Rochinia gracilipes A. Milne Edwards 1875 Pisinae
|
publication ID |
https://doi.org/10.1080/00222933.2016.1190415 |
|
DOI |
https://doi.org/10.5281/zenodo.5195299 |
|
persistent identifier |
https://treatment.plazi.org/id/03B08B0B-FFC2-FFD8-FE64-FFC4D5B1F9CA |
|
treatment provided by |
Plazi |
|
scientific name |
Rochinia gracilipes A. Milne Edwards 1875 Pisinae |
| status |
|
Genus Rochinia A. Milne Edwards 1875 Rochinia gracilipes A. Milne Edwards 1875 View in CoL
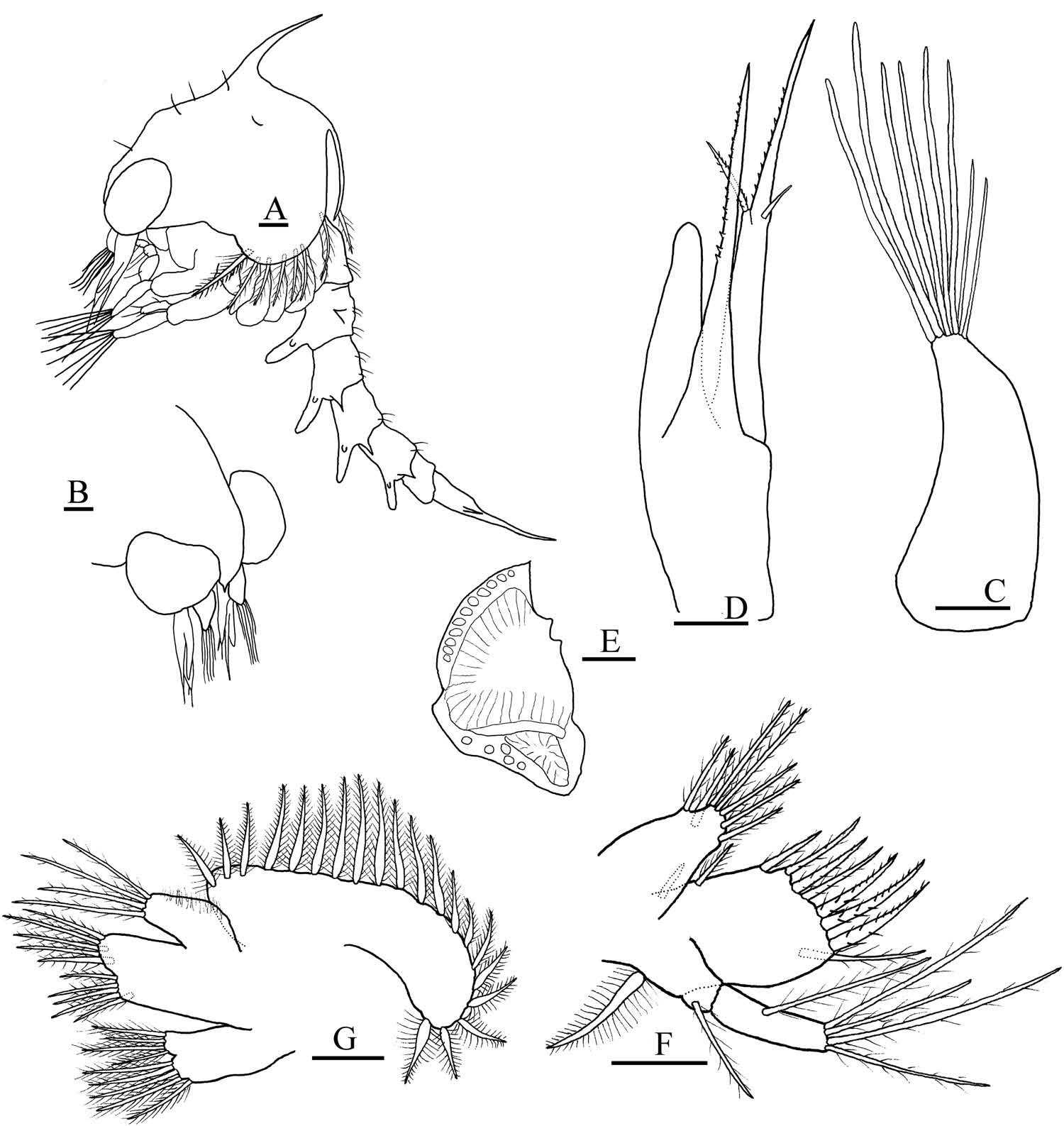
First zoea ( Figures 1 View Figure 1 A – G, 2A – F)
Carapace ( Figure 1 View Figure 1 A, B). Globose, smooth, without tubercles. Dorsal spine well developed, not spinulated. Rostral spine minute. Without lateral spines. One pair of anterodorsal simple setae, one pair of posterodorsal simple setae. Eyes sessile. Ventral margin with densely plumose ‘ anterior seta ’ ( Clark et al. 1998b) followed by three posterior smaller plumose setae.
Antennule ( Figure 1 View Figure 1 C). Uniramous. Endopod absent. Exopod unsegmented, with four aesthetascs (two long and two shorter) and two setae.
Antenna ( Figure 1 View Figure 1 D). Protopod well developed, spinous process armed with strong spines arranged in two lines. Endopod bud aproximately 1/3 of protopod length. Exopod longer than protopod, spinous process armed with strong spines arranged in two lines, one spinulated seta, one simple seta.
Mandible ( Figure 1 View Figure 1 E). Palp absent.
Maxillule ( Figure 1 View Figure 1 F). Coxal endite with three sparsely plumose subterminal setae and four sparsely plumose terminal setae. Basial endite with three (one sparsely plumose, two plumose) subterminal setae and four plumodenticulate cuspidate terminal setae. Endopod two-segmented, one sparsely plumose seta in proximal segment, four terminal sparsely plumose setae on distal segment. Exopod and epipod setae absent.
Maxilla ( Figure 1 View Figure 1 G). Coxal endite bilobed with 4 + 4 plumose setae. Basial endite bilobed with five (two subterminal, three terminal sparsely plumose) and four (two subterminal, two terminal sparsely plumose). Endopod with four setae (two subterminal, two terminal sparsely plumose) setae. Microtrichia on lateral margin of endopod. Scaphognathite with 10 marginal plumose setae and posterior process.
First maxilliped ( Figure 2 View Figure 2 A). Coxa without setae. Basis with 10 plumose setae arranged two, two, three, three. Endopod five-segmented with three, two, one, two and five (one subterminal, four terminal) sparsely plumose, plumodenticulate setae. Exopod twosegmented; distal segment with four long plumose natatory setae with one conspicuous annuli.
Second maxilliped ( Figure 2 View Figure 2 B). Coxa without setae. Basis with three sparsely plumose setae arranged one, one, one. Endopod three-segmented with zero, one (sparsely plumose) and four (one plumodenticulate subterminal, two simple subterminal, one plumodenticulate terminal) setae. Exopod two-segmented; distal segment with four long plumose natatory setae with one conspicuous annulus.
Third maxilliped ( Figure 2 View Figure 2 C). Triramous bud.
Pereiopods ( Figure 2 View Figure 2 D). Buds present, chela bilobed.
Abdomen ( Figure 2 View Figure 2 E). Five somites. Somite 2 with a pair of dorsolateral processes. Two dorsal plumose setae on somite 1. Pair of posterodorsal setae on somites 2 – 5. Somites 3 – 5 with pair of short posterolateral processes. Pleopods absent.
Telson ( Figure 2 View Figure 2 E, F). Bifurcated. Each fork with two lateral spines. Dorsal surfaces of furcae covered by small, disperse spinules; ventral surfaces densely spinulated. Inner margin with two groups of three spinulated setae separated by medial sinus.
Second zoea ( Figures 3 View Figure 3 A – G, 4A – F)
Carapace ( Figure 3 View Figure 3 A, B). Three pairs of anterodorsal simple setae, one pair of posterodorsal simple setae, one pair of simple setae near the base of dorsal spine. Ventral margin with ‘ anterior seta ’ followed by five posterior smaller plumose setae. Eyes stalked. Otherwise as in zoea I.
Antennule ( Figure 3 View Figure 3 C). Exopod with two long, four medium and two shorter aesthetascs, endopod absent.
Antenna ( Figure 3 View Figure 3 D). Endopod bud aproximately 1/2 of protopod length. Otherwise unchanged.
Mandible ( Figure 3 View Figure 3 E). Palp bud absent.
Maxillule ( Figure 3 View Figure 3 F). Coxal endite with seven (four subterminal, three terminal) sparsely plumose setae. Basial endite with four (two plumose, one sparsely plumose, one plumodenticulate) subterminal setae and six (three plumodenticulate, three plumodenticulate cuspidate) terminal setae. Exopod present as a pappose marginal seta. Endopod two-segmented, with one sparsely plumose seta in proximal segment and five (one subterminal, four terminal) sparsely plumose setae on distal segment.
Maxilla ( Figure 3 View Figure 3 G). Coxal endite bilobed with five (one subterminal, four terminal) and four (one subterminal, three terminal) plumose setae. Basial endite bilobed with five (one subterminal, four terminal sparsely plumose) + five (two subterminal, three terminal sparsely plumose) setae. Endopod with four terminal sparsely plumose setae. Microtrichia on lateral margin of endopod. Scaphognathite with 20 marginal plumose setae.
First maxilliped ( Figure 4 View Figure 4 A). Exopod two-segmented with six long plumose natatory setae with two conspicuous annuli. Epipod bud present. Otherwise as in zoea I.
Second maxilliped ( Figure 4 View Figure 4 B). Exopod two-segmented with six terminal longer plumose natatory setae with two conspicuous annuli. Otherwise as in zoea I.
Third maxilliped ( Figure 4 View Figure 4 C). As in zoea I.
Pereiopods ( Figure 4 View Figure 4 D). As in zoea I.
Abdomen ( Figure 4 View Figure 4 E). Six somites. Somite 2 with pair of dorsolateral processes. Somite 1 with three dorsal plumose setae. Somites 2 – 3 with two pairs of dorsal and anterodorsal setae. Somites 4 – 5 with one pair of anterodorsal setae. Somites 2 – 6 with one pair of short or medium posterolateral processes. Pleopod buds present.
Telson ( Figure 4 View Figure 4 E, F). As in zoea I.
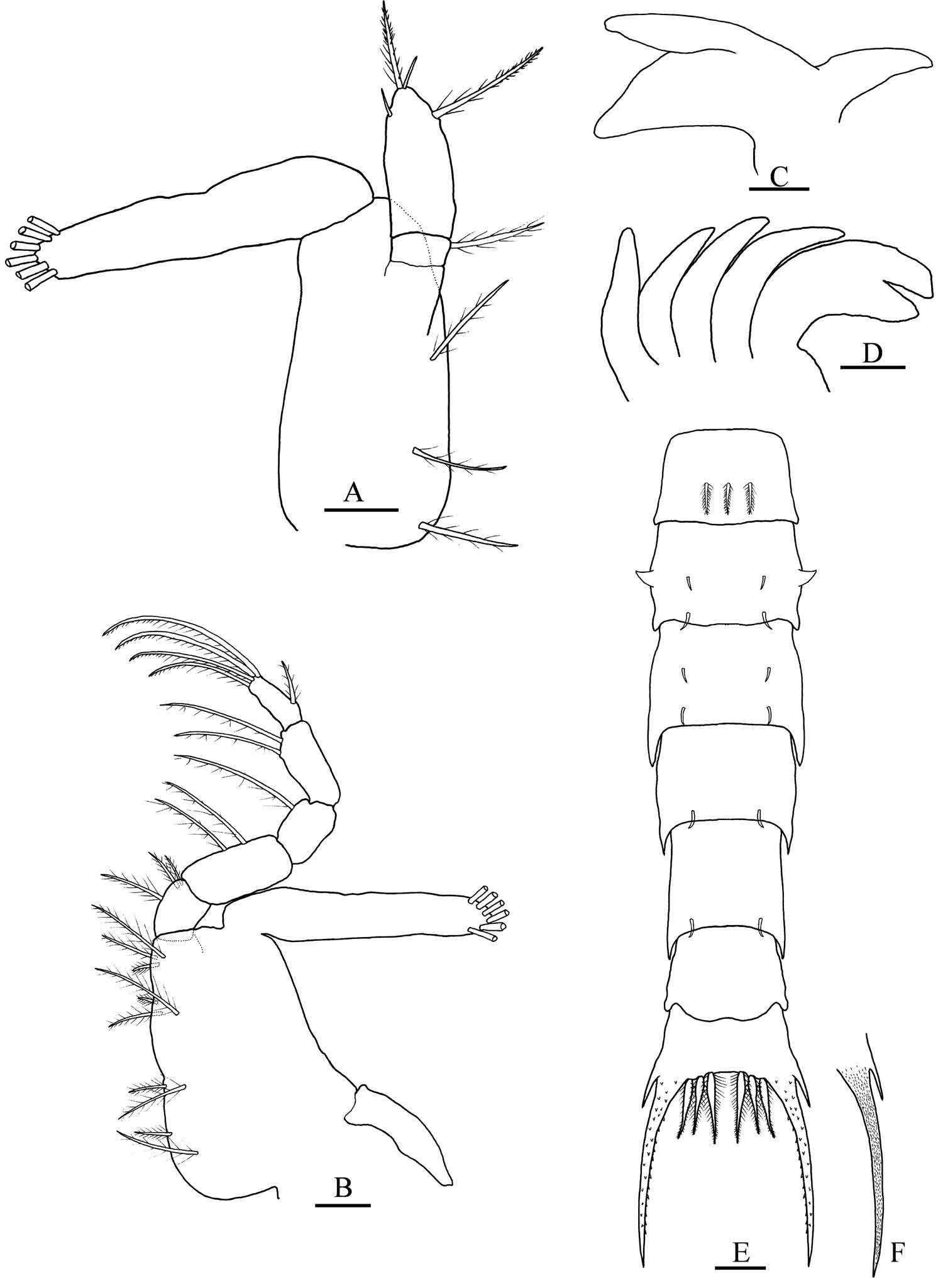
Megalopa ( Figures 5 View Figure 5 A – G, 6A – M, 7A – I)
Carapace ( Figure 5 View Figure 5 A–B). Longer than broad, posteromedial tubercle present, dorsal spine absent. Rostrum ventrally deflected, extending approximately to distal end of segment 3 of antenna. Dorsal surface setose, posterior margin with two groups of plumose setae, other setal arrangement as figured.
Antennule ( Figure 5 View Figure 5 C). Peduncle three-segmented with one, two, and one plumose setae. Endopod unsegmented with one subterminal, two terminal simple setae. Exopod four-segmented with zero, nine (arranged in two tiers), four, one aesthetascs, and zero, one, zero, zero simple setae.
Antenna ( Figure 5 View Figure 5 D). Peduncle three-segmented, with two, two, and three simple setae. Flagellum four-segmented with zero, zero, four and four simple setae.
Mandible ( Figure 5 View Figure 5 E). Palp two-segmented, distal segment with five plumodenticulate setae.
Maxillule ( Figure 5 View Figure 5 F). Coxal endite with five subterminal and five terminal plumose and plumodenticulate setae. Basial endite with 11 subterminal plumose and plumodenticulate and six terminal plumodenticulate cuspidate setae. Endopod unsegmented, one subterminal sparsely plumose, two terminal simple setae. Exopod seta absent.
Maxilla ( Figure 5 View Figure 5 G). Coxal endite bilobed, 6 + 3 plumose setae. Basial endite bilobed with two subterminal plumose and four terminal sparsely plumose setae on proximal lobe, six sparsely plumose setae on distal lobe. Endopod unsegmented without setae; marginal microtrichia present. Scaphognathite with 37 plumose marginal setae and three inner sparsely plumose setae.
First maxilliped ( Figure 6 View Figure 6 A). Epipod with seven long sparsely denticulate setae. Coxal endite with two subterminal plumose, and three subterminal and two terminal plumodenticulate setae. Basial endite with 13 subterminal and terminal plumodenticulate setae. Endopod unsegmented, without setae. Exopod two-segmented, proximal segment with one distal papose seta, distal segment with four long terminal plumose setae.
Second maxilliped ( Figure 6 View Figure 6 B). Epipod bud present. Coxa and basis undifferentiated, one single plumose seta. Endopod four-segmented (ischium and merus undifferentiated), with zero, one, three and six sparsely plumose, plumodenticulate and plumodenticulate cuspidate setae. Exopod two-segmented, distal segment with four terminal plumose feeding setae.
Third maxilliped ( Figure 7 View Figure 7 A). Coxa-epipod joint indistinct. Epipod elongated, six sparsely denticulate setae. Lamellate podobranch well developed. Coxa and basis undifferentiated, nine sparsely plumose and plumose setae. Endopod five-segmented: ischium, merus, carpus, propodus and dactylus with 12, nine, five, six and three sparsely plumose or plumodenticulate setae. Ischium inner margin four-toothed. Exopod two-segmented, distal segment with two subterminal and four terminal plumose feeding setae.
Cheliped ( Figure 7 View Figure 7 B), pereiopods ( Figure 7 View Figure 7 C). Cheliped setae as illustrated. All pereiopod segments well differentiated; dactyli of pereiopods 2 – 5 with three serrulate ventral spine; coxae and ischii without spines.
Sternum (not illustrated). Maxilliped and cheliped sternites fused with six (arranged 2 + 2 + 2) setae; all sternal sutures are medially interrupted.
Abdomen ( Figure 7 View Figure 7 D). Six somites. Somite 1 with two anterodorsal simple setae and two pairs of lateral setae. Somites 2 – 6, proximally to distally with six, six, eight, eight and zero simple setae.
Pleopods ( Figure 7 View Figure 7 E–H). Endopods 1 – 4 unsegmented, two coupling hooks on inner margin. Exopods 1 – 4 with nine, nine, nine and seven long marginal plumose natatory setae, respectively, on distal segments.
Uropods ( Figure 7 View Figure 7 I). Exopod with four natatory setae on distal segment; endopod absent.
Telson ( Figure 7 View Figure 7 D). With two dorsal simple setae.
Discussion
Majoid larval development includes only two zoeal stages and Rochinia gracilipes follows this pattern. The larval morphology of species in Epialtidae , Inachidae , Inachoididae , Majidae and Oregoniidae has been used to propose relationships among families and subfamilies and to construct phylogenies. A recent molecular phylogeny supports several relationships predicted from larval, but not from adult, morphology, suggesting that the adult morphological characters traditionally used may be subject to convergence ( Hultgren and Stachowicz 2008, and references therein). In fact, zoeal morphology may reflect phylogenetic relationships more accurately than adult morphology since the former live in a uniform planktonic environment subjected to relatively constant selection pressures, although it has been recognised that homoplasy is widespread in brachyuran zoeal lineages and many derived characters evolved many times in different clades ( Clark 2009).
Comparison among known Rochinia larvae
The larval morphology of three Rochinia species have been described: R. carpenteri , R. debilis and R. gracilipes ( Ingle 1979; Komatsu and Takeda 2003; this paper) and compared (Supplementary material 1, 2, 3). The zoeae I of R. gracilipes differs from the other two species in having: (1) a minute rostral spine (whereas R. carpenteri and R. debilis have long and short rostral spines, respectively); (2) four setae and two aesthetascs in the antennular protopod (whereas R. carpenteri and R. debilis have two, one and four, one, respectively); (3) antennal exopod longer than protopod (whereas they have similar size in R. carpenteri and R. debilis ); (4) four setae in the distal segment of the endopod of the maxillule (whereas R. carpenteri and R. debilis have six and two setae, respectively); (5) four setae in the endopod of the maxilla (whereas R. carpenteri and R. debilis have six and three setae, respectively); and (6) 10 marginal setae in the scaphognathite of the maxilla (whereas R. carpenteri and R. debilis have 11 and 17 – 18 marginal setae, respectively). Rochinia gracilipes shares with R. debilis the absence of the lateral spine (present in R. carpenteri ), short posterolateral abdominal processes (abdominal process long in R. carpenteri ) and the presence of eight setae in the coxal endite of the maxilla and four setae in the distal segment of the endopod of the 2nd maxilliped (nine and five, respectively, in R. carpenteri ). R. gracilipes share with R. debilis the presence of carapace ventral anterior setae ( ‘ majid setae ’) (present in R. carpenteri ) and with R. carpenteri the absence of pleopod buds (present in R.debilis ).
The zoea II of R. gracilipes and R. carpenteri differ from each other in having in R. gracilipes (1) eight aesthetascs and no setae in the protopod of the antennules (whereas R. carpenteri has five aesthetascs and one seta); (2) nine setae in the basial endite of the maxillule (whereas R. carpenteri has 10 setae); (3) 10 setae in the basial endite, four setae in the endopod and 20 setae in the scaphognathite of maxilla (whereas R. carpenteri has 13, four and 20, respectively); (4) short posterolateral processes in abdominal somites 2 to 6 (whereas they are longer, but are present only in somites 3 to 5 in R. carpenteri ); (5) three, four, four, two and two setae in abdominal somites 1 to 5 (whereas R. carpenteri has two, two, four, four and two setae). In addition, R. gracilipes has no setae near the base of the dorsal spine (whereas R. carpenteri has five) and the palp bud of the mandible is absent (present in R. carpenteri ). The zoea II of R. debilis is presently unknown.
The megalopae of R. gracilipes and R. carpenteri differ from each other in having in R. gracilipes (1) short rostrum and carapace without dorsal spine (long rostrum and carapace dorsal surface provided with spine in R. carpenteri ); (2) one and one setae in the peduncle of antennule; zero, nine, four and one aesthetascs; and zero, one, zero and zero setae in the exopod of the antennule (whereas R. carpenteri has no setae in the peduncle and zero, 10, three and one aesthetascs and zero, zero, zero and one setae in the exopod, respectively); (3) three setae in segment 3 of the peduncle of the antenna, four setae each in segments 3 and 4 of the flagellum of the antenna (whereas R. carpenteri has two setae in the peduncle and three and three setae in the flagellum, respectively); (4) 17 setae in the basial endite of the maxillule and three setae in the distal segment of the endopod of maxillule (whereas R. carpenteri has 10 and four setae, respectively); (5) nine setae in the coxal endite of the maxilla, 37 marginal and three lateral setae in the scaphognathite (whereas R. carpenteri has 12 – 13, 40 and no setae, respectively); (6) seven, seven and 13 setae, respectively in the epipod, the coxal endite and the basial endite of the 1st maxilliped (whereas R. carpenteri has six, six and 10 setae, respectively); (7) three, six and one setae in the propodus and dactylus of the endopod and in the distal segment of the exopod of 2nd maxilliped (whereas R. carpenteri has four, seven and no setae, respectively); (8) six setae in the epipod of the 3rd maxilliped (whereas R. carpenteri has five setae); 12, nine, six and three setae in the ischium, merus, propodus and dactylus of the 3rd maxilliped, four teeth in the ischium of the 3rd maxilliped (whereas R. carpenteri has 11 – 15, 6 – 7, five and four setae, and seven teeth, respectively); podobranchiae on the 3rd maxilliped (podobranchiae putatively lacking in R. carpenteri ); (9) coxae and ischii of pereipods inermis in R. gracilipes (whereas provided with 2 – 4 spines in R. carpenteri ); (10) six, six, six, eight, eight and zero setae in the abdominal somites 1 – 6 (whereas there are four, three, two, two, three and one setae, respectively, in R. carpenteri ); (11) nine, nine, nine and seven setae, respectively, in the exopods of pleopods 2 – 5 (whereas there are 14 – 16 setae in R. carpenteri ); four setae in the exopod of the uropods (whereas R. carpenteri has five setae) and (12) two setae in the telson (whereas R. carpenteri has four setae). The megalopa of R. debilis is presently unknown.
The results of this study pose interesting further questions that should be answered in the context of evolutionary developmental biology. For example, does R. gracilipes have the developmental potential to originate lateral spines or a long rostral spine, as observed in the (alleged) congeneric R. carpenteri ( Ingle 1979) ? In addition, ecological information could help to understand morphological differences between megalopae, a transitional stage that, unlike zoeae, may be subjected to selective pressures related to the change of habitat, from planktonic to benthic (e.g. Martin 1988). For example, are coxal and ischial spines in pereiopods 2 – 4 involved in grasping filamentous substrata, and could the presence of such spines in R. carpenteri , but not in R. gracilipes , be explained by textural differences of megalopal habitats and settlement behaviour between them?
Comparison among Rochinia and Pugettia spp. larval development
The classification of Rochinia in Pisinae was challenged in the description of R. carpenteri larvae by Ingle (1979), who detected affinities with, for example, Oregoniidae (e.g. prominent lateral spines) among other majoid subfamilies. In addition, the genera Rochinia and Pugettia Dana, 1851 were historically considered closely related on the basis of adult morphology although they are currently classified as Pisinae and Epialtinae , respectively ( Colavite et al. 2014). Three species of Pugettia have had their larvae described so far ( P. quadridens , P. marisinica and P. intermedia ) ( Ko 1998; Kornienko and Korn 2004) whose larval morphologies are herein compared with R. gracilipes (Supplementary material 4).
The zoea I of R. gracilipes differs of the zoea of the above-mentioned Pugettia in having (1) a minute rostral spine (versus short rostrum in Pugettia ); (2) the number of aesthetascs and setae in the protopod of antennula (although R. gracilipes shares the number with P. intermedia ); (3) a longer exopod of antenna relative to protopod; (4) the size of the exopod relative to endopod of the antenna (although R. gracilipes shares this character with P. quadridens ); (5) 10 setae in the basis of the 1st maxilliped (versus nine setae in Pugettia spp.); (6) the absence of pleopod buds (present in all Pugettia spp.). Rochinia gracilipes shares with Pugettia spp. the absence of lateral carapace spine, the same number of antero and posterolateral carapace setae, the same setation on the antenna exopod; the absence of palp bud on the mandible; the same setation on the maxillule, maxilla, endopod of the 1st maxilliped, 2nd maxilliped, abdominal somites and telson; the presence of 3rd maxilliped and pereiopod buds; the same size of the dorsolateral and posterolateral processes on the abdomen; and the same size and spinulation of the lateral spines of the telson fork.
The zoeae II of R. gracilipes differs from the zoea of Pugettia , in addition to setation on the protopod of antennula and on the basis of the 1st maxilliped, in having (1) the absence of the endopod bud of antennula and of palp bud of mandible (both present in all Pugettia spp.); (2) nine setae in the basial endite of maxillule (whereas Pugettia spp. have 10); (3) nine setae in the coxal endite of maxilla (whereas Pugettia spp. have 10); (4) the pattern of posterolateral processes of the abdomen; (5) three setae in the proximal somite of abdomen (whereas Pugettia spp. have two). Rochinia gracilipes share with Pugettia spp. the total number of carapace setae; the size of endopod of antenna (relative to exopod); the setation of coxal endite and exopod of maxillule, basial endite, endopod and scaphognathite of maxilla, exopod of 1st and 2nd maxilliped, 2nd to 5th somites of abdomen; and the presence of pleopod buds.
The megalopa of R. gracilipes differs from that of P. quadridens in having (1) one and two setae in segments 1 and 2 of the antennular peduncle, and nine, four and one setae in segments 2, 3 and 4 of the exopod of the antennules (whereas P. quadridens has zero and one setae and eight, zero and zero setae, respectively); (2) two setae in segment 1 of the antennal peduncle (whereas P. quadridens has 1); (3) 10 setae in the coxal endite of the maxillule (whereas P. quadridens has 9); (4) 9 and 12 setae in the coxal and basial endites of the maxilla (whereas P. quadridens has 13 – 15 and 9 – 10 respectively); (5) 13 setae in the basial endite of the 1st maxilliped (whereas P. quadridens has 10 – 11); (6) six setae in the epipod of the 3rd maxilliped (whereas P. quadridens has 10); the presence of podobranchiae on the 3rd maxilliped (podobranchiae putatively lacking in P. quadridens ); four teeth in the ischium of the endopod of the 3rd maxilliped (whereas P. quadridens has 15 – 16); nine and three setae in the merus and dactylus of the endopod of the 3rd maxilliped (whereas P. quadridens has 7 – 8 and four, respectively); (7) absence of the ischial spine of the pereiopods (whereas P. quadridens has two); (8) six, six, six, eight, eight and zero setae in the abdominal somites (whereas P. quadridens has eight, five, five, four, four, and two); (9) nine, nine, nine and seven natatory setae in the pleopods (whereas P. quadridens has 12, 11, 10 and nine); (10) four setae in the exopod of the uropod (whereas P. quadridens has five). Rochinia gracilipes shares with Pugettia quadridens the absence of dorsal spine and of spines in the coxa and ischium of pereiopods; the presence of an epipod bud of the 2nd maxilliped; three spines in the inner margin of dactylus of the pereiopods; and the setation of segment 3 of peduncle, endopod and segment 1 of exopod of the antennules; segments 2 and 3 of peduncle and segment 1, 2, 3 and 4 of flagellum of the antenna; distal segment of palp of the mandible; basial endite and exopod of the maxillule; scaphognathite; epipod, coxal endite, endopod and exopod of the 1st maxilliped; endopod and exopod of the 2nd maxilliped; ischium, carpus and propodus of endopod and both segments of exopod of the 3rd maxilliped; and telson. The megalopae of P. marisinica and P. intermedia are presently unknown.
Comparison among known Pisinae View in CoL larval developments
Phylogenetic reconstructions based on majoid larval characters supported a monophyletic Oregoniidae , a monophyletic Majidae , an Inachidae – Inachoididae clade, and close associations among Epialtinae (Epialtidae) , Pisinae (Epialtidae) , and Mithracinae (Majidae) ( Clark and Webber 1991; Marques and Pohle 1998, 2003; Pohle and Marques 2000). At present, the family Epialtidae includes four recognised subfamilies, and one of them is the previously separated Pisinae (Ng et al. 2008). Colavite et al. (2014, p. 2283) considered that Epialtidae is ‘ probably the most heterogeneous among Majoidea ’ families, that the separation among its subfamilies is sometimes unclear, and that a revision based on adult morphology is needed. Could larval morphology help to understand the relationships within Epialtidae or, at least, within Pisinae ?
An actualised compilation of the larval morphology of Pisinae species with partial or complete descriptions ( Table 1 View Table 1 ), that would help future researchers to compare zoeae I, zoeae II and megalopae is presented here (Supplementary online material 1, 2, 3, respectively). Previous comparisons of Pisinae larvae ( Santana et al. 2004, 2006) included species that are now considered to be within Majidae : Eurynolambrus australis Webber and Wear, 1981 , Eurynome aspera Kinahan, 1858 ; E. spinosa Salman, 1982 and Naxioides serpulifera Rathbun, 1914 = Paranaxia serpulifera . This new comparison of larval morphologies corroborates the previous assertion that larvae of Pisinae are morphologically heterogeneous and that this subfamily cannot be defined on the basis of larval characters ( Santana et al. 2004, 2006), and suggests that larval morphology would not help to accurately understand the phylogenetic relationships of this subfamily of spider crabs.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina [under Grant PIP 11220110100830]; Agencia Nacional de Promoción Científica y Tecnológica, Argentina [under Grant PICT 2013-0763]; and Universidad Nacional de Mar del Plata, Argentina [under Grant EXA 711/14 – 15/E661].
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Majoidea |
|
Family |
|
|
SubFamily |
Pisinae |
|
Genus |