Robertsonidra porifera ( Maplestone, 1909 )
|
publication ID |
https://doi.org/10.1080/00222933.2016.1253797 |
|
DOI |
https://doi.org/10.5281/zenodo.4333633 |
|
persistent identifier |
https://treatment.plazi.org/id/03BE87C2-D13C-5C2D-639A-FA12FC4BFA25 |
|
treatment provided by |
Carolina |
|
scientific name |
Robertsonidra porifera ( Maplestone, 1909 ) |
| status |
|
Robertsonidra porifera ( Maplestone, 1909) View in CoL
( Figure 27 View Figure 27 (d – f))
Schizoporella porifera Maplestone, 1909, p. 416 , pl. 27, fig. 16.
Robertsonidra porifera: Tilbrook 2006, p. 263 View in CoL , pl. 58A – C.
Robertsonidra novella: Ryland and Hayward 1992, p. 261 , fig. 19(a).
Robertsonidra novella: Tilbrook et al. 2001, p. 92 , fig. 18(d).
Material examined
NSMT-Te 1155 ( MIN- 26), bleached, on SEM stub; NSMT-Te 1156, dried specimen, SES site.
Measurements
AzL, 0.50 – 0.69 (0.602 ± 0.066); AzW, 0.37 – 0.50 (0.443 ± 0.037) (n = 15, 1). OrL, 0.10 – 0.13 (0.114 ± 0.009); OrW, 0.13 – 0.15 (0.138 ± 0.006) (n = 15, 1). OvL, 0.26 – 0.38 (0.308 ± 0.040); OvW, 0.31 – 0.40 (0.362 ± 0.029) (n = 9, 1). Largest colony observed 30 × 20 mm.
Description
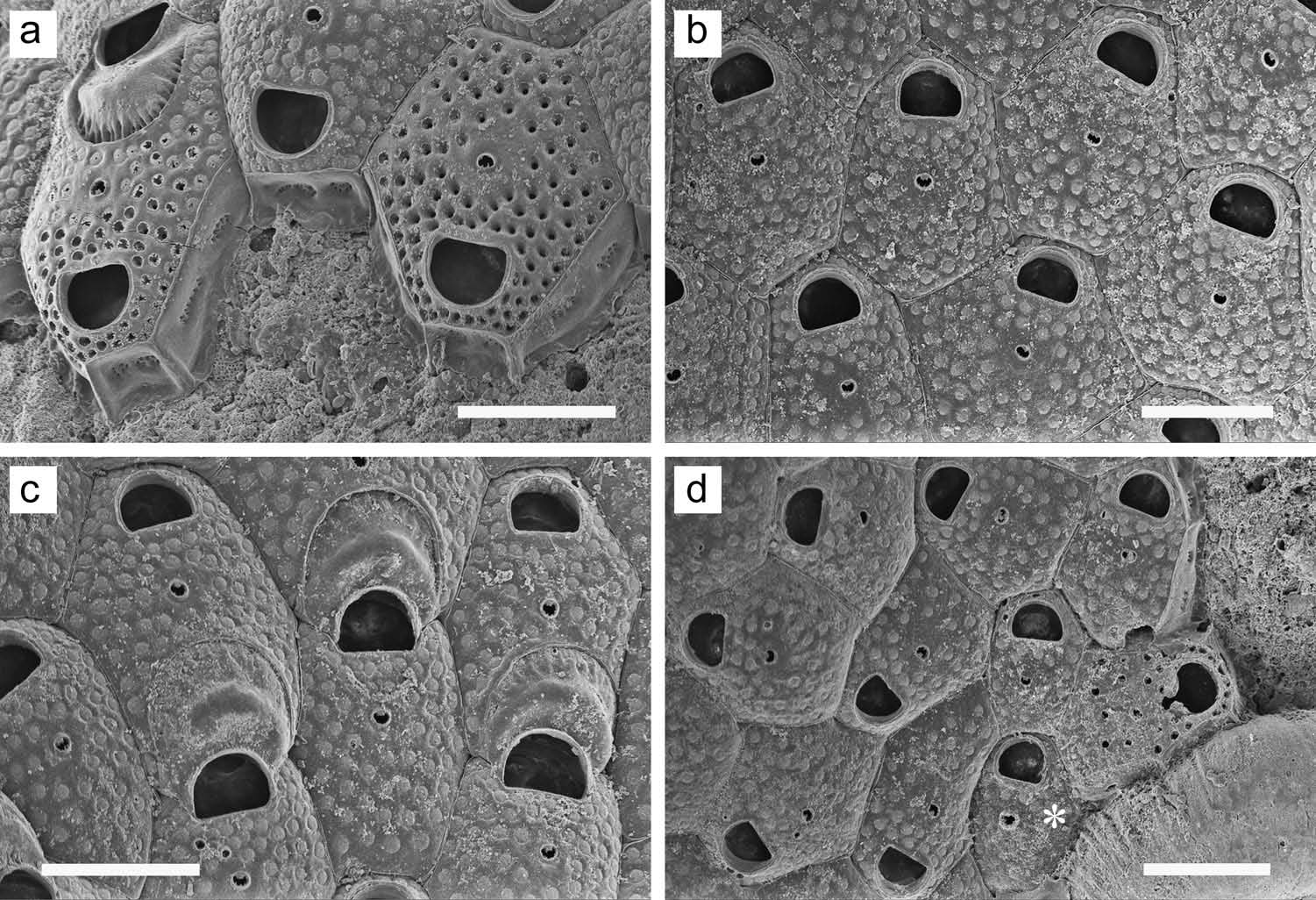
Colony forming a unilaminar, encrusting sheet; white or faintly yellowish in colour. Zooids ( Figure 27 View Figure 27 (d – f)) distinct, delineated by areolae and suture line. Frontal wall weakly convex, without pseudopores, completely and densely covered with small tubercles, with nine to 12 small areolae along each lateral margin. Orifice ( Figure 27 View Figure 27 (d)) transversely elliptical, with a small, shallow, semicircular proximal sinus; surrounded by low, narrow, smooth rim; peristome lacking. Slight umbo often present proximal to orifice. Most autozooids show evidence of one or (more commonly) two small, ephemeral spines distal to orifice; ovicelled zooids often show a pair of more widely spaced spine bases lateral to orifice. Single frontal avicularium uncommonly present proximolateral to orifice. Avicularia either of two types: in one ( Figure 27 View Figure 27 (e)), mandible acute, short-triangular, directed laterally; in other ( Figure 27 View Figure 27 (d)), mandible long, tapering, rounded at tip, directed proximolaterally. No paired avicularia observed, and most zooids lack avicularium. Ovicell ( Figure 27 View Figure 27 (f)) hyperstomial; ooecium globose, more densely tuberculate than frontal wall, covered with minute pseudopores. Ancestrula not observed.
Remarks
Apparently unaware of Maplestone ’ s (1909) description (as Schizoporella porifera ) of this species from the Gilbert Islands, Ryland and Hayward (1992) described the same species as Robertsonidra novella from the Great Barrier Reef. Tilbrook (2006) rectified this apparent error.
Occurrence
We found one colony at the SES site and two at the MIN site. Robertsonidra porifera is broadly distributed in shallow habitats in the subtropical to tropical western Pacific. It has been reported from the Gilbert Islands ( Maplestone 1909), the Great Barrier Reef ( Ryland and Hayward 1992), Vanuatu ( Tilbrook et al. 2001) and the Solomon Islands ( Tilbrook 2006); Tilbrook (2006) noted that it also occurs in the South China Sea and Victoria, southern Australia, but did not give specific records.
Family MICROPORELLIDAE Hincks, 1879
Genus Fenestrulina Jullien, 1888
Fenestrulina parviporus sp. nov.
( Figure 28 View Figure 28 )
Fenestrulina caseola: Ryland and Hayward 1992, p. 280 , fig. 26(d). Tilbrook 2006, p. 217, figs 46(f) and 47(f). Dick et al. 2006, p. 2235, fig. 12(d).
? Fenestrulina catasticos: Scholz 1991, p. 315 , pl. 16, fig. 6, pl. 17, fig. 7.
Not Fenestrulina caseola Hayward, 1988, p. 325 , pl. 10d.
Etymology
The specific name is a noun in apposition combining the Latin parvus (small) and porus (pore), referring to the proportionally small ascopore compared to the congener F. caseola .
Material examined
Holotype: NSMT-Te 1157, dried specimen, SES site . Paratype: NSMT-Te 1158 ( SES- 6), bleached, on SEM stub.
Measurements
AzL, 0.48 – 0.65 (0.571 ± 0.053); AzW, 0.34 – 0.57 (0.431 ± 0.069) (n = 15, 1). OrL, 0.11 – 0.12 (0.113 ± 0.004); OrW, 0.14 – 0.16 (0.147 ± 0.008) (n = 15, 1). OvL, 0.24 – 0.34 (0.28 ± 0.031); OvW, 0.25 – 0.29 (0.27 ± 0.012) (n = 13, 1). Ascopore W 0.026 – 0.036 (0.030 ± 0.003); distance orifice to ascopore, 0.12 – 0.16 (0.137 ± 0.008); ratio between ascopore-to-orifice distance and ascopore width, 3.78 – 5.58 (4.582 ± 0.449) (n = 15, 1). Largest colony observed 11 × 4 mm.
Description
Colony forming a unilaminar, encrusting sheet; white in colour. Zooids ( Figure 28 View Figure 28 (a, b)) distinct, irregularly hexagonal, outlined by a fine incision. Frontal wall moderately convex; completely covered with infundibular pseudopores except in area surrounding ascopore; each pseudopore has a cribriform covering. Orifice ( Figure 28 View Figure 28 (a)) D-shaped, broader than long, proximal margin straight. Ascopore small, transversely elliptical, surrounded by raised rim; inner margin denticulate or smooth, usually with projection from distal side, resulting in lunate opening. Ascopore distance from orifice equal to, or slightly greater than, orifice length. Ovicell ( Figure 28 View Figure 28 (c)) subimmersed. Ooecium with conspicuous, thick, crescent- or chevron-shaped transverse ridge on top, and surrounded by sharp, raised border; periphery inside border depressed, with minute pseudopores separated by ridges. Spines lacking. Zooids interconnect ( Figure 28 View Figure 28 (a)) by two large, circular to oval multiporous septula in transverse wall and two extensive, elongate-oval multiporous septula in each distolateral wall. Ancestrula ( Figure 28 View Figure 28 (d), asterisk) smaller than but otherwise similar to subsequent zooids, budding one daughter zooid distally and another distolaterally, with spiral budding ensuing from the opposite distolateral position.
Remarks
Fenestrulina parviporus sp. nov. is very similar to Fenestrulina caseola Hayward, 1988 , originally described from Mauritius. Our material differs from the latter as follows. The ascopore is proportionally smaller, and is open rather than covered with anastomosing rays or a cribriform plate; there is an imperforate zone surrounding the ascopore; and the orifice is D-shaped, whereas in F. caseola it is broader relative to length, with more sharply rounded lateral margins recurving to the proximal margin. Various Pacific specimens identified as C. caseola in previous studies show characters like those in the Okinawa material: the ascopore is small and lunate, surrounded by an imperforate zone, and the orifice is D-shaped. The average ratio between the ascopore-to-orifice distance and ascopore width is similar in Pacific specimens: 4.44 (n = 2), Great Barrier Reef ( Ryland and Hayward 1992); 4.06 (n = 2), Hawaii Island ( Dick et al. 2006); 4.33 (n = 5), Solomon Islands ( Tilbrook 2006); and 4.58 (n = 15), Okinawa (this study). The ratio is 1.58 (n = 2) in F. caseola from Mauritius ( Hayward 1988), reflecting the greater width of the ascopore.
Another similar species is F. catasticos Gordon, 1984 , described from the Kermadec Islands near New Zealand. That species has a small, lunate ascopore lying at the bottom of a concavity surrounded by a distinct, raised rim; the ooecium is less sunken and has a coarse central umbo from which thick ribs radiate; the pseudopores distal to the orifice are surrounded by a line of calcification, delineating a distinct ‘ panel ’ of pores; and there are three large basal pore-chambers in the distal half of each zooid ( Gordon 1984), whereas F. parviporus has a total of six multiporous septula in the distal half. Material from the Philippines that Scholz (1991) identified at F. catasticos seems more similar to F. parviporus , although the ratio between ascopore-to-orifice distance and ascopore width is less (3.25) in the one mature zooid Scholz illustrated. Scholz did not show or describe the ovicell, which would help identify the Philippine species.
Taxonomically informative characters in the better-studied confamilial genus Microporella include orifice shape and the size, shape, position and covering (or lack thereof) of the ascopore, and these characters are undoubtedly also informative in Fenestrulina . On this basis, we consider material previously described as F. caseola to comprise two species: F. caseola , presently known only from Mauritius but perhaps more broadly distributed in the Indian Ocean, and F. parviporus , broadly distributed in the western and central Pacific.
Occurrence
We found four colonies, all at the SES site. This species is broadly distributed in the western to central, tropical to subtropical Pacific; there are previous records (as F. caseola ) from the Great Barrier Reef, the Solomon Islands and Hawaii.
Family LACERNIDAE Jullien, 1888
Genus Arthropoma Levinsen, 1909
Arthropoma harmelini sp. nov.
( Figure 29 View Figure 29 )
Etymology
The specific name is a Latinised noun in the genitive case, after Dr. Jean-Georges Harmelin, in acknowledgement of his substantial contributions to bryozoology and generous assistance in this study.
Material examined
Holotype: NSMT-Te 1159 ( SES-28 ), bleached, on SEM stub . Paratypes: NSMT-Te 1160, five dried specimens, SES site; NSMT-Te 1161, two dried specimens, SES site; NSMT-Te 1162, three dried specimens, REEF site; NHMUK 2016.5.13.60-71, 12 dried specimens, SES site . For comparison, putative A . cecilii from the vicinity of Marseille , France, collected by J . G . Harmelin : NSMT-Te 1163 (MA-1), Rior Island, 70 m, on dendritic sand, 11 June 1971, bleached, on SEM stub; NSMT-Te 1164 (MA-2), Frioul Island, on dendritic sand, 65 m, 21 April 1971, bleached, on SEM stub .
Measurements
Okinawa: AzL, 0.55 – 0.70 (0.629 ± 0.045); AzW, 0.37 – 0.54 (0.452 ± 0.048) (n = 15, 1). OrL (exclusive of sinus), 0.10 – 0.13 (0.109 ± 0.008); OrW, 0.14 – 0.18 (0.155 ± 0.010) (n = 15, 1). OvL, 0.26 – 0.37 (0.322 ± 0.031); OvW, 0.32 – 0.38 (0.346 ± 0.015) (n = 15, 1). Largest colony observed roughly circular, 25 mm across.
For comparison, measurements from a Mediterranean specimen (vicinity of Marseille, France) presumed to be Arthropoma cecilii ( Figure 30 View Figure 30 ): AzL, 0.66 – 0.79 (0.715 ± 0.040); AzW, 0.42 – 0.55 (0.493 ± 0.043) (n = 15, 1). OrL (exclusive of sinus), 0.13 – 0.16 (0.151 ± 0.008); OrW, 0.17 – 0.21 (0.191 ± 0.009) (n = 15, 1). OvL, 0.40 – 0.47 (0.429 ± 0.025); OvW, 0.37 – 0.41 (0.389 ± 0.016) (n = 8, 1).
Description
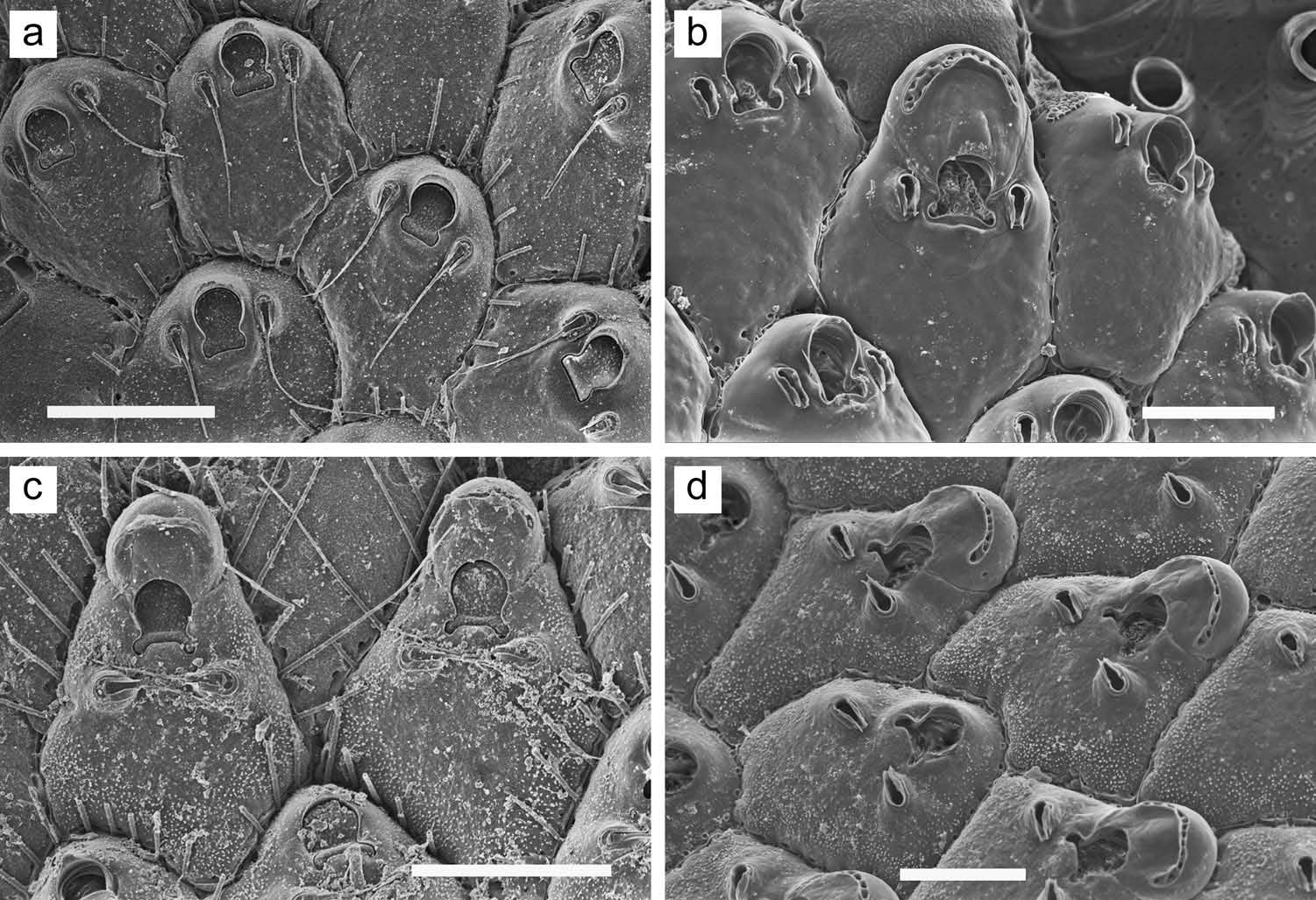
Colony forming a unilaminar, encrusting sheet; light yellowish tan. Zooids ( Figure 29 View Figure 29 (a)) irregularly hexagonal; distinct, delineated by a suture line. Frontal wall mildly convex, completely covered with small pseudopores, although some zooids lack pseudopores ( Figure 29 View Figure 29 (c, d)) in midline proximal to orifice; one to three rows of pseudopores lateral and distal to orifice ( Figure 29 View Figure 29 (a)). Primary orifice ( Figure 29 View Figure 29 (b)) D-shaped, with shallow sinus in midline; sinus usually constricted at opening, circular or transversely oval in outline; row of inconspicuous denticles on proximal orificial margin to each side of sinus. Primary orifice weakly dimorphic in size; generally slightly larger (wider and/or longer) in ovicelled zooids or zooids capable of forming ovicell than in non-ovicelled zooids. Distal margin of orifice raised as a low rim, sharp or thickened. Just proximal to orifice, most zooids have a low, crescentic or chevronshaped umbo, usually with some pseudopores in area between umbo and orifice; in some cases, ends of umbo extend distolaterally to meet circum-oral rim, forming an entire peristomial rim (top central zooid in Figure 29 View Figure 29 (a)). Ovicell ( Figure 29 View Figure 29 (c, d)) hyperstomial. Ooecium without pseudopores, except at periphery; minutely pitted on the surface; with a smooth, broad crescentic or chevron-shaped zone along proximal margin; ooecial periphery wavy at base, with regularly spaced pseudopores. Floor of developing ovicell ( Figure 29 View Figure 29 (e)) meets distal orificial margin, with no intervening pseudopores. Embryo orange in dried specimens. Spines and avicularia lacking. Zooids interconnect ( Figure 29 View Figure 29 (f)) by long zone of up to 20 pores in each of the transverse and distolateral walls. Ancestrula not observed.
Remarks
Our specimens are identifiable with Arthropoma cecilii ( Audouin 1826) sensu lato. This nominal species has a putative global distribution from cool-temperate to tropical waters between about 50°N and 40°S, with records from depths ranging from around 25 m ( Harmer 1957) to as great as 1235 m ( Canu and Bassler 1929). There are records from the Mediterranean, Britain, Brazil, the Galapagos, Costa Rica (Pacific), central California, British Columbia, Japan, the Philippines, the China Sea, the Great Barrier Reef, New Zealand, the Indian Ocean and South Africa ( Canu and Bassler 1929; Osburn 1952; Harmer 1957; Gordon 1984). Harmer (1957, p. 1003) noted, ‘ A. cecilii is so easily recognized that most of the records are probably correct ’. Unfortunately, this perception has resulted in many authors simply reporting this species without description or illustration; there are several such records from Pleistocene and Recent Japan ( Okada 1923; Sakakura 1935, 1938; Okada and Mawatari 1936, 1938; Mawatari 1952, 1963).
One explanation for this apparently global distribution is that A. cecilii has been widely spread on ship hulls over the past several centuries. An alternative explanation is that nominal A. cecilii actually represents a broadly distributed complex of morphologically similar species. In fact, slight morphological differences exist among geographically separate populations, indicative of population isolation and genetic divergence; these differences have to do with the sculpturing on the ooecium, extent of a frontal area proximal to the orifice that lacks pseudopores, depth and shape of the orificial sinus, presence or absence of a median suboral umbo, presence or absence of one or more rows of pseudopores between the orifice and ovicell floor (evident in zooids with incompletely formed ovicells), and zooid size.
Arthropoma cecilii ( Audouin 1826) was originally described from Egypt, but the type locality is unknown. Two of Savigny ’ s plates ( Audouin 1826) show what appear to be Arthropoma specimens. Audouin (1826) designated one of these (Savigny Plate 8) as Flustra ? cecilii and the other (Savigny Plate 10) as Flustra latreillei . The latter probably came from the Red Sea (J.-G. Harmelin personal communication), as Savigny ’ s Plate 10 shows the bryozoan colony encrusting a branch of an Acropora coral. Although d ’ Hondt (2006) considered both plates to represent Arthropoma cecilii , it is possible they represent different species, especially if one specimen came from the Red Sea and the other from the Mediterranean, because there was no connection between the Mediterranean and Red Sea prior to the opening of the Suez Canal in 1869.
British A. cecilii described in Hayward and Ryland (1999) has larger zooids (average ZL about 0.75 mm; average ZW about 0.50 mm) than A. harmelini (comparable values, 0.63 mm and 0.45 mm, respectively) and further differs from the latter in having a deeper, narrower, U-shaped orificial sinus and a small, mammillate umbo in the midline some distance proximal to the orifice. In Hayward and Ryland (1999, fig. 94(a)), zooids appear to have a narrow zone, extending along about two-thirds of the midline proximal to the orifice, that lacks pseudopores. There is no indication whether a zone of pseudopores separates the floor of the ovicell from the distal orificial margin.
In Mediterranean specimens from near Marseille ( Figure 30 View Figure 30 ), which may or may not be conspecific with British A. cecilii populations, zooids are about the same size as indicated for British material but larger than in our specimens (see Measurements above). There is a distinct area, proximal to the orifice, that lacks pseudopores ( Figure 30 View Figure 30 (a, d)), extending about two-thirds the distance to the proximal end of the zooid; the proximal orificial sinus is U-shaped ( Figure 30 View Figure 30 (b)) and longer than broad, with a constricted opening; some zooids show a very slight umbo ( Figure 30 View Figure 30 (c)) just proximal to the orificial sinus; and one or two rows of frontal pseudopores are evident between the distal margin of the orifice and the floor of the ovicell ( Figure 30 View Figure 30 (a, c, d)). Some ooecia in the Marseille population show a distorted lateral expansion ( Figure 30 View Figure 30 (a)), a trait also occasionally observed in the Okinawan specimens ( Figure 29 View Figure 29 (c)).
There appear to be several Arthropoma species in Japan. Ortmann (1890) described (as Schizoporella cecilii ) a new variety (now A. cecilii var. mediolaevis ) from 40 fathoms ( 73 m) depth in Sagami Bay, on the basis of its having on the frontal wall a more extensive median area lacking pseudopores than in the typical form; presumably Ortmann had seen Atlantic or Mediterranean A. cecilii and this character struck him as different. Hirose ’ s (2010, pl. 189A, B) SEM images of a Sagami Bay specimen identified as A. cecilii show an extensive area lacking pseudopores, and small, paired tubercles lateral to the orifice; zooids are large (average ZL × ZW, 0.73 × 0.56 mm) and appear to lack a frontal umbo. Zooids in two other panels in the same plate ( Hirose 2010, pl. 189C, D) have a shorter, rounder suboral sinus, raising the question whether they are from the same colony as the first two panels. Hirose (2010, pl. 190) also illustrates from Sagami Bay another two colonies identified as ‘ Arthropoma n. sp. ’ that have much smaller zooids (average ZL × ZW, 0.43 × 0.40 mm). It is not clear that these two colonies are conspecific with one another. The specimen in his pl. 190A, B has relatively few, large, densely packed pseudopores; a distinctly U-shaped sinus; and one row of large pseudopores between the orifice and the floor of the ovicell. The other (his pl. 190 C) has a shallower sinus and a large frontal area that lacks pseudopores. Grischenko and Mawatari (2006, fig. 1(g)); illustrate a subtidal Sagami Bay specimen identified as A. cecilii in which zooids have a narrow non-pseudoporous zone occupying only half the frontal length proximal to the orificial sinus, which is oval or teardrop-shaped. This species, with an extensive zone of pores evident between the distal orificial margin and the ovicell floor, is clearly different from that in Hirose ’ s (2010, pl. 189A – B) specimen in which the floor of the ovicell arises directly from the orificial margin, with no intervening pseudopores.
In summary, SEM images in Hirose (2010) and Grischenko and Mawatari (2006) indicate at least three species of Arthropoma in Sagami Bay ; it is unclear which, if any, represents Ortmann ’ s (1890) A. cecilii var. mediolaevis . Arthropoma harmelini differs from two of these species ( Grischenko and Mawatari 2006, fig. 1(g); Hirose 2010, pl. 190A, B) in lacking pseudopores between the orificial margin and floor of the developing ovicell; from the third ( Hirose 2010, pl. 189A, B) in sinus shape; and from all three in having a crescentic suboral umbo that sometimes contributes to a continuous peristomial rim. Our specimens might be conspecific with Arthropoma cecilii reported by Kataoka (1961) from the Pleistocene of Kikai Island, roughly 300 km north-east of our study site. While Kataoka did not provide a description, a low-magnification photomicrograph appears to show a short, broad sinus and a median frontal zone lacking pseudopores; zooid measurements are similar to those in our material (by chance, the mean zooid length is identical).
Occurrence
Arthropoma harmelini was abundant at the SES site and common at the REEF site ( Table 1); these are the only known localities.
Family ESCHARINIDAE Tilbrook, 2006
Genus Bryopesanser Tilbrook, 2006
Bryopesanser latesco Tilbrook, 2006
( Figure 31 View Figure 31 )
Bryopesanser latesco Tilbrook, 2006, p. 255 , pls. 55D, 56A – C. Tilbrook 2012, p. 46, figs 11 – 16. Bryopesanser serratus Dick, Tilbrook, and Mawatari, 2006, p. 2233 , fig. 5(d – f).
For additional synonyms, see Tilbrook (2012).
Material examined
NSMT-Te 1054 (MIN-Thal1), bleached, on SEM stub (with Thalamoporella stapifera ); NSMT- Te 1100 ( MIN- 13), bleached, on SEM stub (with Crepidacantha poissonii and Parasmittina serrula ); NSMT-Te 1103 ( MIN- 12), bleached, on SEM stub (with Parasmittina alanbanneri ); NSMT-Te 1165, dried specimen, MIN site (with Cribralaria curvirostris ); NSMT-Te 1167 ( MIN- 19), bleached, on SEM stub (with Crepidacantha longiseta ).
Measurements
AzL, 0.52 – 0.64 (0.579 ± 0.041); AzW, 0.30 – 0.46 (0.376 ± 0.063) (n = 10, 1). OrL, 0.08 – 0.09 (0.083 ± 0.005); OrW (excluding sinus), 0.09 – 0.11 (0.105 ± 0.007) (n = 7, 1). OvL, 0.11 – 0.21 (0.141 ± 0.030); OvW, 0.16 – 0.24 (0.204 ± 0.024) (n = 15, 1).
Description
Colony forming a unilaminar, encrusting sheet that is typically small; one of those illustrated ( Figure 31 View Figure 31 (d)) measures 3 × 1.5 mm, with 32 zooids, 19 of which have ovicells. Zooids ( Figure 31 View Figure 31 (a)) distinct, delineated by a groove. Frontal wall convex, finely tuberculate, covered with minute pseudopores. Orifice (exclusive of sinus) D-shaped ( Figure 31 View Figure 31 (b)); proximal sinus deep, usually constricted at opening and flaring proximally. Condyles sloping proximomedially; each condyle is serrate on frontal face; proximal margin of orifice to each side of sinus is similarly serrate. Marginal zooids have seven coarse spines ( Figure 31 View Figure 31 (a)) laterally and distally around orifice; three pairs are evident lateral to orifice in ovicelled zooids. Orifice in nonovicelled zooids is not deeply immersed. Proximal to orifice is a raised, flared peristomial collar, with the frontodistal surface smooth; outer edge of collar usually sharp, sometimes with median projection. Avicularia small; single or paired lateral to orifice; rostrum truncate, directed distomedially; cross-bar complete. Mandible not observed. Ovicell ( Figure 31 View Figure 31 (c, d)) terminal. Ooecium broader than long, without pseudopores; surface weakly rugose or tuberculate, often with low transverse ridge that sometimes bears a small median umbo. Ancestrula not observed.
Remarks
Bryopesanser is morphologically a rather uniform genus, with 16 Recent species known at present from tropical and subtropical areas. Colonies are typically small; those in B. latesco can reach up to roughly 1 cm across, while most other species have smaller maximum size. The most conspicuous character for identification of B. latesco is the broadly flared peristomial rim that delineates an extensive smooth area proximal to the orifice ( Figure 31 View Figure 31 (b, c)). Another character is having a ‘ drop-shaped ’ oral sinus that is broader than long ( Tilbrook 2012); while this is the case in some zooids in our specimens ( Figure 31 View Figure 31 (d)), other zooids have the sinus clearly longer than broad ( Figure 31 View Figure 31 (b)).
Occurrence
We found two colonies at SES and 10 at MIN. This is a widely distributed Indo-Pacific species, documented from the Red Sea, the Gulf of Aden, the Great Barrier Reef, Southeast Asia, the Philippines, Taiwan, Melanesia and Hawaii; there is also a disjunct record from the Caribbean coast of Panama ( Tilbrook 2012).
Superfamily MAMILLOPOROIDEA Canu and Bassler, 1927 Family CREPIDACANTHIDAE Levinsen, 1909
Genus Crepidacantha Levinsen, 1909
Crepidacantha longiseta Canu and Bassler, 1928 sensu lato
( Figure 32 View Figure 32 (a, b))
? Crepidacantha longiseta Canu and Bassler, 1928, p. 135 , pl. 21, figs 3 and 4.? Crepidacantha longiseta: Brown 1954, p. 251 , fig. 1(f).
Crepidacantha longiseta: Tilbrook et al. 2001, p. 92 , fig. 16(b). Dick et al. 2006, p. 2239, fig. 13(g, h).
Material examined
NSMT-Te 1167 ( MIN- 19), bleached, on SEM stub (with Bryopesanser latesco ); NSMT-Te 1168 ( MIN- 35), bleached, on SEM stub (with Hippopodina adunca ); NSMT-Te 1169 ( SES- 37), bleached, on SEM stub; NSMT-Te 1170, MIN site, three dried fragments; NSMT-Te 1171, dried colony, SES site.
Measurements
AzL, 0.39 – 0.54 (0.466 ± 0.049); AzW, 0.32 – 0.52 (0.374 ± 0.054) (n = 15, 1). OrL, 0.10 – 0.12 (0.104 ± 0.006); OrW, 0.07 – 0.09 (0.084 ± 0.006) (n = 15, 1). OvL, 0.199 – 0.227 (average = 0.210); OvW, 0.209 – 0.213 (average = 0.211) (n = 3, 2). Largest colony observed 4.7 × 3.6 mm.
Description
Colony forming a unilaminar, encrusting sheet. Zooids ( Figure 32 View Figure 32 (a)) distinct, delineated by a sharp incision. Frontal wall convex, tumid, without pseudopores, smooth or minutely granulate in texture. Circular or slit-shaped marginal areolae present but rarely evident with light microscopy and often scarcely with SEM. Orifice subterminal, raised, hoof-shaped, with subcircular anter separated from equally broad poster by conspicuous, low, rounded-triangular condyles; proximal margin straight or slightly convex. Operculum with granular surface. Orifice not immersed; proximal to orifice is a small, low, mammillate or transversely crescentic projection. Zooids have 8 – 11 (mode = 10) long, thin, straight spines around distal and lateral margins, each spine tilted against frontal wall of adjacent zooid. Frontal avicularia paired, lateral to orifice, directed proximally; rostrum shorter than autozooidal orifice; mandibular part of rostrum elongate, tapering, with end forming trough; mandible ( Figure 32 View Figure 32 (a)) setiform, very long, sometimes reaching proximal border of zooid. Ovicell ( Figure 32 View Figure 32 (b)) terminal, about as broad as long; closed by operculum. Ooecium with smooth ectooecium; flattened on top, with low median ridge in proximal half evident in cleaned specimens; crescentic foramen of non-calcified ectooecium around frontodistal periphery of ooecium exposing up to 20 pseudopores in a single or double row. Ancestrula, poorly visible in one specimen, appears to be smaller than but of same form as later zooids, with spiral budding pattern, although these details are ambiguous.
Remarks
This species has the proximal margin of the orifice straight or nearly so; around 10 marginal spines; and small, paired frontal avicularia lateral to the orifice that have a long, setose mandible directed proximally. Although the original description mentions a concave poster, the proximal orificial margin appears straight in at least some zooids illustrated by Canu and Bassler (1928, pl. 21, fig. 4). The original description also mentions incomplete calcification of the ectooecium in a frontal area on the ovicell, and a small mucro proximal to the orifice. In his revision of Crepidacantha, Brown (1954) illustrated the frontal fenestra on the ooecium in a specimen from Brazil as being transversely oval and positioned atop the ooecium, clearly a different condition than in Pacific material, where the frontal area has been described as horseshoe shaped ( Tilbrook et al. 2001) or comprising a crescent near the distal end ( Dick et al. 2006). Brown (1954) made no mention of a proximal mucro, and this character seems variable among Pacific populations; it is present in specimens from Hawaii and Okinawa, but appears to be lacking in those from Vanuatu ( Tilbrook et al. 2001).
Canu and Bassler (1928) originally described this species from coral and hydroid substrata at depths of 67 – 201 fathoms ( 123 – 368 m) in the western Atlantic (Strait of Florida, off Cuba). Subsequent reports have indicated a much broader range, including Brazil ( Brown 1954), Mauritius ( Hayward 1988), Vanuatu ( Tilbrook et al. 2001) and Hawaii ( Dick et al. 2006). It seems unlikely that all these far-flung populations have maintained reproductive continuity, especially between the Pacific and Atlantic, and also unlikely that this species has been spread anthropogenically, as it is typically uncommon in natural habitats, rather than occurring in abundance in harbours. We thus expect that the nominal species will eventually prove to encompass more than one species, with western Pacific material requiring a different name from that originally described from the western Atlantic. For the time being, we use the name C. longiseta sensu lato for the specimens from Okinawa.
Occurrence
We found six colonies, at the SES and MIN sites. Although Crepidacantha longiseta appears to have a circumglobal distribution in the subtropical and tropical zones ( Tilbrook et al. 2001), this is probably not the case, as discussed above.
| MIN- |
University of Minnesota |
| SES |
Southeastern Shanxi Teachers School |
| SES- |
Southeastern Shanxi Teachers School |
| NHMUK |
Natural History Museum, London |
| MIN |
University of Minnesota |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Inovicellina |
|
SuperFamily |
Buguloidea |
|
Family |
|
|
Genus |
Robertsonidra porifera ( Maplestone, 1909 )
| Dick, Matthew H. & Grischenko, Andrei V. 2016 |
Robertsonidra porifera
| : Tilbrook 2006: 263 |
Robertsonidra novella :
| Tilbrook 2001: 92 |
Robertsonidra novella :
| Ryland and Hayward 1992: 261 |
Schizoporella porifera
| Maplestone 1909: 416 |