Baetis (Rhodobaetis) vadimi, Godunko, Roman J., Palatov, Dmitry M. & Martynov, Alexander V., 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3948.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:9BB1C85A-E8C5-4A12-AD23-2FEE26FFE431 |
|
DOI |
https://doi.org/10.5281/zenodo.5624728 |
|
persistent identifier |
https://treatment.plazi.org/id/03B3A476-FF8D-876D-FF2C-9CBA9D46FC8C |
|
treatment provided by |
Plazi |
|
scientific name |
Baetis (Rhodobaetis) vadimi |
| status |
sp. nov. |
Baetis (Rhodobaetis) vadimi sp. nov.
Figures 1–41 View FIGURES 1 – 3 View FIGURES 4 – 9 View FIGURES 10 – 15 View FIGURES 16 – 21 View FIGURES 22 – 24 View FIGURES 25 – 31 View FIGURES 32 – 40 View FIGURE 41
? Baetis gemellus Etn. : Kazancı, 1984, Aquatic Insects, 6 (4): 254 [partim]
? Baetis gemellus Eaton, 1885 View in CoL : Kazancı, 2001, Türkiye iç Suları Arastırmaları Dizisi, 6: 25, 36; Kazancı & Türkmen, 2012, Review of Hydrobiology, 5 (2): 147; Türkmen & Kazancı, 2013, Review of Hydrobiology, 6 (1): 36, fig. 2A, B [partim]? Baetis (Baetis) gemellus Eaton, 1885 View in CoL : Novikova, 1987, Podenki semeistva Baetidae (Ephemeroptera) View in CoL fauny SSSR, 70, 225 [partim]
Types. HOLOTYPE: larva, TURKEY, Kaçkar Mountains, Rize il [Province], Ardeşen ilçe [Ardeshen District], unnamed brook, small right-side tributary of upper part of Fırtına Deresi [Fırtına stream], h ~ 2540 m a.s.l., 41°04'29.91"N, 41°18'0.61"E, 26.viii.2012, leg. D.M. Palatov.
PARATYPES: 31 larvae (14 larvae mounted with Liquide de Faure: 9 larvae in SMNH NASU and 5 larvae in MSU collections), same locality and date as holotype, leg. D.M. Palatov; 50 larvae (4 larvae mounted with Canada balsam—slides nr. 579, 583– 585 in NMNH NASU), GEORGIA, Adzharia [Autonomous Republic of Adjara], Kobulety District, Kintrishi State Nature Reserve, between Narusala and Sarbiela Mts., limnocrene and spring (left tributary of Cherulisghele River), 41°45'40"N 42°06'29"E, h ~ 2305 m a.s.l., 10.vi.2013, leg. A.V. Martynov; 5 larvae, GEORGIA, ibid, flank of Narusala Mt., stream—source of Cherulisghele River, 41°46'21"N 42°05'39"E, h ~ 2160 m a.s.l., 11.vi.2013, leg. A.V. Martynov; 16 larvae, GEORGIA, ibid, flank of Narusala and Khino Mts., stream—source of Bzhudzha River (near snowfields), 41°46'00"N 42°06'59"E, h ~ 2260 m a.s.l., 10.vi.2013, leg. A.V. Martynov.
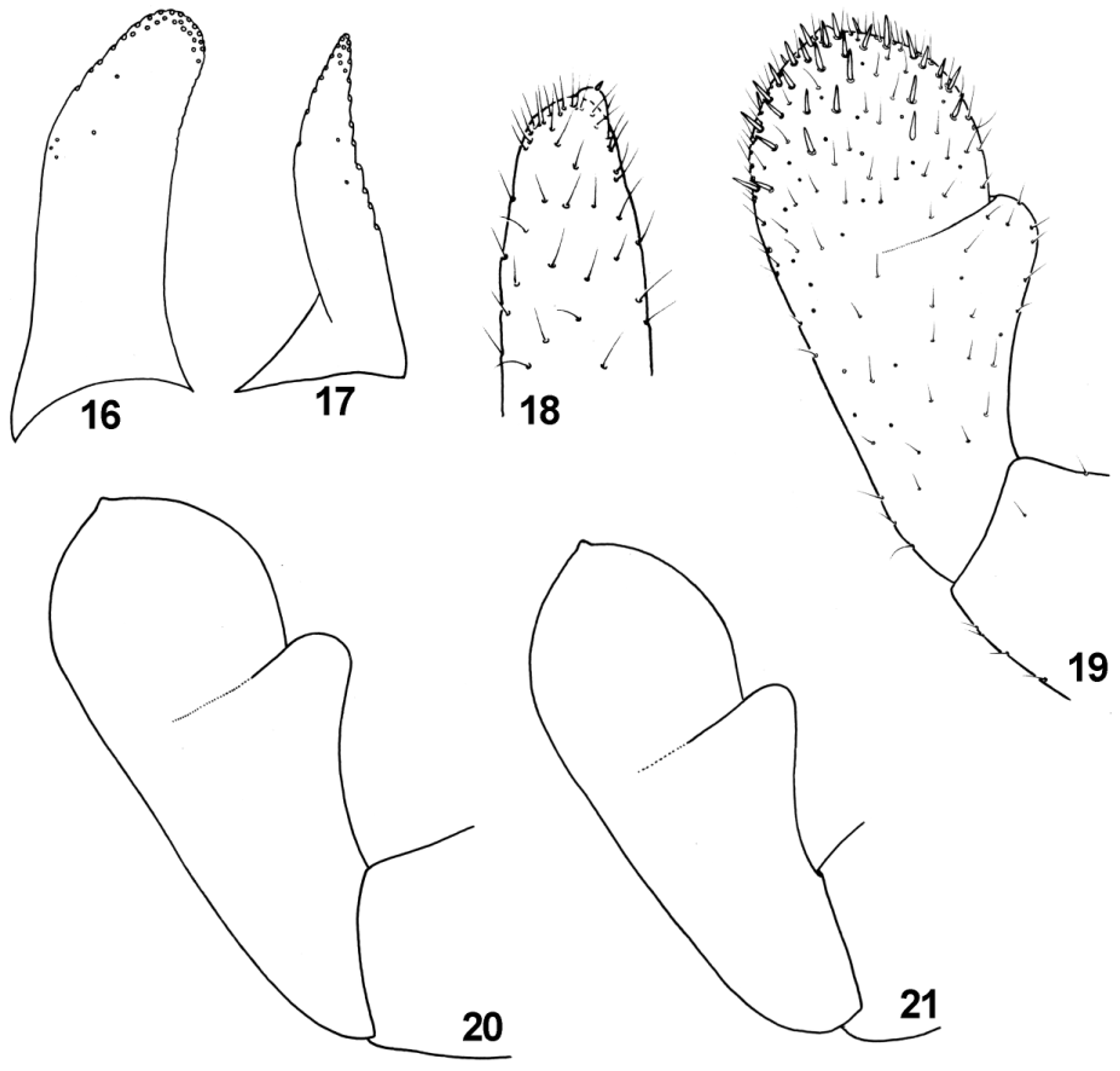
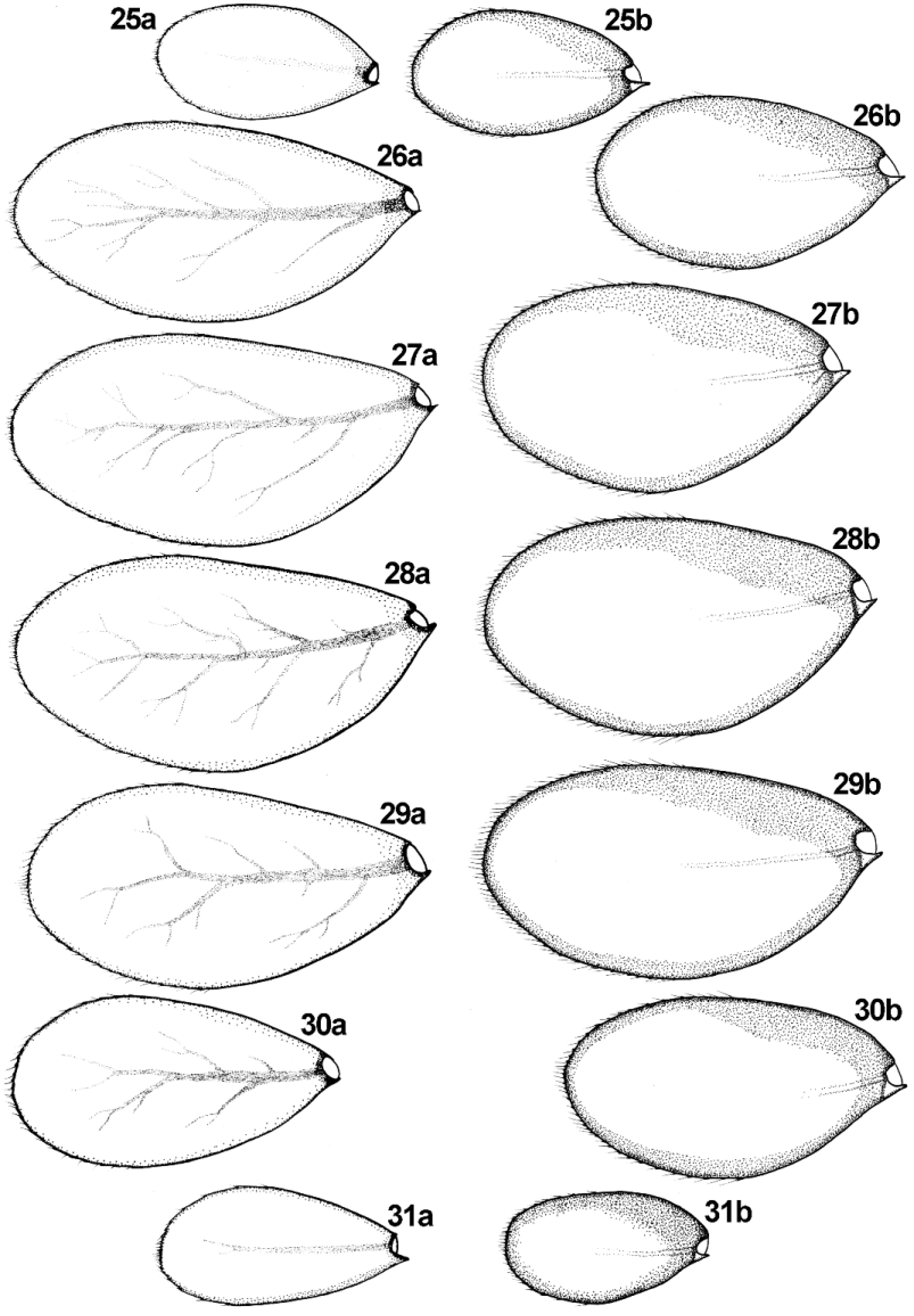
Diagnosis. The new species can be distinguished from other representatives of the subgenus Rhodobaetis by the combination of following characters: (i) paraglossae with two irregular rows of long stout bristles apically ( Fig. 16 View FIGURES 16 – 21 ); (ii) segment III of labial palps almost symmetrical ( Figs 19−21 View FIGURES 16 – 21 ); (iii) posterior margin of abdominal terga III −V without triangular spines ( Figs 33, 36, 37 View FIGURES 32 – 40 ); tergum VI mainly without marginal spines; tergum VII with occasional 1−2 small spines; (iv) stout spatulate setae of posterior margin of abdominal terga relatively slender, more sparsely scattered centrally; distances between individual stout setae equal to the width of 1/3 to 4/1 full seta base ( Figs 32−37 View FIGURES 32 – 40 ); (v) surface of abdominal terga covered with nearly equilateral triangle-shaped large scales bluntly pointed apically, and their semilunar sockets ( Figs 32−37 View FIGURES 32 – 40 ); (vi) gill margins without stout setae ( Figs 25 View FIGURES 25 – 31 a, b −31a, b); (vii) gills moderately elongated, pairs I, VI and VII nearly symmetrical, and only pairs II–V slightly asymmetrical ( Figs 25 View FIGURES 25 – 31 a, b −31a, b); (viii) paraproct plate with numerous (up to 27) marginal spines (mainly 18−25) ( Figs 39, 40 View FIGURES 32 – 40 ); (ix) surface of paraproct covered with numerous (more than 25) sockets; (x) tarsal claw without subapical tiny setae ( Fig. 24 View FIGURES 22 – 24 ); (xi) paracercus 1/4–1/2 of cerci length ( Figs 1, 2 View FIGURES 1 – 3 ).
Description. Larva. Size: female body length: 6.5−10.0 mm, cerci length: 5.0− 6.5 mm, paracercus: 2.4–3.2 mm; male body length: 5.0− 7.5 mm; cerci length: 4.6−6.0 mm, paracercus: 2.2–2.8 mm. General body colour more or less evenly light brown to brown.
Cuticular coloration ( Figs 1−3 View FIGURES 1 – 3 ). Head and thorax with more or less evenly light brown to brown cuticle, wherein lighter and darker areas alternate. Pronotum with large dark maculae centrally and laterally. Mesonotum dark, with two distinct light spots near wing pads’ base ( Fig. 1 View FIGURES 1 – 3 ). Thoracic pleura slightly darker than terga; sterna unicoloured yellow to light brown; occasionally paler diffuse spot occurs on mesonotum centrally. Abdominal terga with contrasting pattern including darker terga II −III and VI −VIII ( Fig. 3 View FIGURES 1 – 3 ; for more details see below). Abdominal sterna light brown; with a pair of paler oblique strokes and dots on sterna II–VIII anteriorly ( Fig. 2 View FIGURES 1 – 3 ). All femora with diffuse brownish spot centrally. Terminal filaments yellowish brown, slightly darker distally.
Hypodermal coloration. Hypoderm without contrasting markings.
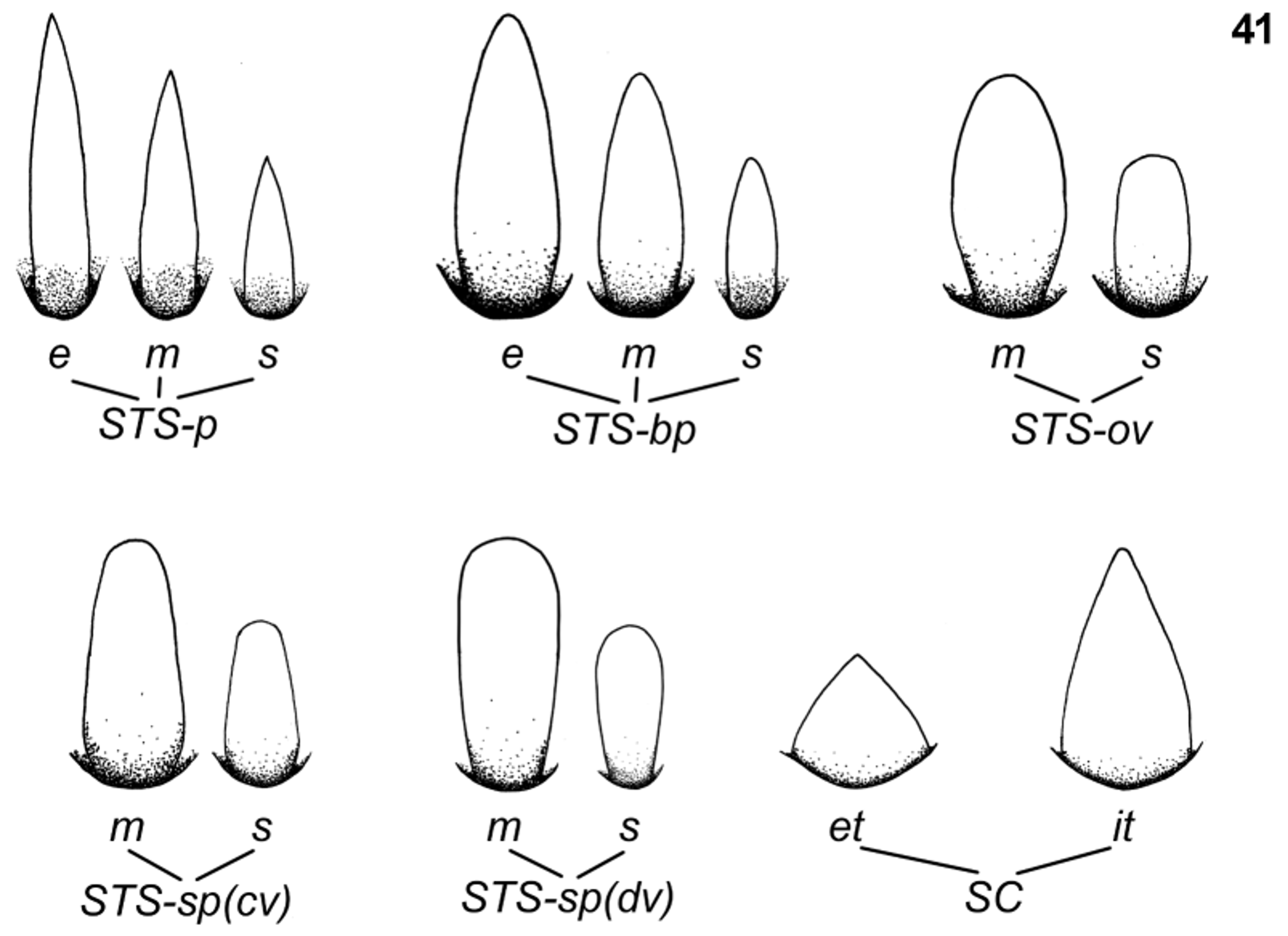
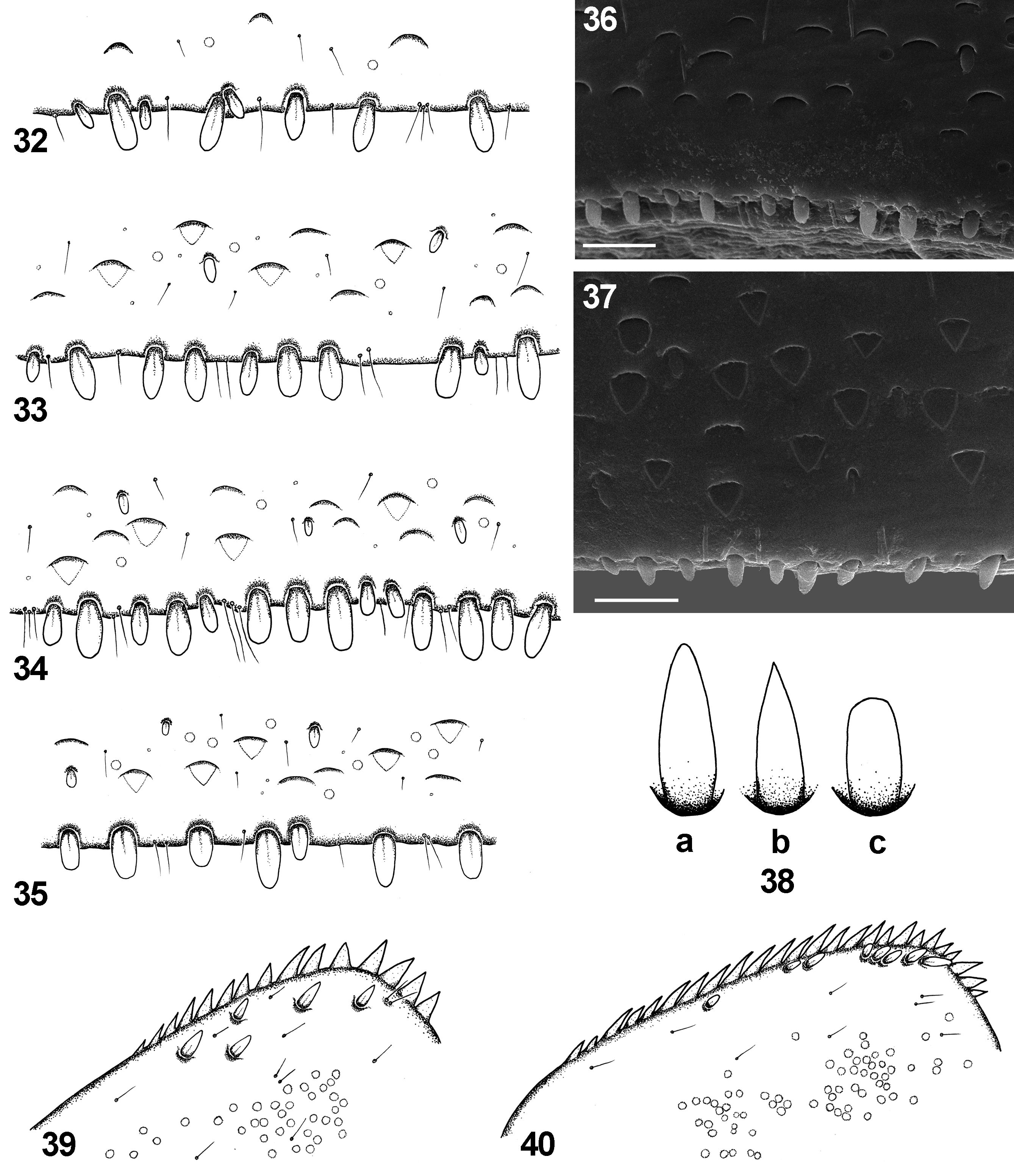
Setation (see Table 1, 2 View TABLE 2 and Fig. 41 View FIGURE 41 ). Surface of body covered with several types of setae and scales:
Stout setae (sensillum chaeticum s. Gaino & Rebora 1998) ( Fig. 41 View FIGURE 41 ): (i) elongated (more than 15 µm in length), middle (8.5−14.9 µm) and small (up to 8.4 µm) pointed setae with distinctly convergent margins, widest at the base [STSe-p, STSm-p and STSs-p, respectively; the same size groups exist for other types of stout setae]; (ii) middle and small setae, blunt or bluntly pointed apically, with smoothly convergent margins, widest at the base or near to the base [STSm-bp and STSs-bp, respectively]; (iii) middle and small oval-shaped setae rounded apically, widest near to the middle of the seta length [STSm-ov and STSs-ov, respectively]; (iv) middle and small spatulate setae rounded apically, with nearly parallel margins proximally and smoothly convergent margins in distal half of seta length, widest at the base [STSm-sp(cv) and STSs-sp(cv), respectively]; (v) middle and small spatulate setae rounded (or truncated straight) apically, with parallel or slightly divergent apically margins, widest centrally and apically [STSm-sp(dv) and STSs-sp(dv), respectively]. All types of setae listed above have more or less serrated margins mainly near their tip.
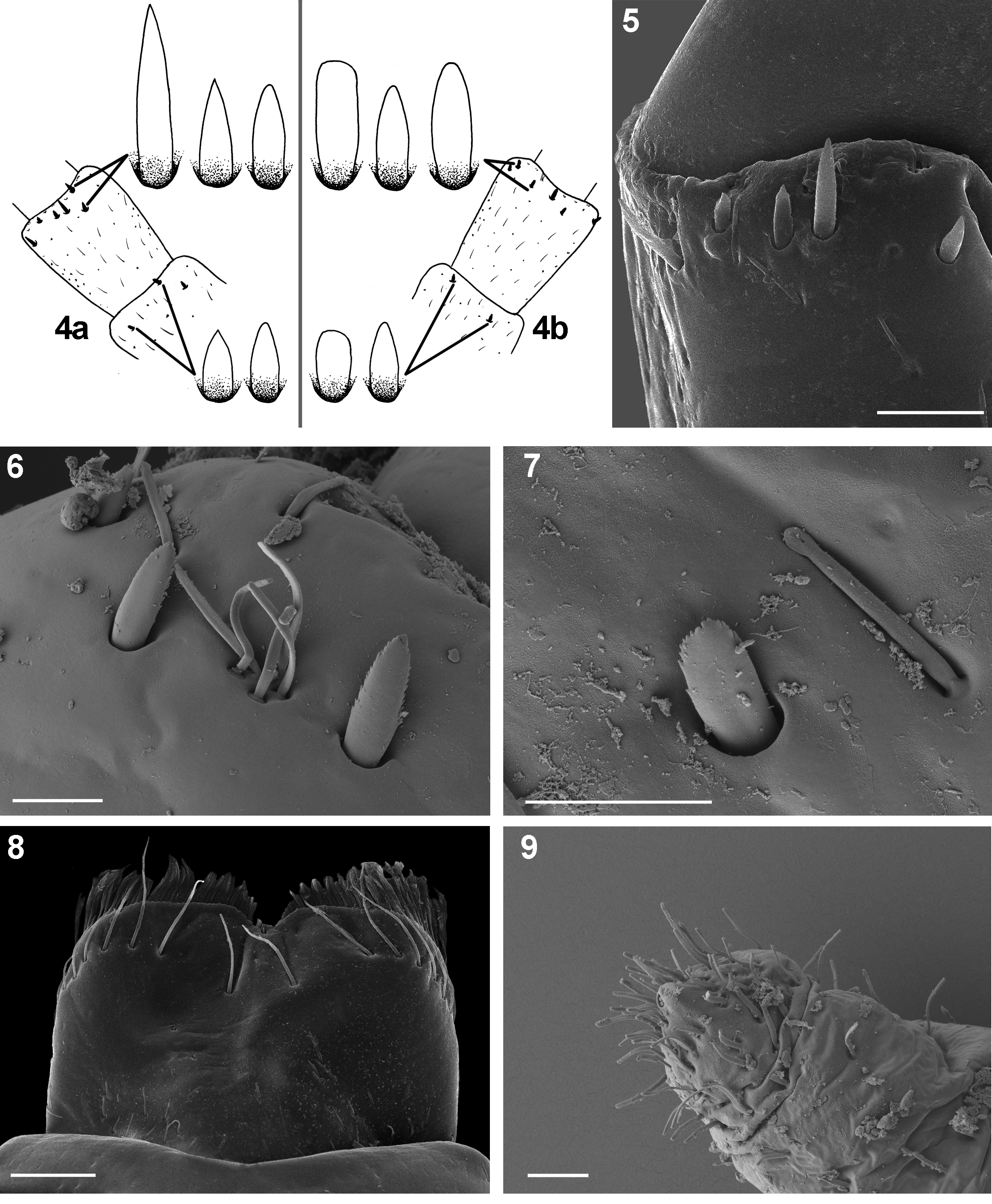
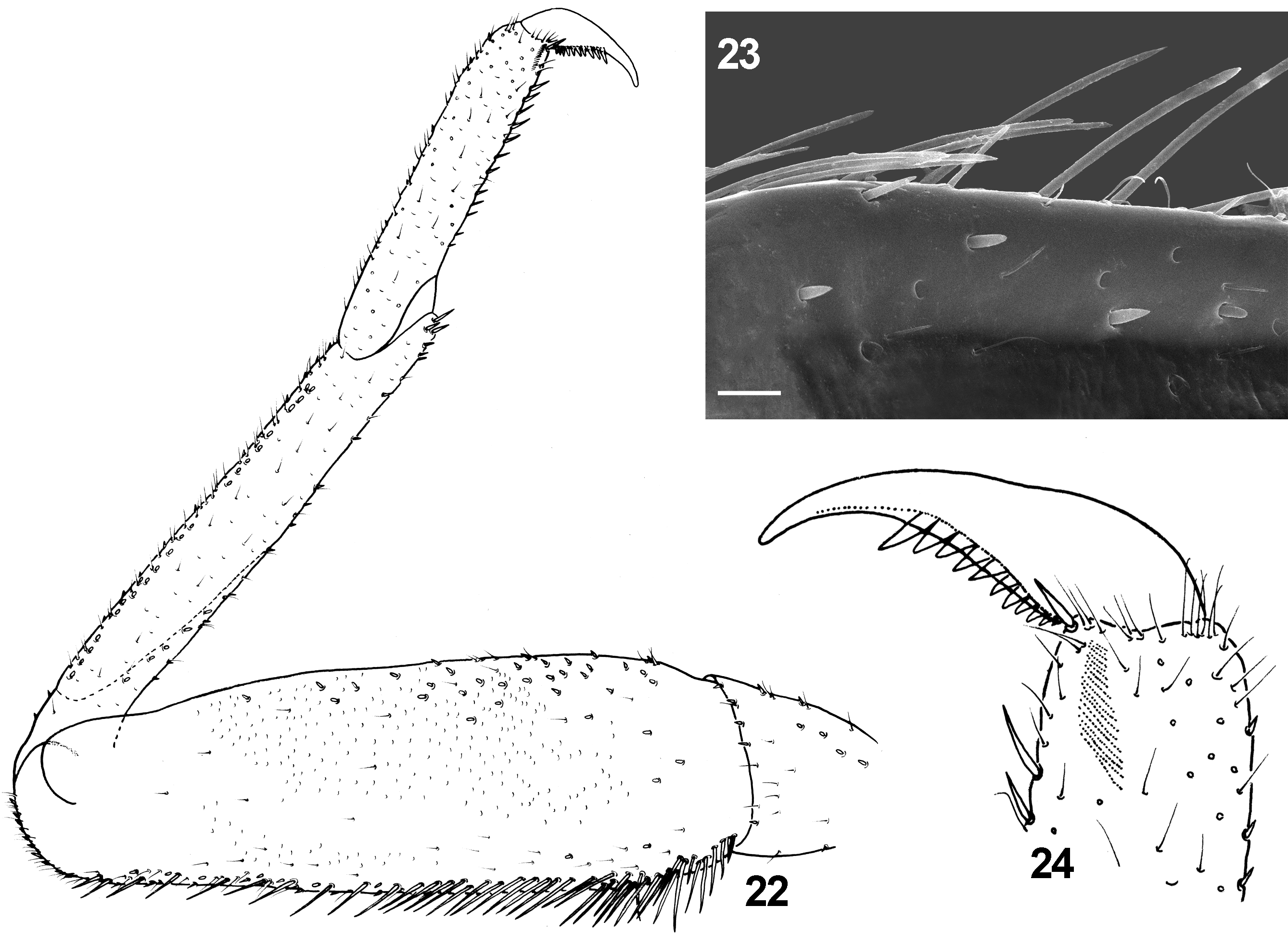
Tiny setae (see Gaino & Rebora 1996, 2003): (vi) flat-tipped sensillum, scattered throughout all the surface of the body; the most abundant type of tiny setae [FT; 8−13 µm in length] ( Figs 7 View FIGURES 4 – 9 , 36, 37 View FIGURES 32 – 40 ); (vii) sensillum basiconicum, not numerous, isolated or appearing in groups of 2−6 setae, alternating with FT [B; 10−18 µm in length] ( Fig. 6 View FIGURES 4 – 9 ); (viii) long thin hair-like setae, sparsely distributed mainly on the surface of legs; more abundant along legs margins [Hr; 16−27 µm in length] ( Fig. 23 View FIGURES 22 – 24 ).
Scales and their sockets (see Gillies & Thorpe 1996). Body surface covered with articulated triangular scales: (ix-a) nearly equilateral triangle-shaped scales, bluntly pointed apically [SC-et; 8.2−10.0 µm in length]; (ix-b) semilunar sockets of this type of scales [SCS-et]; (x-a) nearly isosceles triangle-shaped scales, bluntly pointed apically [SC-it (6.5−7.5 µm in length); (x-b) semilunar sockets of this type of scales [SCS-it].
Head. Colour brown with paler frons and genae. Surface of clypeus and frons covered with numerous FT and sparse B and Hr setae, and occasional SCS; additionally, a few STSs-sp(dv) and STSs-ov setae on clypeus ( Fig. 7 View FIGURES 4 – 9 ). Surface of larval turbinate eyes moderately brown to reddish-brown. Antennae distinctly paler than head, yellowish to yellowish brown; scape and pedicel darker than flagellum. Antennae slightly longer than 1/2 of body length. Occiput with brownish maculation.
Scape with 1–4 STSs-p and STSs-bp setae distally (occasionally scape without any setae); surface covered with sparse FT setae; a few B setae concentrated along distal margin of segment, occasionally grouped with 2−6 setae ( Fig. 4 View FIGURES 4 – 9 a, b); ventral side mainly with FT setae only.
Pedicel with 3−7 STSm-bp, STSs-bp and STSm-sp(dv) setae near distal margin of segment dorsally (occasionally with STSe-p and STSm-p setae); several FT (rarely B) setae scattered on surface; tiny setae markedly longer than on scape ( Figs 4 View FIGURES 4 – 9 a, b, 5, 6).
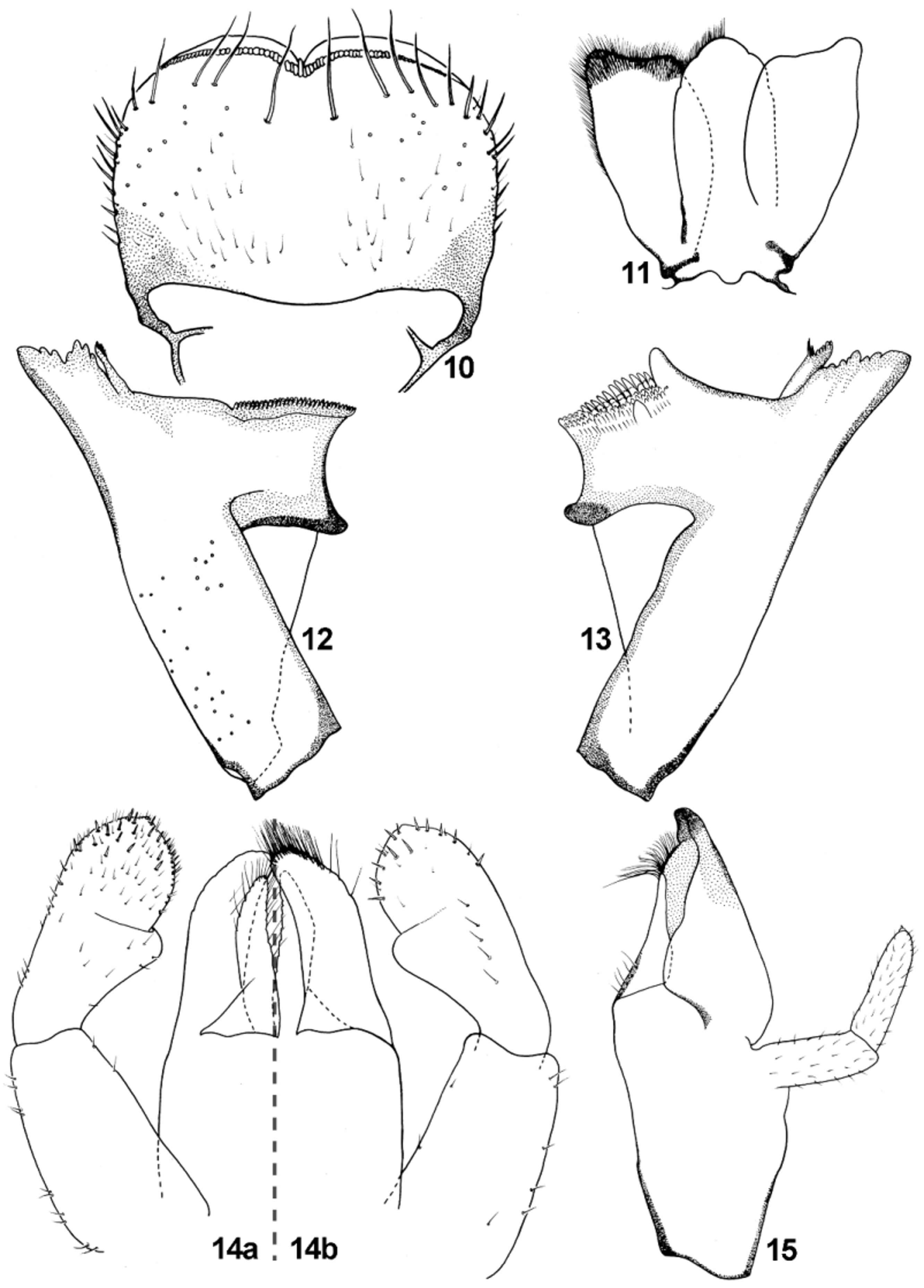
Labrum ( Figs 8 View FIGURES 4 – 9 , 10 View FIGURES 10 – 15 ) more or less oblong-shaped with width/length ratio 1.41–1.57; dorsal surface with 1 + 6– 8 (occasionally 9) long stout setae, arranged mainly in one regular row; 4–7 smaller setae laterally on both margins; dorsal surface covered with sparsely arranged setae [B]; ventral side with 3–4 small pointed setae anterolaterally.
Hypopharynx with a trilobed lingua; central lobe broad, not well separated from lateral lobes; superlingua approximately triangular-shaped with marked hump anterolaterally ( Fig. 11 View FIGURES 10 – 15 ); numerous minute setae along distal margin of lingua and superlinguae.
Right mandible with 7 incisors, without setae on outer margin; row of tiny setae on outer margin of outer of incisors; prostheca slender, not distinctly extended apically ( Fig. 12 View FIGURES 10 – 15 ). Left mandible with 6 incisors; prostheca with 5 teeth (first two larger than other ones), extended apically ( Fig. 13 View FIGURES 10 – 15 ).
Maxillae with 4 teeth (three canines and distal dentiseta), none of them opposed to others. Maxillary palp two segmented; segment I slightly shorter than second one; segment II with pronounced tip and one stout scale ( Fig. 18 View FIGURES 16 – 21 ); numerous tiny setae (uniporous basiconic sensillum s. Gaino & Rebora 2003: 449, figs 19−21) on segment II; surface of segment I with several tiny setae [FT]; setation clearly denser on distal part of segment II ( Figs 9 View FIGURES 4 – 9 , 15 View FIGURES 10 – 15 ).
Labium with glossae shorter than paraglossae ( Fig. 14 View FIGURES 10 – 15 a, b). Glossae relatively slender with 9–10 stout bristles on inner margin, and 7−9 bristles along outer margin; surface covered with sparse B and Hr setae; paraglossae with two irregular rows of long stout bristles apically; two shortened stout subapical setae near tip dorsally ( Figs 16, 17 View FIGURES 16 – 21 ). Segment II of labial palp with rounded apicomedial projection slightly expanded laterally; dorsal surface of segment II with row of 5–6 long setae centrally ( Fig. 14 View FIGURES 10 – 15 b). Segment III nearly symmetrical, slightly elongated ( Figs 19−21 View FIGURES 16 – 21 ); quotient q ranges from 1.19 to 1.43 (see Sroka et al. 2012b, 29, 31: fig. 2); only few strong pointed setae on surface and tip of segment III (long and short hairs s. Gaino & Rebora 2003).
Thorax. Pronotum light brown to brown; brownish large macula near anterior margin; two paired brown maculae laterally at each side; dorsal surface covered with SC-it, FT, occasional SC-it and Hr setae. Mesonotum of the same colour, light brown to brown; two light spots near base of wing pads; brown narrow area along anterior margin and anterolaterally. Metanotum mostly of same colour than mesonotum; two diffuse pale spots anteriorly. Lateral sides of thorax yellow to intensively brown, small brown spots around thoracic spiracles. Ventral side of thorax unicoloured dark yellow to light brown; occasionally paler diffuse spot on mesonotum centrally.
Legs pale, yellow to greyish-brown and brown. Femora yellowish brown; diffuse brownish spot centrally. Femora relatively large; the widest part near proximal half. Tibia mainly of the same colour as femora, yellowish brown to dirty brown, darker distally. Tarsi yellowish brown, with light brown or yellowish smudge centrally (two brownish rings basally and apically); apical ring markedly visible, relatively wide; basal ring narrow. Tarsal claw light brown to brown.
Bluntly pointed long bristles along outer margin of femora; bristles slightly expanded in subapical part; occasionally solitary pointed bristles alternating with bluntly pointed bristles along outer margin ( Fig. 23 View FIGURES 22 – 24 ). Femoral long bristles arranged in two irregular rows more proximally; shorter and more robust bristles situated distally; one row of bristles near distal end; occasionally stout bristles arranged in one more or less sparse row ( Fig. 22 View FIGURES 22 – 24 ). Long marginal bristles alternating with STSm-p, STSm-bp, and Hr setae; submarginal area covered with STSm-p, STSmbp, FT, Hr and occasional STSm-ov setae. Inner margin of femora with STSm-p, STSm-bp alternating with FT and sparse Hr setae. Dorsal surface of femora covered with occasional SC-et and Hr, and more abundant FT, SC-it setae and their sockets; stout setae presented by (i) STSm-p, STSm-bp and occasional STSm-ov setae scattered mainly near margins, and (ii) few STSm-bp, STSm-sp(dv) and occasional STSs-ov setae concentrated centrally. Femora with STSm-p and STSs-p setae (predominantly near inner margin), and FT setae ventrally.
Both margins of tibia covered with STSm-p and STSm-bp setae (on inner margin slightly longer than on outer margin), together with FT and Hr setae; a group of 4–6 stout STSe-p setae at the tip of inner margin of tibia. Dorsal surface of tibia with sparse STSm-bp, STSs -bp, FT and Hr setae, SC-it setae and their sockets; occasional STSm-p, STSs-p setae near inner margin of tibia ventrally.
Tarsus with group of 5−8 elongated B setae along the distal end; 7–12 stout (from STSm-p to STSe-p) setae along of inner margin; STSm-bp setae alternating with Hr setae along the outer margin. Dorsal surface of tarsi only with a few STSs-bp and more abundant FT setae (occasionally grouped by 2−4 setae). Tarsal claw moderately hooked with row of 9–13 (mainly 12–13) teeth, without subapical tiny setae ( Fig. 24 View FIGURES 22 – 24 ).
Abdomen. Terga yellow to light brown. Tergum I brownish; diffuse pale spot centrally near anterior margin. Tergum II with diffuse dark spot centrally near posterior margin; broader brownish smudge centrally. Tergum III with distinct brown spot centrally surrounded by light brown diffuse background. More or less uniform drawing on terga IV–V, with distinct broad pale field centrally, framed anteriorly by narrow brown band. Terga VI −VIII with previous type of colour pattern, but more intensively pigmented, darker; central brownish spot well visible. Tergum IX yellowish centrally with brownish edging laterally and anteriorly; additionally two brown spots centrally.
Tergum X light brown; two triangular brown spots anteriorly and two dark dots centrally. Each terga II −VIII with more or less visible longitudinal large spot laterally; additionally two oblique strokes and two brownish dots on terga II–VI (occasionally II −VIII) centrally ( Figs 1, 3 View FIGURES 1 – 3 ).
Posterior margin of terga I −II with sparse irregular row of STSm-sp(cv), STSs-sp(cv), and solitary STSm-sp(dv), STSs-sp(dv) slender setae, accompanied with solitary FT setae and few elongated B setae (sometimes grouped by 2−3 setae) ( Fig. 32 View FIGURES 32 – 40 ); terga III −IV with same arrangement of slender stout setae; distances between individual stout setae are equal to the width of 1/3 to 4/1 seta base ( Fig. 33 View FIGURES 32 – 40 ); terga V −VII mainly with row of STSm-sp(cv) and STSs-sp(dv) setae, and less frequent STSm-sp(cv) and STSs-sp(dv) setae, alternating with solitary B setae; stout setae sparsely arranged in irregular row centrally (distances between individual setae are equal to the width of 1/2 to 2/1 seta base), and densely grouped laterally; occasional 1−2 small hardly visible spines on tergum VII. Surface of terga covered with a few Hr setae, middle and small STS-sp(cv), STS-sp(dv) and STS-ov setae, and regular FT setae; numerous SC-et and SCS-et setae over whole surface of terga ( Figs 34−37 View FIGURES 32 – 40 ).
Sterna pale, mainly unicoloured light brown ( Fig. 2 View FIGURES 1 – 3 ); sterna VIII–IX occasionally paler than others, yellowishbrown to yellow; a pair of paler oblique strokes and dots on sterna II–VIII anteriorly. Posterior margin of sterna covered with one irregular row of STSm-bp setae and occasional Hr, and without large spines; predominance of SC- et, SCS-et and Hr setae on sternal surface; only STSm-p setae and occasional Hr setae on lateral sides of sterna.
Surface of paraproct covered with 2−6 STSm-p, STSm-bp and/or STSs-ov setae (occasionally with 8−12 middle and small STS-sp(cv) setae) ( Fig. 38 View FIGURES 32 – 40 ). Additionally to stout setae, a few SC-et and numerous sockets are scattered over the plate surface, alternating with FT setae ( Figs 39, 40 View FIGURES 32 – 40 ). Presence of 14–27 (mainly 18–25) stout spines on posterior half of paraproct inner margin; a few small spines subapically; lack of tiny setae among stout spines ( Figs 39, 40 View FIGURES 32 – 40 ).
Gills moderately elongated. Gills I, VI and VII nearly symmetrical; gills II–V slightly asymmetrical or occasionally almost oval, yellowish to dirty yellow or dirty brown, with brownish edging; tracheation yellowish to yellowish brown, occasionally poorly visible in material from crenal zone; gills I–VI approximately 1.90–1.98 times longer than wide (approximately 2.00 for gill VII) ( Figs 25 View FIGURES 25 – 31 a, b–31a, b); both gill margins without articulated stout setae; serrated margins of gills well marked in material from crenal zone ( Figs 25 View FIGURES 25 – 31 b −31b); serrated margins of gills with B and Hr setae, inserted in small not articulated spines bases; surface of gills covered with FT setae.
Cerci pale, yellowish brown, slightly darker distally; swimming bristles small, missing approximately at 1/3–1/2 of cerci length. Paracercus well developed, not vestigial, as long as 1/4–1/2 of cerci length; well developed swimming bristles on both sides of paracercus along whole filament.
Indexes Acronyms 1. Stout setae STS 1.1. Pointed apically STS-p 1.1.1. Elongated, with distinctly convergent margins, widest at the base STSe-p 1.1.2. Middle, with distinctly convergent margins, widest at the base STSm-p 1.1.3. Small, with distinctly convergent margins, widest at the base STSs-p 1.2. Blunt or bluntly pointed apically STS-bp 1.2.1. Elongated, with smoothly convergent margins, widest at the base or near to the base STSe-bp 1.2.2. Middle, with smoothly convergent margins, widest at the base or near to the base STSm-bp 1.2.3. Small, with smoothly convergent margins, widest at the base or near to the base STSs-bp 1.3. Rounded apically STS-ov, STS-sp 1.3.1. Oval-shaped STS-ov 1.3.1.1. Middle, widest near to the middle of the seta length STSm-ov 1.3.1.2. Small, widest near to the middle of the seta length STSs-ov 1.3.2. Spatulate STS-sp 1.3.2.1. Middle, with nearly parallel margins proximally and smoothly convergent margins in distal STSm-sp(cv) half of seta length, widest at the base
1.3.2.2. Small, with nearly parallel margins proximally and smoothly convergent margins in distal half STSs-sp(cv) of seta length, widest at the base
1.3.2.3. Middle, with parallel or slightly divergent apically margins, widest centrally and apically STSm-sp(dv) 1.3.2.4. Small, with parallel or slightly divergent apically margins, widest centrally and apically STSs-sp(dv) 2. Scales SC 2.1. Nearly equilateral triangle-shaped, bluntly pointed apically SC-et 2.2. Nearly isosceles triangle-shaped, bluntly pointed apically SC-it Male and female adults. Unknown.
Etymology. The new species is named in honour of Vadim V. Marinskiy, research worker of the Department of Hydrobiology, Moscow State University ( Russian Federation) and well-known specialist on the Central Asia invertebrate fauna. Baetis vadimi sp. nov. was “comprehended” by us a as new species at his birthday.
Affinities. The subgenus Rhodobaetis ( rhodani -Gruppe as defined by Müller-Liebenau 1969: 91) can be characterized by the presence of articulated stout setae of various shapes and sizes on the body surface in larvae and by the shape of forceps in male imagines ( Jacob 2003: 122; Bauernfeind & Soldán 2012: 158). Godunko et al. (2004b) introduced a standard set of 26 morphological characters, for both larval and adult stages, useful for the identification of 13 West Palaearctic species of Rhodobaetis . This set of features, among others, also included characters of larval chaetotaxy, i.e. the presence and shape of stout setae on antennae, abdominal terga, paraproct plates and gills. In the same work, five species of the subgenus Rhodobaetis , which lack the stout setae on gill margins, were characterized (i.e. B. baksan Soldán, 1977 , B. bisri Thomas & Dia, 1983 , B. braaschi Zimmermann, 1980 , B. gadeai Thomas, 1999 and B. canariensis Müller-Liebenau, 1971 ). Three other species, which lack the stout pointed, bluntly pointed or spatulate setae along one or both gill margins, were described later from the Republic of Khakassia in Russian Federation ( B. khakassikus Beketov & Godunko, 2005 ), Madeira in Portugal (B.
enigmaticus Gattolliat & Sartori, 2008 View in CoL ) and Talas Region in Kyrgyzstan ( B. taldybulaki Sroka, Godunko, Kluge & Novikova, 2012 View in CoL ).
Baetis vadimi sp. nov. clearly differs from B. canariensis View in CoL , B. enigmaticus View in CoL and B. taldybulaki View in CoL by the absence of subapical tiny setae on tarsal claw (see character x in Diagnosis above). Additional distinguishing characters between the new species and B. canariensis View in CoL and B. taldybulaki View in CoL are the number and shape of large spines of posterior margin of terga, number of long bristles on paraglossae and shape of labial palps (see characters i −iii; Müller-Liebenau 1971: 24, 27, figs 19e −g, 21; Sroka et al. 2012a: 52−57, figs 19−23, 39, 49).
The isolated position of Baetis enigmaticus View in CoL is confirmed on the basis of morphological and genetic evidences (see Gattolliat et al. 2008; Rutschmann et al. 2014). Baetis vadimi sp. nov. can be easily separated from B. enigmaticus View in CoL by the characters i, ii, iii, viii, ix, x and xi. Additionally, the larvae of B. enigmaticus View in CoL can be distinguished by the features of body colour (Gattolliat et al. 2008: 59−60, figs 24−27, 29, 30).
Baetis khakassicus View in CoL markedly differs from the new species firstly by the arrangement of outer margin of larval tibia (see Beketov & Godunko 2005: 10, fig. 9), with presence of elongated pointed stout setae alternating with smaller ones. Both species also could be separated by combination of characters i, iii, ix and xi listed in Diagnosis above.
Baetis bisri and B. vadimi sp. nov. are well distinguishable on the basis of the structure and shape of apical part of paraglossae and theirs setation, surface and posterior margin of terga and their stout setae, paraproct plates and gills, as well as the length of terminal filament (see characters i, iii −v, vii −ix, xi; Thomas & Dia 1983: 213−216, figs 5, 11−13).
We have investigated in detail the specimens of B. braaschi View in CoL from several regions within Pontic basin, using a methodological approach combining morphological and molecular data; as a result, the intraspecific variability in several morphological characters was recognized and summarized in an updated table of standard set of characters for both larval and adult stages (for comparison see Godunko et al. 2004b: 244, table 1; Sroka et al. 2012b: 37, 38, table 2). Nevertheless, despite these differences between populations of B. braaschi View in CoL , the new species described here can be clearly separated by the shape of third segment of labial palps, arrangement of spines and setae on surface and posterior margin of abdominal terga, shape of the gills, setation of surface of paraproct plates and length of terminal filament (characters ii −v, vii, ix, xi, respectively). Both species also clearly differs by the colour pattern of abdominal terga and the shape of long stout setae at the outer margin of femora. In a previous paper (character 14 in Sroka et al. 2012b) it was noticed that articulated stout setae (originally marked as “spines”) are absent on the gills margins in specimens of B. braaschi View in CoL from the Caucasus, Crimea and Central Asia, however they are occasionally present in specimens from the east of Ukraine. The similar occasional setae are present on the gills of B. baksan View in CoL from the Caucasus (according to Godunko et al. 2012b, viz. 1−3 solitary setae can be found on outer margin). The new species markedly differ from B. baksan View in CoL by the number of long stout setae on the dorsal surface of labrum and stout scales at the tip of second segment of maxillary palps (see Soldán 1977: 230, 232, fig. 1; Godunko et al. 2004b: 244, table 1). Additionally, B. vadimi sp. nov. can be distinguished by the combination of diagnostic characters iv, v, vii −ix.
Baetis vadimi sp. nov. is morphologically very similar to the European species B. gadeai View in CoL (= B. gemellus View in CoL sensu auct. [1999 antae]; see Bauernfeind & Soldán 2012: 165−166). The similarity of the species is shown in some aspects of the body colour pattern and structure of mouthparts. Clear differences between these species can be found in the shape and arrangement of stout setae and scales on the body surface, features of the legs, terga and paraprocts’ structure. Detailed comparison of both species is summarized in Table 2 View TABLE 2 .
Used acronyms are discussed above (see chapters of Material and methods and Description; Table 1, Fig. 41 View FIGURE 41 ). STSe-bp type of stout setae is not described for B. vadimi sp. nov., but recognized in B. gadeai View in CoL .
... ...continued on the next page In the recent review of Turkish mayfly fauna, Kazancı & Türkmen (2012) recorded five species belonging to the subgenus Rhodobaetis Jacob, 2003 ( Baetidae View in CoL : Baetis View in CoL ). Besides B. braaschi View in CoL and B. rhodani (Pictet, 1843) View in CoL , which distribution in Asia Minor and the Caucasus is doubtless for us and has been confirmed by numerous material (see Godunko et al. 2004a; Kazancı 2001; Bauernfeind & Soldán 2012; Türkmen & Kazancı 2013), occurrence of other Rhodobaetis species is questionable and requires to be confirmed. Baetis milani Godunko, Prokopov & Soldán, 2004 View in CoL was initially reported from Western Turkey based on a single larva ( Türkmen & Özkan 2011), found at a considerable distance from Crimea ( Ukraine).This species was described as possible endemic of the Crimean Peninsula, found in streams and rivers of northern and southern slopes of the Crimean Mountains ( Godunko et al. 2004b). It was keyed and depicted by Türkmen & Kazancı (2013) in the larval key of the mayflies of the Eastern Black Sea Basin within Turkey. However, confirmation of its occurrence in Western Anatolia is pending.
Baetis gemellus View in CoL auct. Kazancı (1984, 2001), Novikova (1987) and Türkmen & Kazancı (2013) was mentioned for Central and Eastern Anatolia, Central Caucasus ( Russian Federation, Georgia) and Armenia. Perhaps, it is better to speak about several undescribed species which lack the pointed setae at the gill margins, though indications from North-Eastern Turkey may partially concern the new species Rhodobaetis described here (see synonymy below).
Two other species of the subgenus Rhodobaetis were mentioned by Kazancı (2009) from Eastern Anatolia.
Baetis bisri , described from Lebanon, was identified basing on three larvae from Hakkâri il [Province], but this record with high probability is questionable, from our point of the view (on the base of investigation of Rhodobaetis material from this region). It should be noted that subsequently this species is not cited in the Turkey mayfly checklist published by Kazancı & Türkmen (2012). The second record belongs to B. pseudogemellus Soldán, 1977 View in CoL , described from Sudan and collected within Şırnak il [Province] (probably from the Resor deresi [stream]); it may actually refer to an undescribed species of the subgenus Rhodobaetis , which is also characterized by the absence of the pointed setae at the gill margins.
In their work on the distribution and biogeography of Palaearctic species of the subgenus Rhodobaetis, Soldán & Godunko (2008) allocated Baetis ilex Jacob & Zimmermann, 1978 View in CoL and B. baksan View in CoL to a separate subgroup of Caucasian endemics. The first species was recorded from numerous localities in the Central and Southern Caucasus ( Russian Federation, Georgia, Armenia, Azerbaijan and Iran) ( Jacob & Zimmermann 1978; Novikova 1987; Palatov 2013). The recent distribution of B. baksan View in CoL within the Caucasus is insufficiently studied. Nevertheless, B. baksan View in CoL is perhaps characterized by the same area of distribution as B. ilex View in CoL (for the present moment known from Great and Lesser Caucasus), and it predominantly occupies the epirhithral section of small and middle-sized streams and rivers ( Palatov 2013; unpublished personal data). There is a possibility, that these species were also found in Northern Iraq (Euphrate [Firat] river-basin) (see “ Baetis View in CoL ex gr. rhodani View in CoL ” in Al-Zubnidi et al. 1987: 180), at localities quite isolated by geographical barriers from the north slope of the Caucasus. However, such “disjunctivity” may be simply explained also by a lack of knowledge, as shown for numerous representatives of Rhodobaetis ( Soldán & Godunko 2008) . Finally, it should be noted that B. ilex View in CoL and B. baksan View in CoL are not recorded from Turkey hitherto.
Only one species of the subgenus Rhodobaetis , i.e. B. rhodani View in CoL , which is recorded from the Caucasus and Asia Minor (according to the published data analysed above), can be placed into the group of the species with large, Transpalaearctic or Palaearctic distribution, according to Soldán & Godunko (2008) (see also Soldán et al. 2009; Bauernfeind & Soldán 2012). However, the taxonomic status of known populations attributed to “ B. rhodani View in CoL ” remains unclear. Williams et al. (2006), and later Lucentini et al. (2011) have established the existence of cryptic species within European materials formally identified as “ B. rhodani View in CoL ”, based on presence of marginal stout setae on the gills. As a result of these investigations, 7 to 11 putative species could be found in Europe, supporting the hypothesis of B. rhodani View in CoL being a complex of cryptic species. Moreover, the existence of cryptic species was used as an explanation of the wide distribution of the “ B. rhodani View in CoL ” s.l. ( Lucentini et al. 2011).
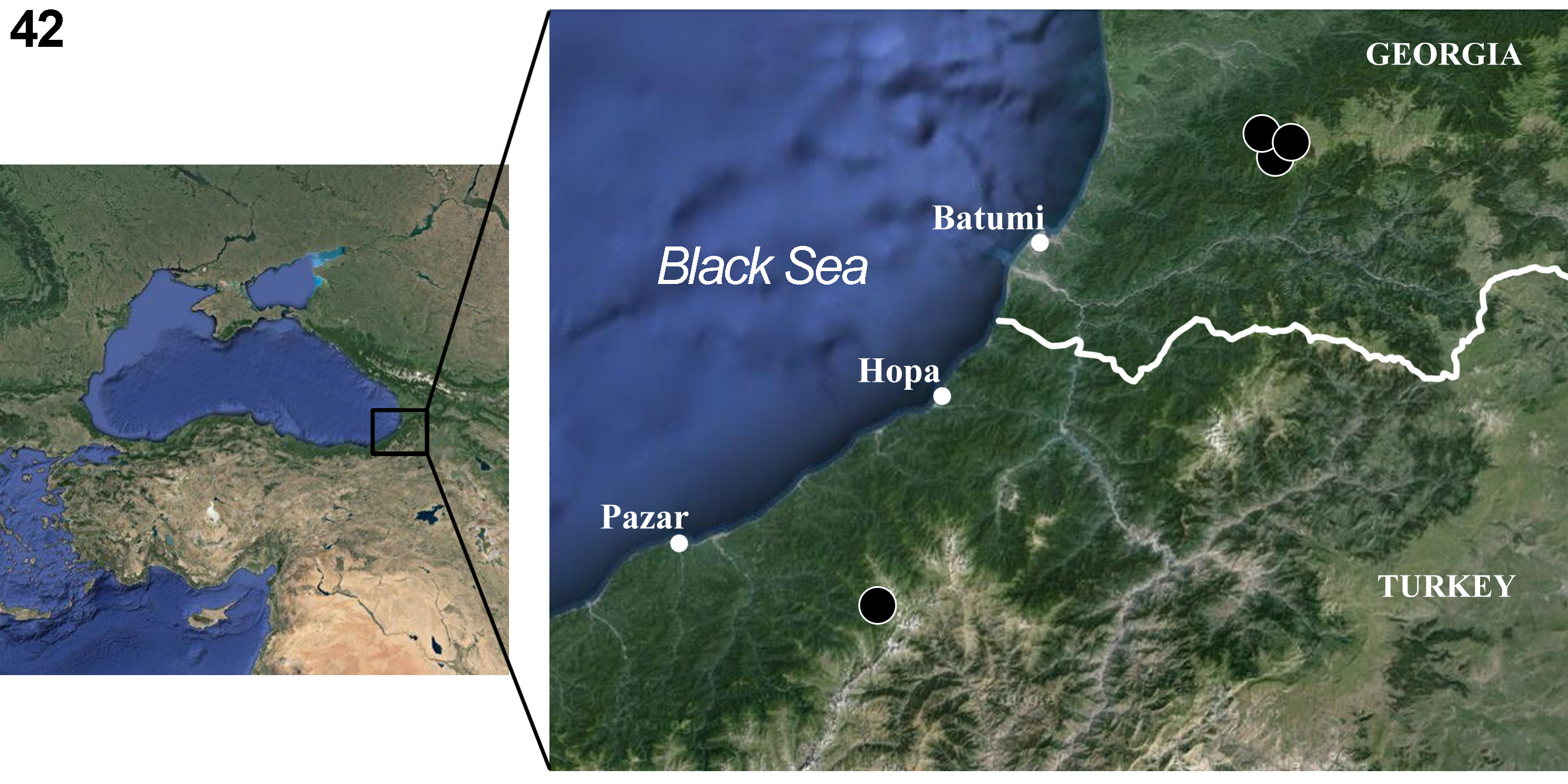
Distribution and biology. So far, Baetis vadimi sp. nov. is known only from the type locality in Turkey and additional localities at the Kintrishi State Nature Reserve (Adjara, Georgia) ( Fig. 42 View FIGURE 42 ). There is a possibility that Baetis vadimi sp. nov. is very rare within the whole species range, where it may be represented by a few populations. The nymphs were found in upper parts of the streams where crenal, epi- and/or metarhithral sections of the rapids occur along alpine and subalpine areas (2000−2600 m a.s.l.) of principal mountain ranges of the South-Western Caucasus. We can preliminary consider this new species as an endemic of the Lesser Caucasus (including the Pontic Mts.).
At the type locality ( Fig. 43 View FIGURES 43 – 44 ) the larvae were observed in the flow, staying on stones and mosses ( Fontinalis sp.) in central part of the stream or in littoral, never occurring at places with extremely turbulent flow. Baetis vadimi sp. nov. was found in streams up to 1.5−2.0 m wide and up to 0.5 m deep; larvae were recorded in water where current velocity ranged from 0.2 to 0.5 m /sec; water temperature during the observation period was 8−12°C. The taxocenes of mayflies associated with the new species were dominated by Heptageniidae ( Iron , Electrogena , Ecdyonurus and Rhithrogena spp.) and Baetidae ( Nigrobaetis (Takobia) and Acentrella spp.).
For the present moment, throughout the territory of Adjara B. vadimi sp. nov. was recorded only at Adjara- Imereti (Meskhetian) Range. Here the species was found at approximately 2150−2300 m a.s.l. The new species inhabits crenal and epirhithral zones of springs, streams and upper courses of rivers (sometimes already at the border of snowfields) ( Figs 44−48 View FIGURES 43 – 44 View FIGURES 45 – 48 ). All these watercourses are characterized by relatively low current velocity (no more than 0.5 m /s) and small stream discharge. However, a few larvae of B. vadimi sp. nov. were recorded once in limnocrene (area about 0.5 m 2, depth— 0.1–0.15 m, with flowing out stream). The mayfly taxocene of B. vadimi sp. nov. in Adjaria was represented by the Electrogena and Nigrobaetis (Takobia) spp.
TABLE 2. Morphological characters to distinguish Baetis (Rhodobaetis) vadimi sp. nov. from B. gadeai: concept proposed by Godunko et al. (2004 b), with additions and corrections. Larval characters according to Novikova (1987), Godunko et al. (2004 b) and Gattolliat & Sartori (2008); new characters are marked by asterisk.
| N° Characters | Baetis vadimi sp. nov. | B. gadeai View in CoL (coding according to Godunko et al. 2004b, with additions and corrections) |
|---|---|---|
| Head | ||
| 1. Pedicel: shape of stout setae | STSm-bp, STSs-bp, STSm- sp(dv), occasional STSe-p, STSm-p | STSe-bp, STSm-bp, STSs-bp, occasional STSm-ov |
| 2. Scape: shape of stout setae | STSs-p, STSs-bp | STSm-sp(cv), STSs-sp(cv), occasional STSs-ov |
| 3. Surface of clypeus: shape of stout setae and scales* | SCS-it, occasional STSs-sp(dv), STSs- ov | SCS-it, occasional SC-it and STSs-bp |
| 4. Surface of frons: shape of stout setae and scales* | absent, occasional SCS-it | SCS-it, occasional SC-it and STSs-bp |
| Mouthparts | ||
| 5. Labrum: mean width/length ratio | 1.38–1.57 | 1.30−1.34 |
| 6. Labrum: number of long submarginal setae | 1 + 6–9 | 1 + 7−10 |
| 7. Maxillary palps: apical part of distal segment | with one pointed scale | with one pointed scale |
| 8. Paraglossae: number of regular rows of apical bristles | 2 | 3 |
| 9. Paraglossae: number of medium tiny setae on ventral surface* | 2−5 | 4−7 |
| 10. Labial palps: number of medium tiny setae on dorsal surface of segment II* | 5−6 | 5−6 |
| 11. Labial palps: shape of segment III | nearly symmetrical, slightly elongated | nearly symmetrical, relatively wide |
| 12. Labial palps: degree of asymmetry [quotient q]* | 1.19−1.43 | 1.22−1.36 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Baetis (Rhodobaetis) vadimi
| Godunko, Roman J., Palatov, Dmitry M. & Martynov, Alexander V. 2015 |
B. taldybulaki
| Sroka, Godunko, Kluge & Novikova 2012 |
enigmaticus
| Gattolliat & Sartori 2008 |
Rhodobaetis, Soldán & Godunko (2008)
| Soldan & Godunko 2008 |
Rhodobaetis ( Soldán & Godunko 2008 )
| Soldan & Godunko 2008 |
Baetis milani Godunko, Prokopov & Soldán, 2004
| Godunko, Prokopov & Soldan 2004 |
Rhodobaetis
| Jacob 2003 |
Baetis ilex
| Jacob & Zimmermann 1978 |
B. pseudogemellus Soldán, 1977
| Soldan 1977 |
Baetis gemellus
| Eaton 1885 |
Baetis (Baetis) gemellus
| Eaton 1885 |
B. rhodani
| Pictet 1843 |