from the positions of pm2 nuclei, the relative positions of the other radial cell nuclei to one another are relatively conserved with C. elegans , with pm3 most anterior and pm4 most posterior, although the positions of these relative to epithelial cell nuclei is more variable between taxa. Furthermore, all nuclei except those of putative e1 and e3 are confined to the metacorpus, whereas in C. elegans they are distributed along the entire corpus. This observation is not surprising if the metacorpus and most of the procorpus of C. elegans are considered homologous to the metacorpus of A. avenae . Apparently more conserved are the positions of nuclei with respect to their own cells; namely, pm1 and pm2 have their nuclei in posterior processes, whereas pm3 and pm4 lack such processes and contain their nuclei within the contractile part of the cell. If homologies of pharyngeal radial and marginal cells can be most reliably based on their position lining the cuticle of the pharyngeal lumen, as results herein suggest, the positions of nuclei raise interesting questions of cell extension during development.
Pharyngeal neurones
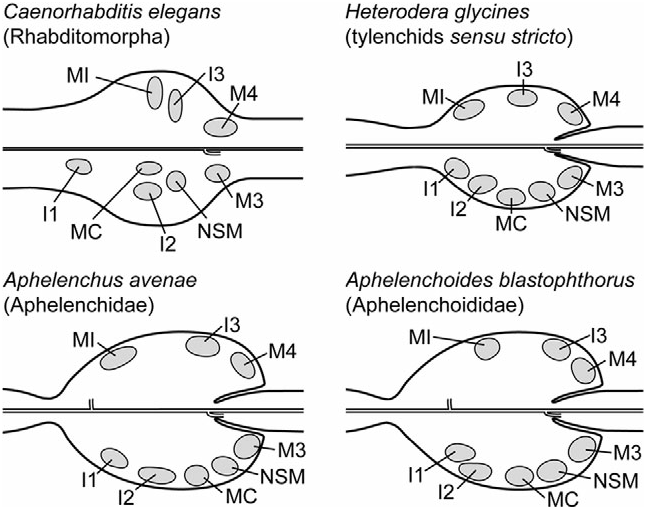
Relative positions of neuronal nuclei are highly conserved across representatives of Rhabditomorpha, Diplogasteromorpha, Cephalobomorpha, and Panagrolaimorpha ( Chiang et al., 2006), and thus provide a strong basis for assigning neurone identities in A. avenae ( Fig. 8 View Figure 8 ). Although neurones and their interconnections were not fully reconstructed in the present study, many details are apparent that support homology predictions. Findings for A. avenae confirm conservatism of the relative positions of all nuclei as predicted for the corpus. Polarities of all neurones identified are also consistent between taxa. In the context of the entire pharyngeal corpus, identification of homologous neurones provides additional tests of similarity to other classes of cells to which they connect, including all four classes of pm cells. Descriptions herein of putative neurone homologues are, unless otherwise stated, of features common to both A. avenae and C. elegans . Comparison with C. elegans is based on reconstruction of the pharyngeal nervous system by Albertson & Thomson (1976).
Motor neurones: Motor neurone M1 has the most anterior terminus of the dorsal pharyngeal neurones. Its slightly asymmetrical position between the dorsal radial cells and right subdorsal marginal cells is conserved. Although this cell is associated with the dorsal pm1 cells, no ventral circling of the terminus was observed in A. avenae . Divergence in the morphology of this neurone in A. avenae with respect to C. elegans is particularly striking in its anterior branch. The anterior process extends into the procorpus and terminates with prominent swelling, including a process into the dorsal stylet protractor muscle (e1). The exaggerated presence of microtubules in its terminus in A. avenae ( Ragsdale et al., 2008) suggests a distinct modification of function of this neurone, perhaps to include mechanoreception (e.g. Perkins et al., 1986). The additional association of this neurone with homologues of the e1, as assigned herein, is also novel in A. avenae .
The morphology of the M3 motor neurones is complex, with two processes, the anterior of which has multiple branches, patterns of which in at least C. elegans vary amongst individuals. Consistent in A. avenae and various individuals of C. elegans is an anterior, subcuticular terminus at the subventral gland orifices. Also conserved is the dorsal circling of the anterior process as well as a branch extending into the subventral neurone bundles. The posterior processes are simple, extending partially into the isthmus along the distal, subventral margins.
The single M4 motor neurone, the cell body of which is at the level of the commissure, extends two lateral processes ventrally into the latter.
The MC motor neurones have anterior processes that terminate subventrally against or near the cuticle of the lumen, immediately posterior to the pm3 muscle cells. Whereas in C. elegans the termini are directly between the insertions of pm3 and pm4, this configuration is precluded in A. avenae by the complete enclosure of the luminal cuticle by the metacorpus marginal cells (mc1) posterior to putative pm3. Notwithstanding the peculiarity of the marginal cells in A. avenae , the termini are between the same two sets of muscles, pm3 and pm4, given the present assignments of homology.
Interneurones: The I1 interneurones are bipolar, with an anterior process extending to connect to the somatic input neurones, the RIP interneurones, at which point they terminate. In A. avenae these anterior processes are diminutive, possibly because of the short distance between the I2 cell bodies and the point of entry of the RIP into the pharynx. A difference observed in these putative homologues is the lack of subcuticular anterior termini in A. avenae .
Interneurones I2 have the most anterior subventral termini, which prominently abut against the cuticle and attach to the subventral pm1 muscles, supporting the homology of the latter. Unique to A. avenae is the connection of the anterior processes of I2 to the RIP interneurones.
The I3 interneurone has an anterior process that terminates subcuticularly, immediately anterior to the DGO. Furthermore, the anterior process inserts on two sets of adjacent, dorsal radial cells, the putative homologues of pm1 and pm2, supporting homology of the latter with the metacorpus constraining muscles and anterior valve muscles, respectively.
Other neurones: From the cell body of each NSM neurone, a single process enters the commissure, where a branch crosses ventrally to the other lateral side and connects to subventral isthmus muscle cells on both sides. Association of NSM with pm5 is conserved. The motor-interneurone MI has a single posterior process that enters the commissure. The connections of this neurone in C. elegans are known to be variable amongst individuals, but they are apparently consistent in including those to the other dorsal neurones of the corpus, M1 and I3, anterior to entering the commissure; associations with putative homologues of the latter are observed in A. avenae . The entry points of the somatic RIP neurones with respect to muscle cells lend support to homology of pm1 with the constraining muscles.
COMPARISON WITH THE PHARYNX IN TYLENCHIDS
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.