Parastacus gomesae, Huber & Araujo & Ribeiro, 2022
|
publication ID |
https://doi.org/10.11646/zootaxa.5168.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:3624B353-61FF-4A2E-8583-453822F49948 |
|
DOI |
https://doi.org/10.5281/zenodo.6903184 |
|
persistent identifier |
https://treatment.plazi.org/id/654BFFA1-6A3B-4EFE-BD8C-F3E323C9D183 |
|
taxon LSID |
lsid:zoobank.org:act:654BFFA1-6A3B-4EFE-BD8C-F3E323C9D183 |
|
treatment provided by |
Plazi |
|
scientific name |
Parastacus gomesae |
| status |
sp. nov. |
Parastacus gomesae sp. nov. Huber, Araujo & Ribeiro
( Figs. 1 View FIGURE 1 , 5 View FIGURE 5 , 7–10 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 )
Type series. Holotype: adult male, Brazil, Rio Grande do Sul, São Jerônimo, Horto Florestal Quitéria , riacho de primeira ordem ( 30°29’05.8”S, 52°04’09.9”W) 21/X/2020, coll. A.F. Huber, K.M. Gomes & F.B. Ribeiro (MNRJcarcino 030203) GoogleMaps . Paratype 1: 1 female, same data as holotype (MNRJcarcino 30204) GoogleMaps ; Paratypes 2 and 4: 1 male and 1 females, same data as holotype ( UFRGS 6933 View Materials ) GoogleMaps ; Paratype 3: 1 female, same data as holotype (MNRJcarcino 30205); 5 GoogleMaps : 1 female, Brazil, Rio Grande do Sul, São Jerônimo ( 30°29’05.0”S; 52°04’11.0”W) VII/2011, coll. K.M. Gomes & C. Sokolowicz ( UFRGS 5339 View Materials ) GoogleMaps .
Etymology. Named in honor to Dra. Kelly Martinez Gomes from Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil. Kelly is one of the greatest astacologist in South America and has been contributing for several years in taxomy and biology research of the freshwater crayfish from the Neotropical region, especially genus Parastacus . She was also responsable for the first sample of this species in an expedition in 2011. We also suggest the common name “Quitéria crayfish” for this species.
Diagnosis. Narrow front with short triangular rostrum. Rostral apex shaped as inverted “U”. Suborbital angle>90° and unarmed. Postorbital carinae moderately prominent in anterior and middle portions. Cervical groove weakly V-shaped. Areola narrow. Telson subrectangular with small sharp spines on lateral margins. Epistome anteromedian lobe heptagonal. Mandible with caudal molar process bicuspidate with one cephalodistal cusp and one smaller distoproximal cusp; incisive lobe with seven teeth (the second tooth from the anterior margin is the largest). S2 pleurae high and moderate with moderately deep groove parallel to margin. Thoracic sternites SPL7 with strongly concave, median keel inflated with shallow longitudinal groove.
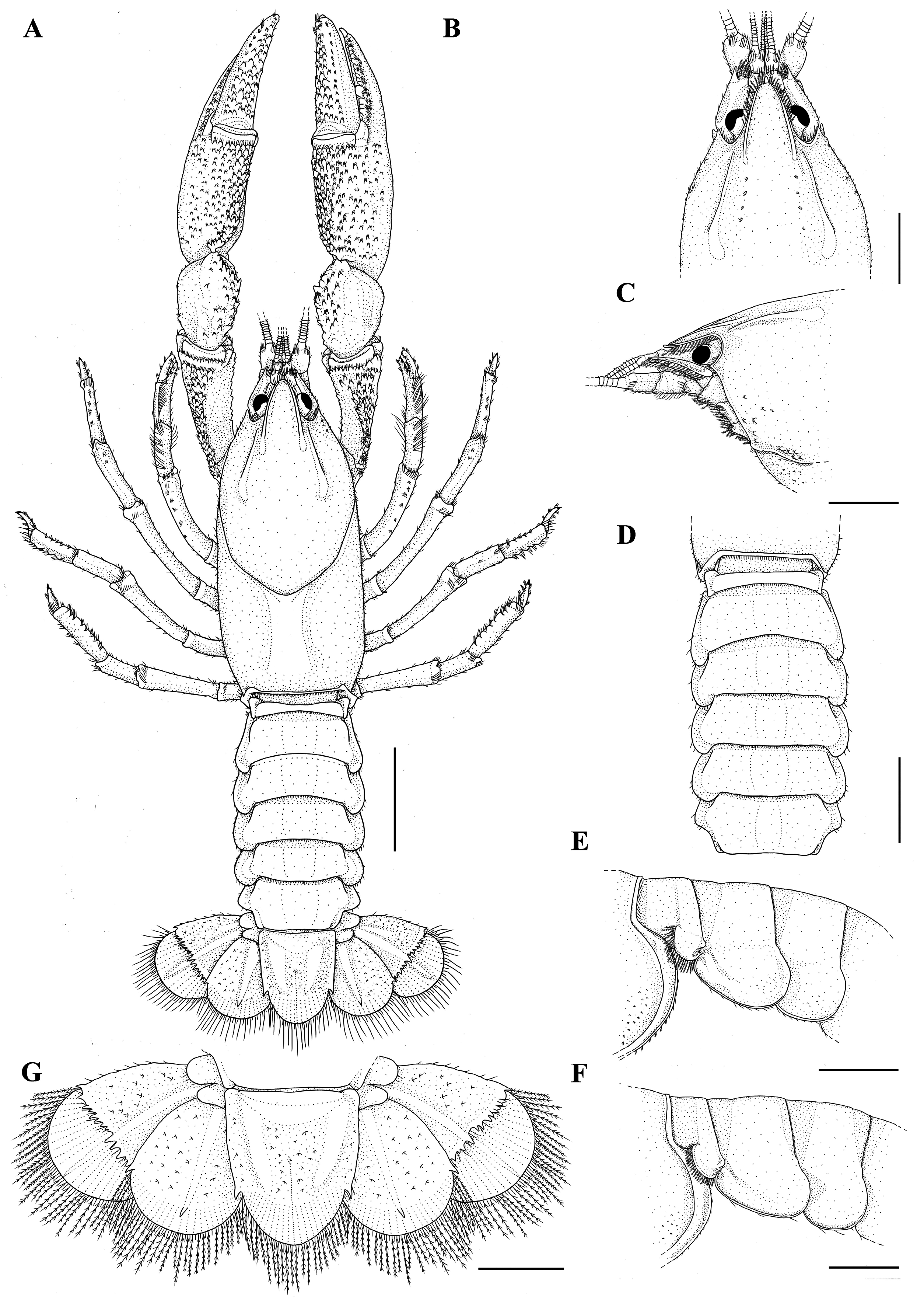
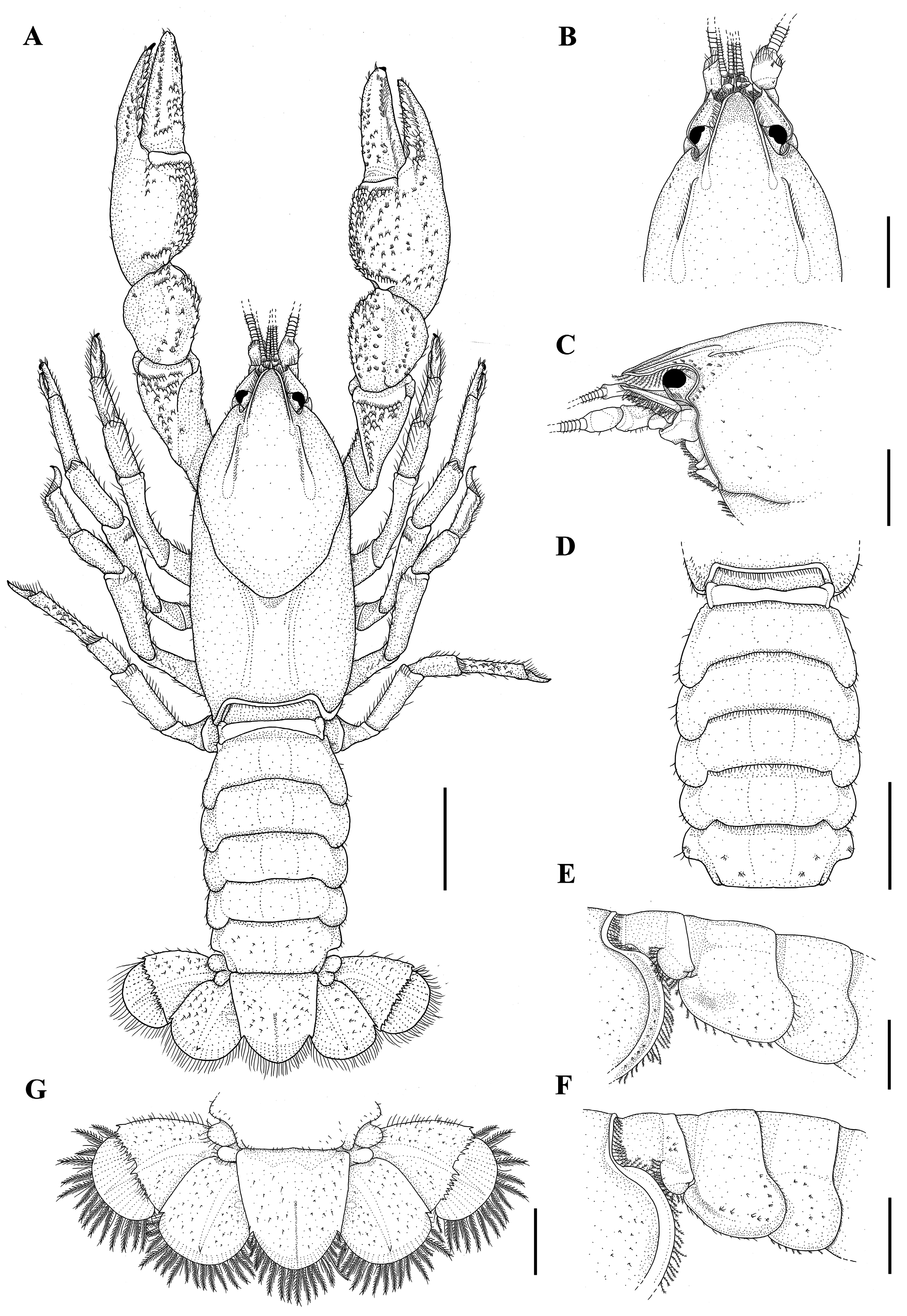
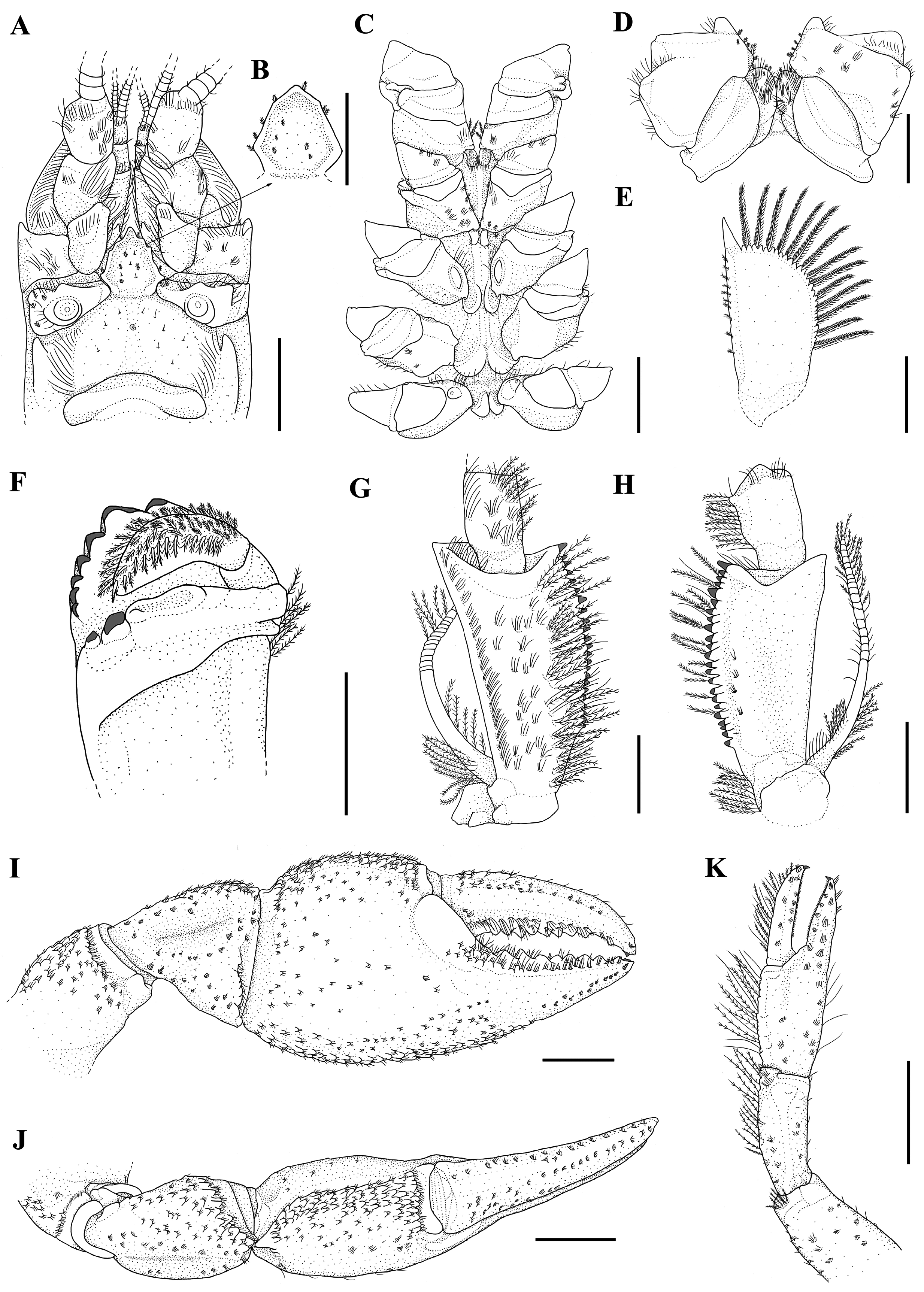
Description of holotype. Rostrum: triangular, a little wider than long (RW 1.04X of RL), short (RL 13.1% of CL), reaching distal portion of the second article of the antennular peduncle ( Fig. 7A–C View FIGURE 7 ). Dorsum slightly concave in the anterior portion, apex inverted “U”-shaped and smooth ( Fig. 7A–C View FIGURE 7 ). Few plumose setae on lateral margins ( Fig. 7C View FIGURE 7 ). Carinae almost straight, prominent and narrow, extending back to carapace, surpassing rostral basis; rostral carinae sides convergent and rostral carinae basis convergent ( Fig. 7A, B View FIGURE 7 ).
Cephalon: Carapace lacking spines or tubercles. CeL 67% of CL. Eyes small (CMW 62.6% of OW); suborbital angle>90°, unarmed ( Fig. 7C View FIGURE 7 ). Front narrow (FW 39.9% of CW). Postorbital carinae longer than rostral carinae (RCL 82.8% of POCL), prominent in anterior and middle portions. Lateral cephalic edge with few setation ( Fig. 7A–C View FIGURE 7 ).
Thorax: carapace laterally compressed, deep and narrow (CD 52.1% of CL; CW 46% of CL). Cervical groove weakly V-shaped. Branchiocardiac grooves inconspicuous ( Fig. 7A View FIGURE 7 ). Areola narrow, 2.08 x as long as wide (AreL 24.9% of CL) ( Fig. 7A View FIGURE 7 ).
Pleon: lacking spines or tubercles, short and wide (PL 77.5% of CL; PW 91.6% of CW), smooth and pleural margins with sparse small setae ( Fig. 7A, E View FIGURE 7 ). Pleural somites with rounded posterior margins. S1 pleurae with a small distal lobe partially overlapped by S2 pleurae. S2 pleurae high and moderate, with a moderately deep groove parallel to margin ( Fig. 7E View FIGURE 7 ).
Tailfan: telson more calcified in the proximal portion and weakly in the distal portion, subrectangular, longer than wide (TeW 79.9% of TeL), with small sharp spines on lateral margins; rounded distal margin with abundant long plumose setae and short simple setae. Dorsal surface with tufts of short setae and inconspicuous dorsomedian longitudinal groove ( Fig. 7A, G View FIGURE 7 ). Uropod protopod bilobed, with rounded and unarmed margins; proximal lobe largest. Exopod lateral margin bears a small and sharp spine, mid-dorsal carina few prominent, ending in a sharp spine. Transverse suture (diaeresis) straight, with nine dorsolateral spines (outer) and nine dorsolateral spines (inner) on right exopod and nine dorsolateral spines (outer) and eight dorsolateral spine (inner) on the left exopod. Endopod, mid-dorsal carina weakly prominent, ending in a small sharp spine; lateral margin with one small sharp spine at level of exopod transverse suture ( Fig. 7G View FIGURE 7 ).
Epistome: anterolateral section with conical projection on both sides and small circular median concavity. Posterolateral section smooth and with lateral grooves converging to the basis of the anteromedian lobe. Median section with a longitudinal depression. Anteromedian lobe with irregular margins, but in paratypes heptagonal with straight margins, 1.07x longer than wide, apex acute with some sparse serrated and simple setae, surpassing median part of antepenultimate article of antennal peduncle; dorsal surface concave with grooves parallels to margins from the basis to the apex and basis with a moderately deep groove ( Fig. 8A,B View FIGURE 8 ).
Thoracic sternites: SLP4 smaller than SPL 6, 7, and 8, very close to each other, median keel present and not inflated; SLP5 smallest and close to each other, median keel present and not inflated; SLP6 larger than SLP4, 5 and 8, separe to each other, with a concave surface, median keel inflated; SLP7 largest and with surface with a strongly concave, median keel inflated with a shallow longitudinal groove, bullar lobes absent; SLP8 smaller than SPL 6 and 7, median keel absent, vertical arms of paired sternopleural bridges separated to each other, bullar lobes separeted and clearly visible ( Fig. 8C, D View FIGURE 8 ).
Antennule: internal ventral border of basal article with a blunt spine in the proximal region ( Fig. 8A View FIGURE 8 ).
Antenna: when extended back surpasses the posterior margin of the carapace and reaches the posterior margin of the SII. Antennal scale wider at the midlength, reaching proximal margin of third antennal article, ASW 54.6% of ASL ( Fig. 8A, D View FIGURE 8 ), lateral margin straight and distal sharp spine well developed. Coxa with weakly prominent carina above nephropore and two blunt spines with similar sizes laterally distributed. Basis unarmed ( Fig. 8A View FIGURE 8 ).
Mandible: cephalic molar process molariform, caudal molar process bicuspidate with one cephalodistal cusp and one distoproximal cusp. Incisive lobe with seven teeth. The second tooth from the anterior margin is the largest ( Fig. 8F View FIGURE 8 ).
Third maxilliped: ischium, ventral surface with tufts of long and simple setae on the middle portion and inner margin, and setiferous punctuations on outer margin ( Fig. 8G View FIGURE 8 ); dorsal surface with tufts of simple and short setae parallel to the inner margin ( Fig. 8H View FIGURE 8 ); crista dentata bearing 25 teeth in both right and left ischia. Merum, dorsal surface glabrous with some tufs of simple and short setae on distal margin; ventral surface sparsely covered by long and short simple setae in the median region and on the inner margin ( Fig. 8G View FIGURE 8 ); Exopod longer than ischium, with flagellum reaching the proximal ⅓ of merum, with tufts of long and composed setae in the last articles ( Fig. 8G, H View FIGURE 8 ).
First pair of pereiopods (chelipeds): large and subequal, laterally flattened (RPrT 29% of RPrL; LPrT 28.1% of LPrL) ( Fig. 7A View FIGURE 7 ; Fig. 8 View FIGURE 8 IJ). Ischium ventral surface with 12 and 13 tubercles in the right and left respectively. Merus: right merus (RML) 57.8% of propodus length (RPrL); left merus (LML) 57.3% of propodus length (LPrL); ventral surface with two longitudinal series of tubercles: inner series with 17 tubercles, outer 13, plus 24 mesial tubercles irregularly distributed on right merus; inner series with 16 tubercles, outer 15, plus 27 mesial tubercles irregularly distributed on left merus. Dorsal spine absent and midventral spines present and blunt. Carpus with dorsomedial surface divided longitudinally by a groove ( Fig. 7A View FIGURE 7 ; Fig. 8I, J View FIGURE 8 ). Internal dorsolateral margin with row of tubercles, increasing in size distally; inner surface with 19 and 14 small mesial tubercles in the left and right carpus respectively. Carpal spine absent ( Fig. 8J View FIGURE 8 ). Propodus width (RPrW and LPrW) 54.7% of length in right cheliped and 53.2% in left cheliped. Dorsal surface of palm with 3–4 rows of verrucous tubercles irregularly distributed ( Fig. 8I, J View FIGURE 8 ). Inner margin with few small tubercles. Ventral surface bearing two rows of squamose tubercles, reaching the beginning of the fixed finger ( Fig. 8I View FIGURE 8 ). Dactylus: moving subvertically, right dactylus (RDL) 56.3% of propodus length (RPrL), left dactylus (LDL) 56.1% of left propodus (LPrL); dorsal surface with few squamose tubercles in the proximal portion reaching the middle of dactylus ( Fig. 8J View FIGURE 8 ). Cutting edge of fingers visible. Fixed finger with nine teeth, third teeth the largest in the right cheliped. Dactylus with ten teeth in the right chelip, third tooth is the largest ( Fig. 8I, J View FIGURE 8 ). Nine teeth in the fixed finger of the left chelip, third tooth the largest. Dactylus of the left cheliped with 13 teeth, third tooth the largest.
Second pair of pereiopods: dorsal surface of dactylus and ventral and dorsal surface of carpus and propodus with moderate covering of long simple and composed setae ( Fig. 8K View FIGURE 8 ).
Gonopores: Presence of both genital apertures on coxae of third and fifth pairs of pereiopods. Female gonopores semi-ellipsoidal (maximum diameter 2.15 mm) with well-calcified membrane. Male gonopores rounded, opening onto the apical end of a small, fixed, calcified and truncated phallic papilla, close to the inner border of the ventral surface of coxae of the fifth pair of pereiopods. Male cuticle partition present ( Fig. 2B View FIGURE 2 ).
Branchial count: 20 + epr + r. Branchial arrangement follows the same described by Huxley (1879) and Hobbs (1991) with the epipod of the first maxilliped with rudimentary podobranchial filaments.
Measurements. Holotype male, CL 33.23 mm and TL 67.5 mm. In type series, CL ranges from 17.64 to 44.74 mm (27.70 ± 10.52 mm). FW/CW: 0.43 ± 0.03 (min: 0.39; max: 0.47). RW/RL: 0.95 ± 0.06 (min: 0.88; max: 1.04). CMW/OW: 0.72 ± 0.12 (min: 0.61; max: 0.93). Postorbital carina longer than rostral carina in all specimens analyzed. CW/PW: 1.05 ± 0.05 (min: 1.00; max: 1.13). AreW/RW: 0.87 ± 0.03 (min: 0.84; max: 0.93) ( Table 3 View TABLE 3 ).
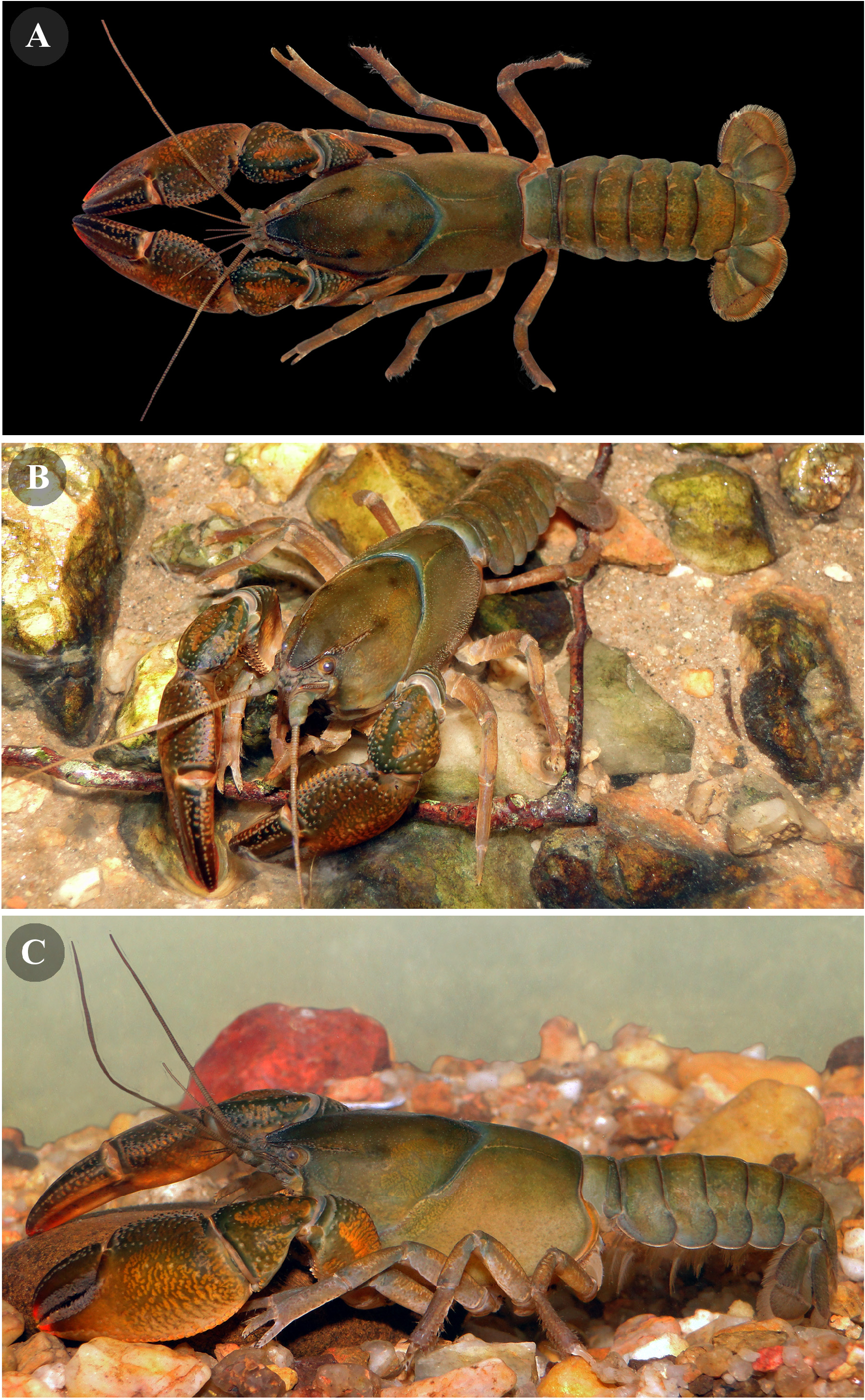
Color of living specimens. Rostrum darkish brown. Cephalothorax anterior and lateral regions dark brown to greenish brown with shades of light brown. First pair of pereiopods dark brown in the dorsal and light brown in the middle and in the ventral of the external surface; light brown to light orange in the internal surface; the apex of the chela has bright red tones. Pereiopod pairs 2–5 dark brown to light brown. Dorsal pleon and taifan dark brown with shades of light brown ( Fig. 9 View FIGURE 9 ).
Variations in paratypes. All paratypes present both masculine and feminine gonopores in the same individual. Male paratypes also present female gonopores semi-ellipsoidal (average maximum diameter 1.0 mm) covered by a calcified membrane. Male gonopores are very similar in all male paratypes. Rostrum longer than wide in paratypes 1, 2, 4 and 5, and reaches the third article of antennule (long) in paratype 4. Apex of the rostrum with a tiny blunt spine in paratypes 3 and 4. Rostral carina bases parallel in paratype 4. Eyes large in paratypes 2–5. Front wide in paratype 5. Pleon long in paratypes 2 and 4. Number of spines in exopod of tailfan varies between six to nine in inner and outer dorsolateral spines on right side and six to nine dorsolateral spines (outer) and five, six or eight dorsolateral spines (inner) on the left side. Antenna of paratype 5 do not reaches the posterior margin of cephalotorax, probable broken; antenna almost reach the anterior margin of S2 pleura in paratypes 2–4. Antennal scale reaches the median region of the third antennal article in paratypes 2, 3 and 5. Number of teeth in the crista dentata ranges from 21 to 25 in the left ischium and from 21 to 25 in the right ischium of the third maxilliped in the paratypes. Number of teeth in chelipeds in paratypes ranges from 7–11, 7–11, 7–8 and 6–10 in left dactylus, left propodus, right dactylus and right propodus, respectively. Paratype 1 has the rostral carenas orange.
Remarks. Parastacus gomesae sp. nov. is morphologically similar to P. brasiliensis , P. buckupi , P. guapo sp. nov., P. macanudo and P. promatensis in general shape of rostrum, rostral carinae length and in the shape of telson (subrectangular) ( Fig. 7A–C, G View FIGURE 7 ; Table 2 View TABLE 2 ). However, P. gomesae sp. nov. differs from: P. brasiliensis in the sub-orbital angle> 90°, postorbital carinae prominent in anterior and middle portions, epistome anteromedian lobe heptagonal, 3–4 rows of verrucose tubercles irregularly distributed in the palm dorsal surface of chelipeds, areola narrow and pleon short ( Fig. 7 View FIGURE 7 ; 8A, B, I View FIGURE 8 ; Table 2 View TABLE 2 ); P. buckupi in the sub-orbital angle> 90°, postorbital carinae prominent in anterior and middle portions, epistome anteromedian lobe heptagonal, mandible caudal molar process bicuspidate and 3–4 rows of verrucose tubercles irregularly distributed in the palm dorsal surface of chelipeds ( Fig. 7A–D View FIGURE 7 ; 8A, B, F, I, J View FIGURE 8 ; Table 2 View TABLE 2 ); P. guapo sp. nov. in the carpal spine absent, telson with small lateral spines, 3–4 rows of verrucose tubercles irregularly distributed in the palm dorsal surface of chelipeds and internal surface of chelipds palm smooth ( Fig. 7G View FIGURE 7 ; 8A, B, J View FIGURE 8 ; Table 2 View TABLE 2 ); P. macanudo in the postorbital carinae prominent in anterior and middle portions, epistome anteromedian lobe heptagonal, carpal spine absent, 3–4 rows of verrucose tubercles irregularly distributed in the palm dorsal surface of chelipeds, pleon short, telson with small lateral spines and the habitat ( Fig. 7A–D, G View FIGURE 7 ; 8A, B, I, J View FIGURE 8 ; Table 2 View TABLE 2 ); P. promatensis in the apex of rostrum rounded, sub-orbital angle> 90°, postorbital carinae prominent in anterior and middle portions, cervical groove “V” shaped, epistome anteromedian lobe heptagonal, pleon short, telson with small lateral spines and the habitat ( Fig. 7A–D, G View FIGURE 7 ; 8A, B View FIGURE 8 ; 10A, B View FIGURE 10 ; Table 2 View TABLE 2 ). Parastacus gomesae sp. nov. also differs from all these species for rostral spine absent, epistome anteromedian lobe heptagonal and mandible incisive process with seven teeth (the second tooth from the anterior margin is the largest) ( Fig. 7A–C View FIGURE 7 ; 8A, B, F View FIGURE 8 ; Table 2 View TABLE 2 ).
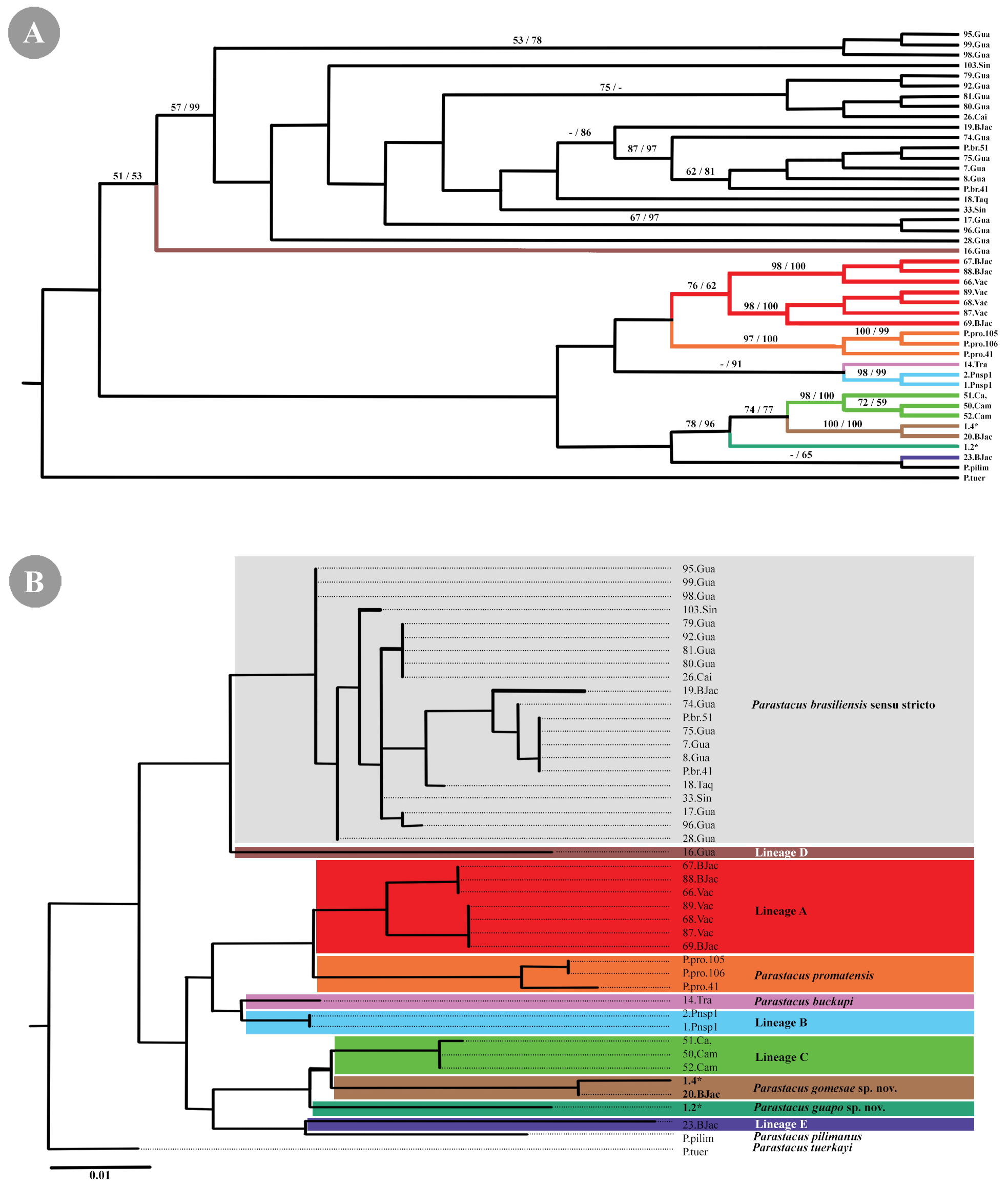
Phylogenetic position. The phylogenetic analysis suggests, with high values of reliability (bootstrap and posterior probability), that P. gomesae sp. nov. is a distinct species of P. brasiliensis s.s., Lineages A–E, and other species of the genus included ( Fig. 5 View FIGURE 5 ). The genetic distances estimated between P. gomesae sp. nov. and P. brasiliensis s. s. was 5.14% (Sup. Table. 2 View TABLE 2 ). Besides that, between the lineages range from 2.71% (Lineage C) to 6.50% (Lineage D), and of other species from 4.45% ( P. buckupi ) to 5.88% ( P. tuerkayi ) (Sup. Table. 2 View TABLE 2 ).
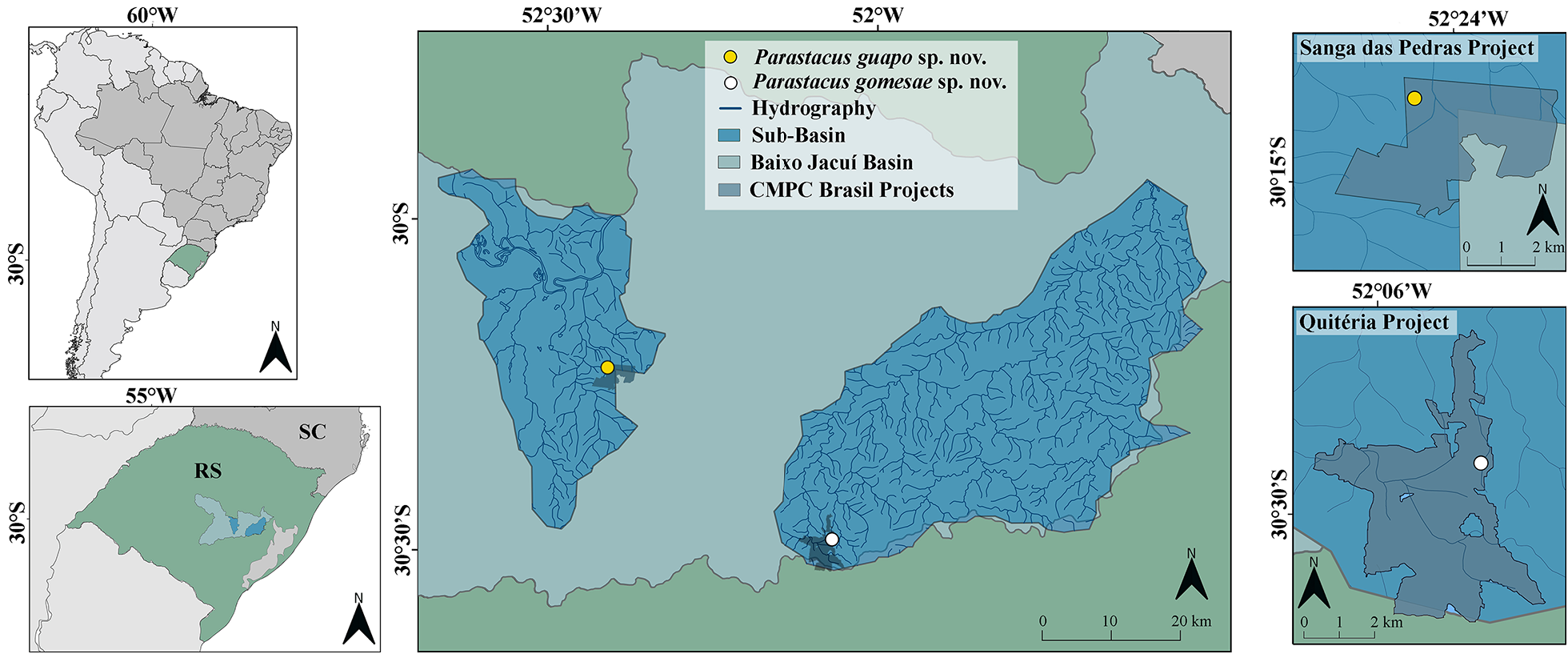
Habitat and ecology. Parastacus gomesae sp. nov. was collected in a stream and the margins of a small spring, both located inside the Quitéria project of CMPC ( Fig. 1 View FIGURE 1 ; 10A, B View FIGURE 10 ). This forestry area is located in the Baixo Jacuí hydrographic basin in the central region of the state of Rio Grande do Sul ( Fig. 1 View FIGURE 1 ). It is also part of the physiographic region of Planalto Sul Rio Grandense region, border with the northern edge of the Serra do Sudeste, and the Pampa biome ( CMPC Brasil 2018; IBGE 2004a; 2004b; UFRGS-IB-Centro de Ecologia 2016).
Vegetation. The region has as predominant vegetation grassy-woody steppe with gallery forests ( Fig 10A, B View FIGURE 10 ). The steppe grassy-woody vegetation is composed predominantly by grasses from the families Asteraceae ( Eupatorium sp. L., Baccharis sp. L.—“Carqueja”) and Poaceae ( Andropogon lateralis Nees. — “Capim Caninha”, Aristida sp. L.—“Capim Barba de Bode”, Paspalum notatum Flüggé — “Grama Forquilha” and Axonopus fissifolius (Raddi) Kuhlm. – “Capim Tapete”) ( CMPC Brasil 2018; Teixeira et al. 1986; Veloso et al. 1991). According to Veloso et al. (1991), the gallery forests are distributed along the watercourses and on hillsides, they are composed of trees like Anacardiaceae ( Lithraea brasiliensis (L.) Marchand — “Aroeira Brava”), Euphorbiaceae ( Sebastiania commersoniana Baill. ) L.B. Sm. & Downs—“Braquilho”) and Rhamnaceae ( Scutia buxifolia Reissek. —“Espinho de Touro”).
Soil. The soil is classified as Distro-Umbric Regolithic Neosol associated with rocky outcrop and it is characterized as recent, undeveloped, shallow depth, low fertility, generally sandy texture, and with high erodibility, especially on slopes ( CMPC Brasil 2018; Santos et al. 2021b; Streck et al. 2002; 2008). The use of this soil is restricted due to the relief and shallow depth, which requires severe conservation measures. In general, areas are used for permanent pastures, regions with strong undulated relief for reforestation and fruit growing, and the steepest areas should be destined for permanent preservation (AGEITEC 2021b; Santos et al. 2011; 2021a; 2021b; Streck et al. 2002; 2008).
Burrowing behavior and burrow structure. According to observations of burrows structure and characteristics of the habitat, P. gomesae sp. nov. can be classified as a primary burrower with types 1a, b burrows, due to the construction of complex tunnels close and with direct and indirect connections to permanent water bodies (Hobbs’, 1942; Horwitz and Richardson’s, 1986; Richardson, 2007). This species builds burrow galleries of 1–5 interconnected tunnels, which can reach a horizontal depth up to 1m and a vertical depth of ± more than 70cm ending in a life chamber. The entries of the galleries are delimited by simple holes or by chimneys, which can reach up to 10 cm high and wide ( Fig. 10 C, D View FIGURE 10 ).
Distribution. Parastacus gomesae sp. nov. has a limited distribution, being registered only in the municipality of São Jerônimo, state of Rio Grande do Sul, southern Brazil ( Fig. 1 View FIGURE 1 ).
Conservation status. Data Deficient. The EOO and AOO were estimated as approximately 2.107 km ² and 23, 9 km ² (or 10, 5 km ² considering only preserved areas), respectively ( Fig. 1 View FIGURE 1 ). This species has a similar situation to P. guapo sp. nov., it also occurs inside a large forestry area and that is the only known location for it. Furthermore, it also can only be included in sub-item b (iii), continuous decline observed in habitat quality and should be classified as DATA DEFICIENT.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Astacidea |
|
SuperFamily |
Parastacoidea |
|
Family |
|
|
Genus |