Micrommata Latreille, 1804
|
publication ID |
https://doi.org/10.11646/zootaxa.5352.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:ED680310-AF88-4A95-A436-40E7B276A79F |
|
DOI |
https://doi.org/10.5281/zenodo.8411405 |
|
persistent identifier |
https://treatment.plazi.org/id/03B087BB-FFA9-C32E-52CE-F93674E74F4A |
|
treatment provided by |
Plazi |
|
scientific name |
Micrommata Latreille, 1804 |
| status |
|
Micrommata Latreille, 1804 View in CoL View at ENA
Type species: Aranea smaragdula Fabricius, 1793 = Micrommata virescens ( Clerck, 1757) (see Jäger 1999a: 6).
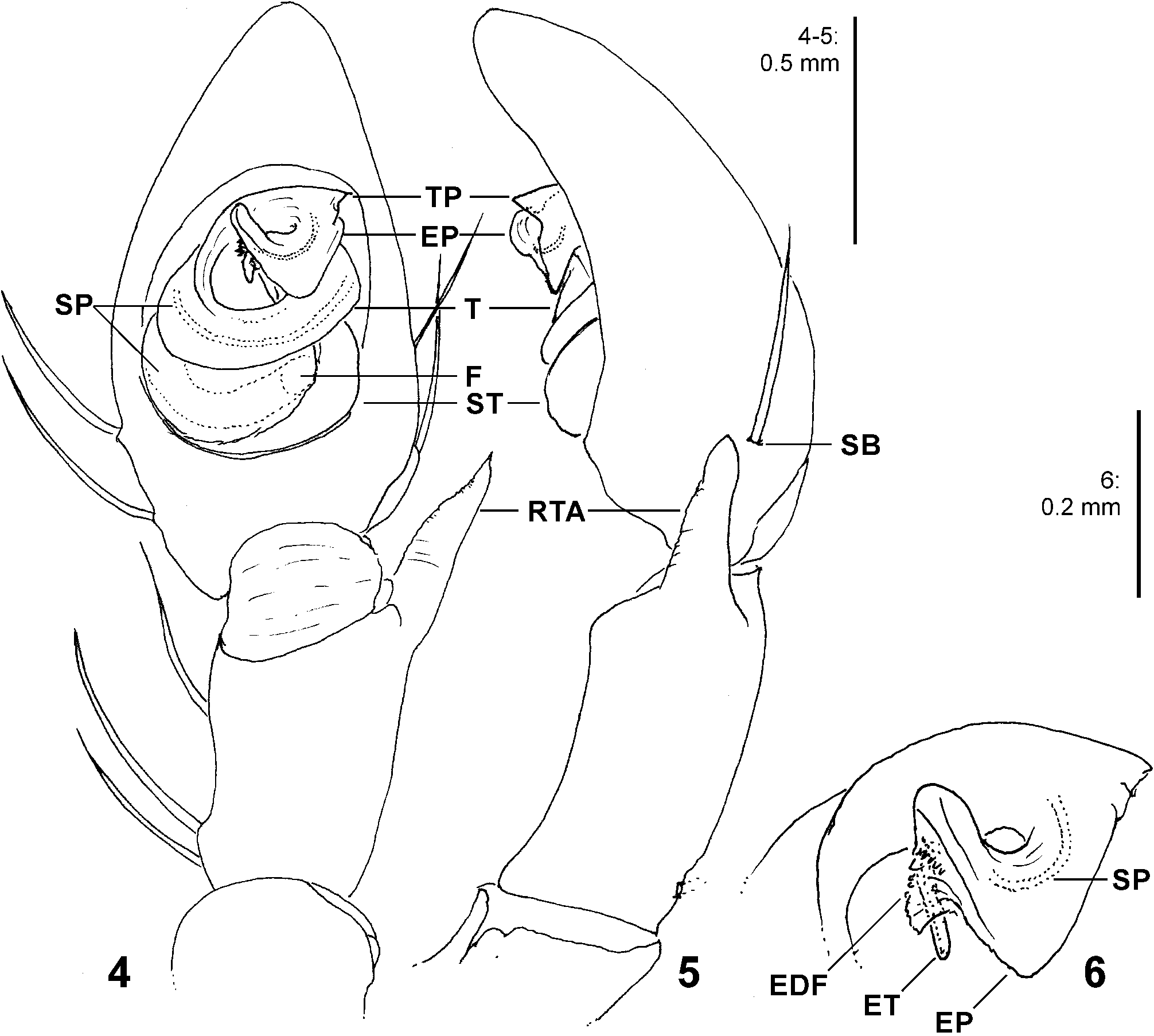
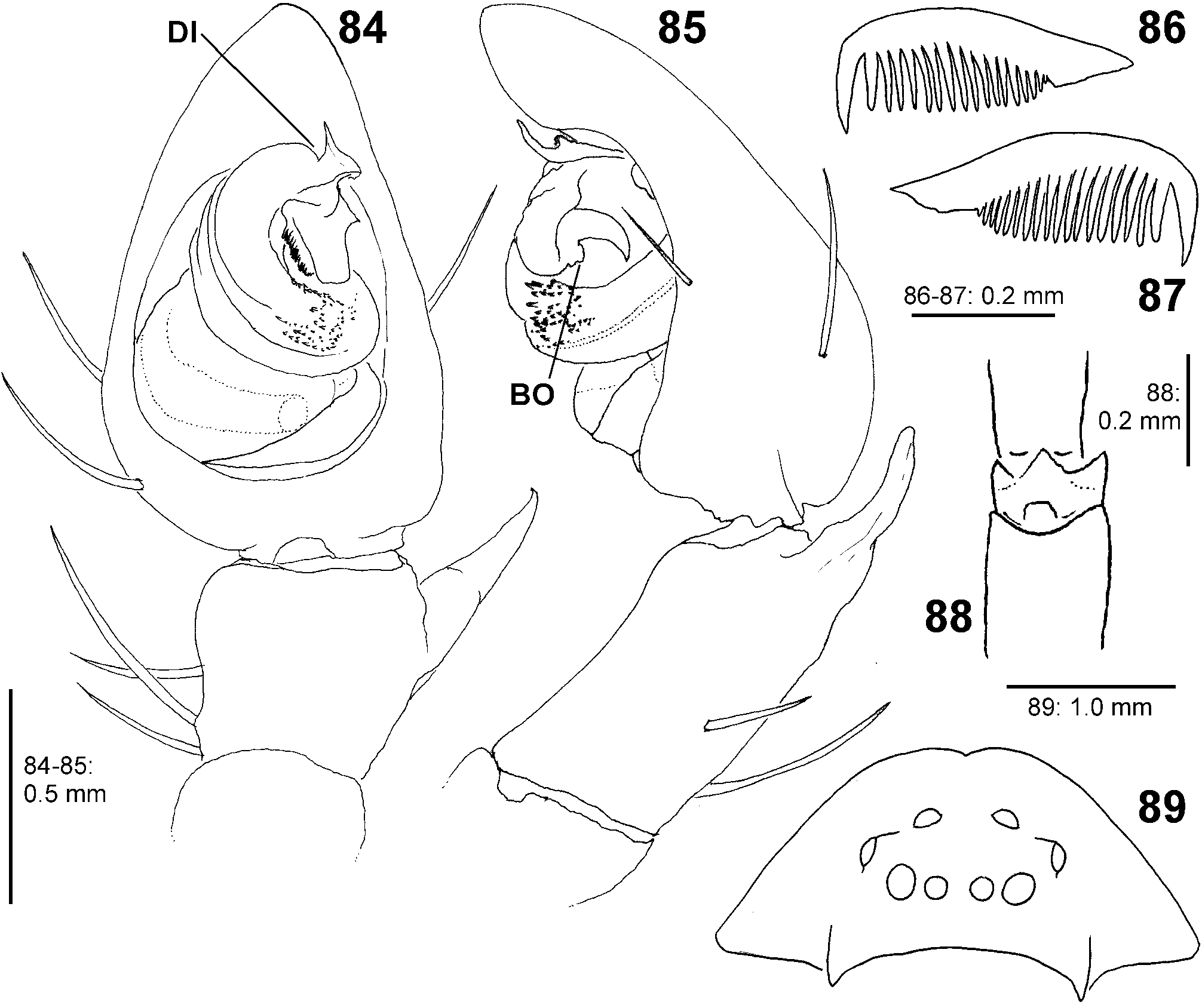
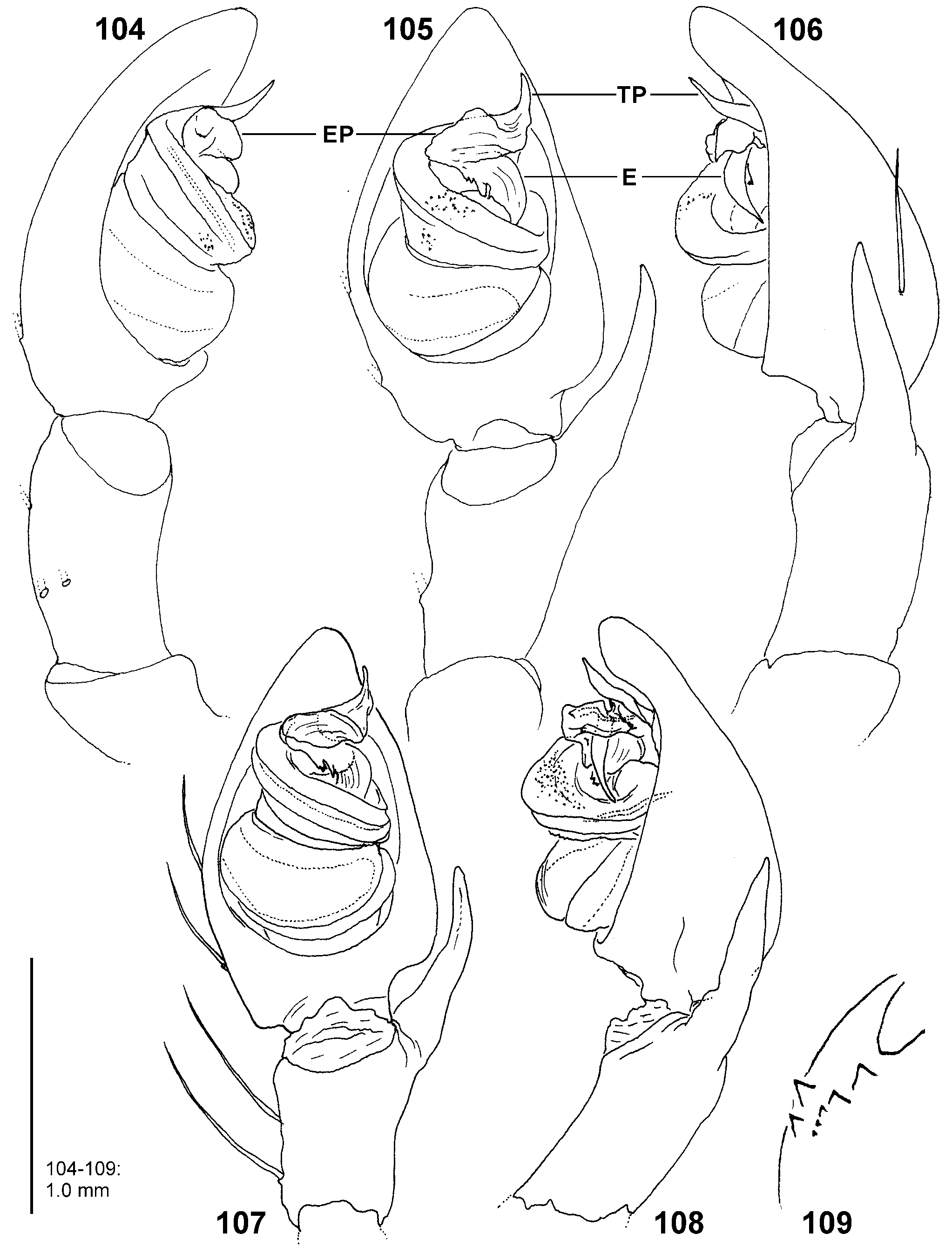
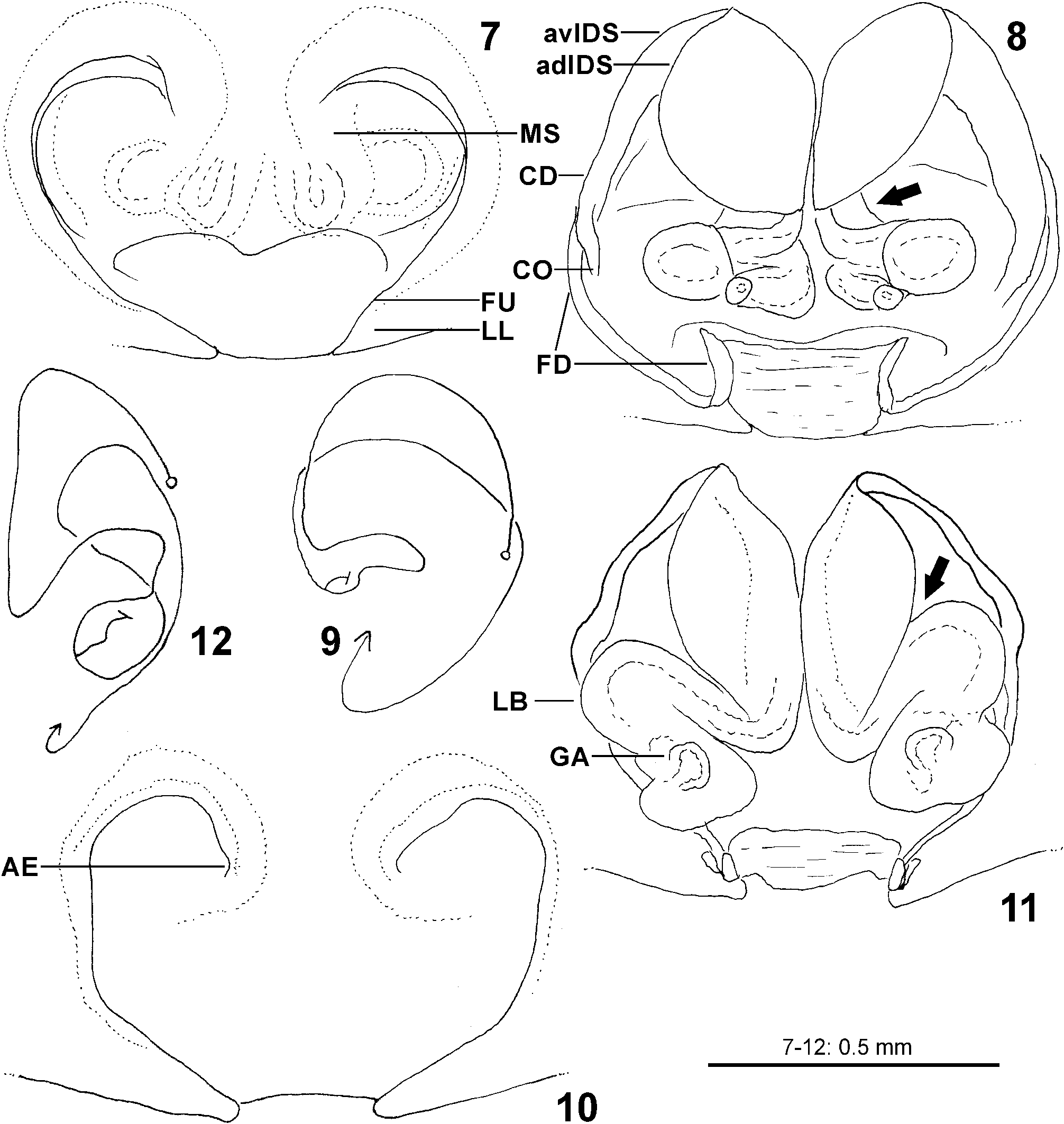
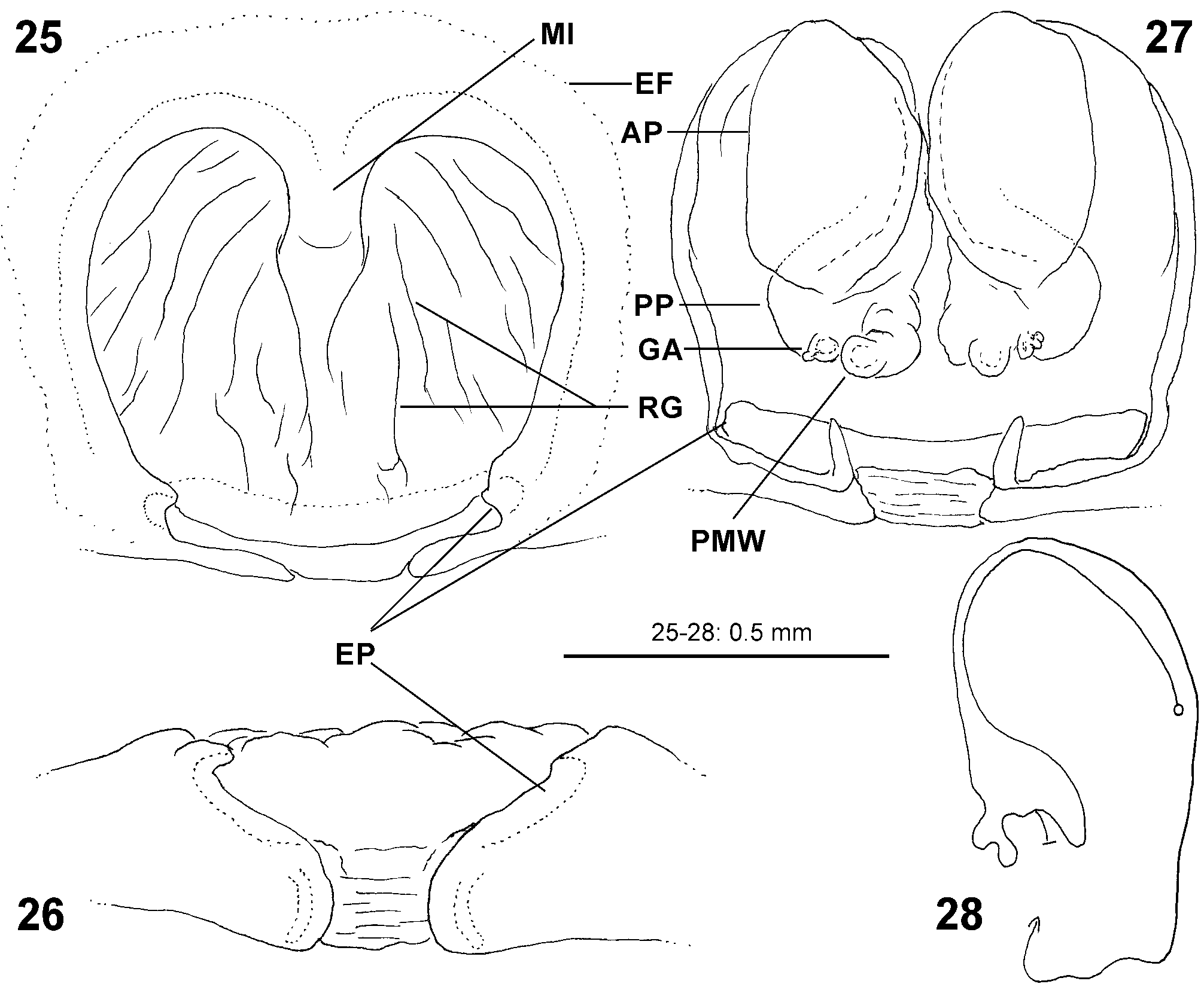
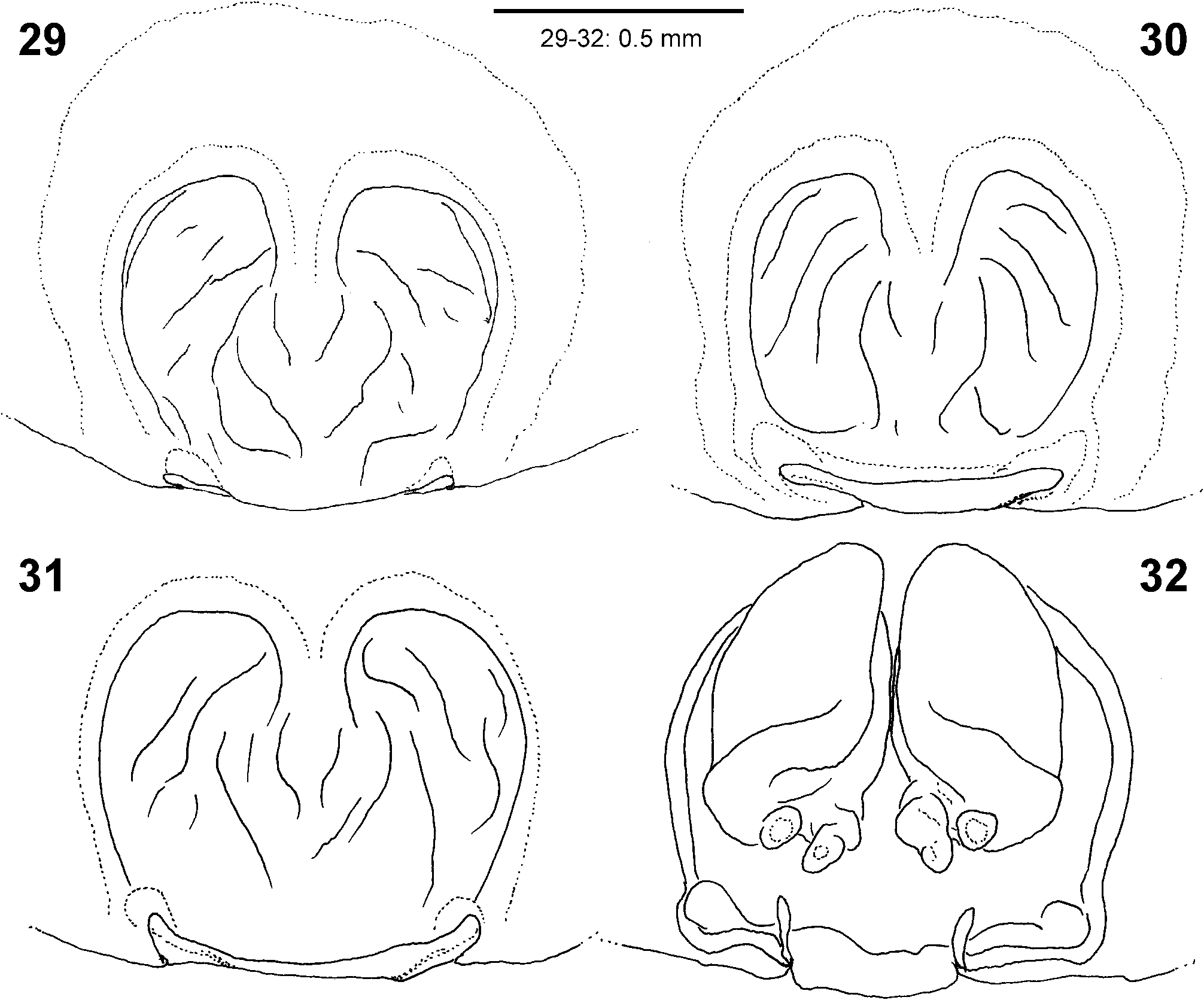
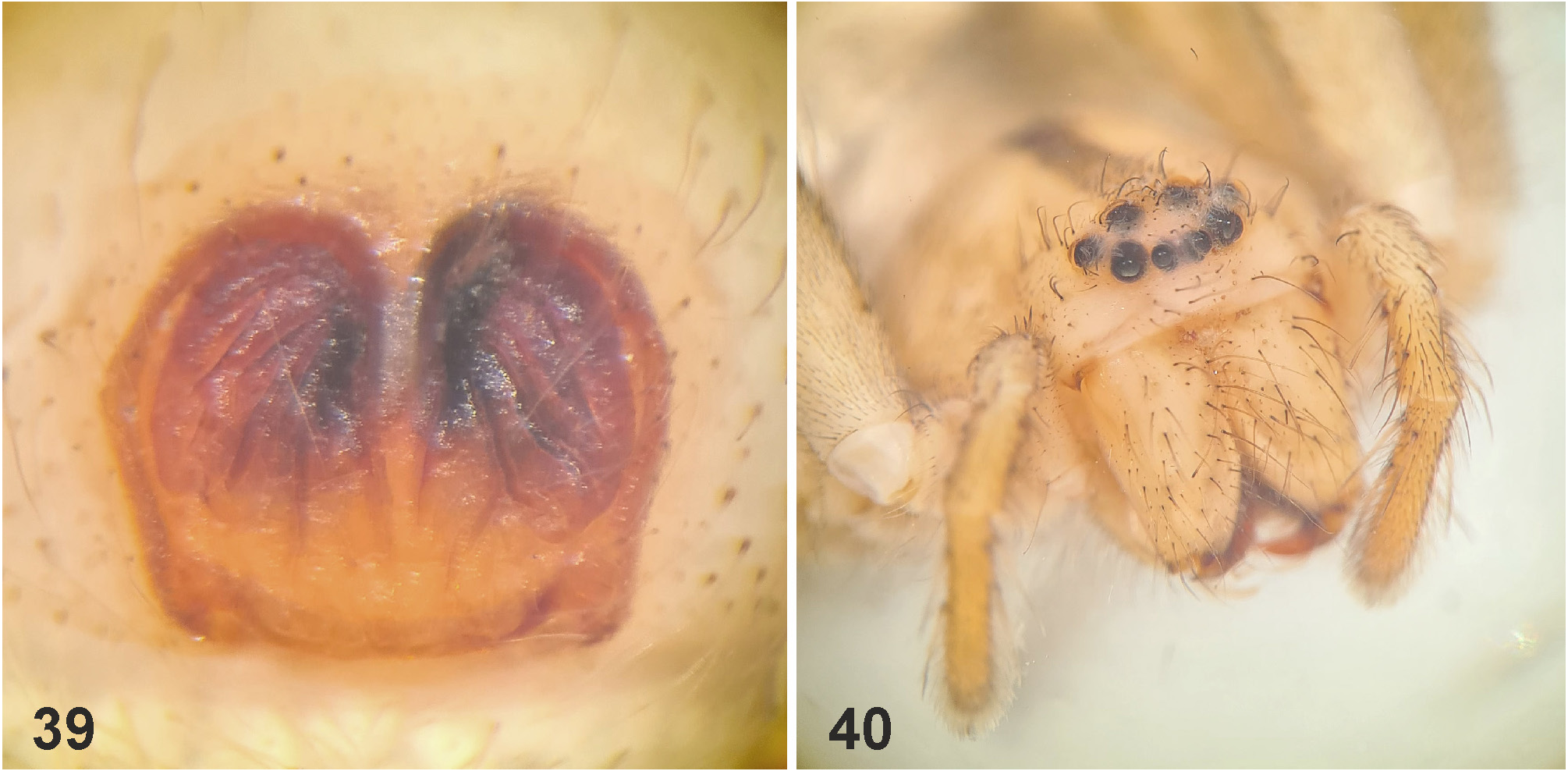
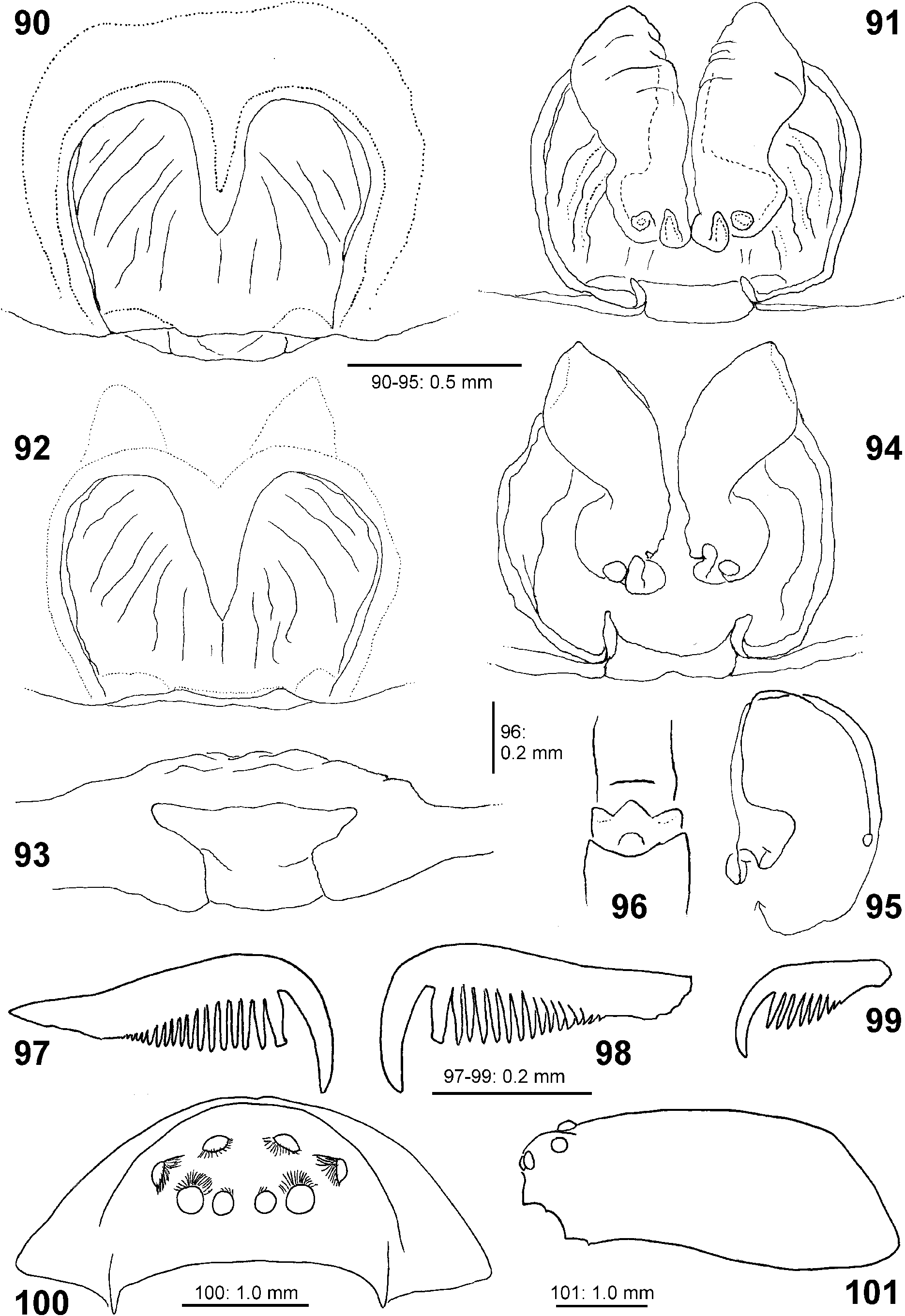
Diagnosis. Males ( Figs 4–6 View FIGURES 4–6 , 19–24 View FIGURES 19–24 , 41–43 View FIGURES 41–47 , 56–61 View FIGURES 56–61 , 84–85 View FIGURES 84–89 , 104–108 View FIGURES 104–109 ) are easily distinguished from all other Sparassidae by the spiralled tegulum, the tegular prong and the embolic division with plate, embolus and denticle field; the embolic division is connected to the distal tegulum by a flexible membranous part and is therefore mobile [males of Macrinus Simon, 1887 , Nolavia Kammerer, 2006 and Vindullus Simon, 1880a in the Neotropics also have a spiralled tegulum and a tegular prong, but the long flagelliform embolus is not mobile ( Jäger 2020b: figs 393, 398, 412) and the spiders are not green-coloured or diurnal ( Rheims 2010, 2019; Rheims & Jäger 2008)]. In addition, male genital bulbs lack a conductor [this character state is known only from a few species of the Heteropodinae genus Pseudopoda Jäger, 2000a , that is easily distinguished from Sparassinae , i.e. Micrommata , by the presence of intermarginal denticles and 3 promarginal teeth on the chelicerae ( Jäger 2000a, 2015; Jäger et al. 2015), and most species of the genus Chrosioderma Simon, 1897a and all species of the genus Cebrennus Simon, 1880b ; the latter two distinguished by their brown to yellowish colour ( Silva-Dávila 2005; Jäger 2000c, 2014); and Origes Simon, 1897b , distinguished by the presence of 1 promarginal tooth and 2 retromarginal teeth on chelicerae or the brownish colour pattern ( Jäger & Rheims 2008)]. Females ( Figs 7–12 View FIGURES 7–12 , 25–32 View FIGURES 25–28 View FIGURES 29–32 , 39 View FIGURES 39–40 , 46–51 View FIGURES 41–47 View FIGURES 48–53 , 62–72 View FIGURES 62–67 View FIGURES 68–72 , 90–95 View FIGURES 90–101 , 115–128 View FIGURES 115–120 View FIGURES 121–128 ) are distinguished from all other Sparassidae by their roughly heart-shaped, sclerotised median septum (with or without folds), the two distinct pockets at the posterior margin of the epigyne, and the internal duct system with broad membranous parts of the intromittent ducts, a sub-central set of glandular appendages and tight loops, from which long and thin fertilisation ducts emerge, running ventrally from the membranous part in a large semicircle to the epigastric furrow. A superficially similar arrangement can be observed in females of the Neotropical species “ Olios ” erroneus O. Pickard-Cambridge, 1890 , but the two epigynal pockets as well as the glandular appendages or tight loops are missing ( Jäger 2020b: figs 281–286) and the spiders are neither green nor diurnal.
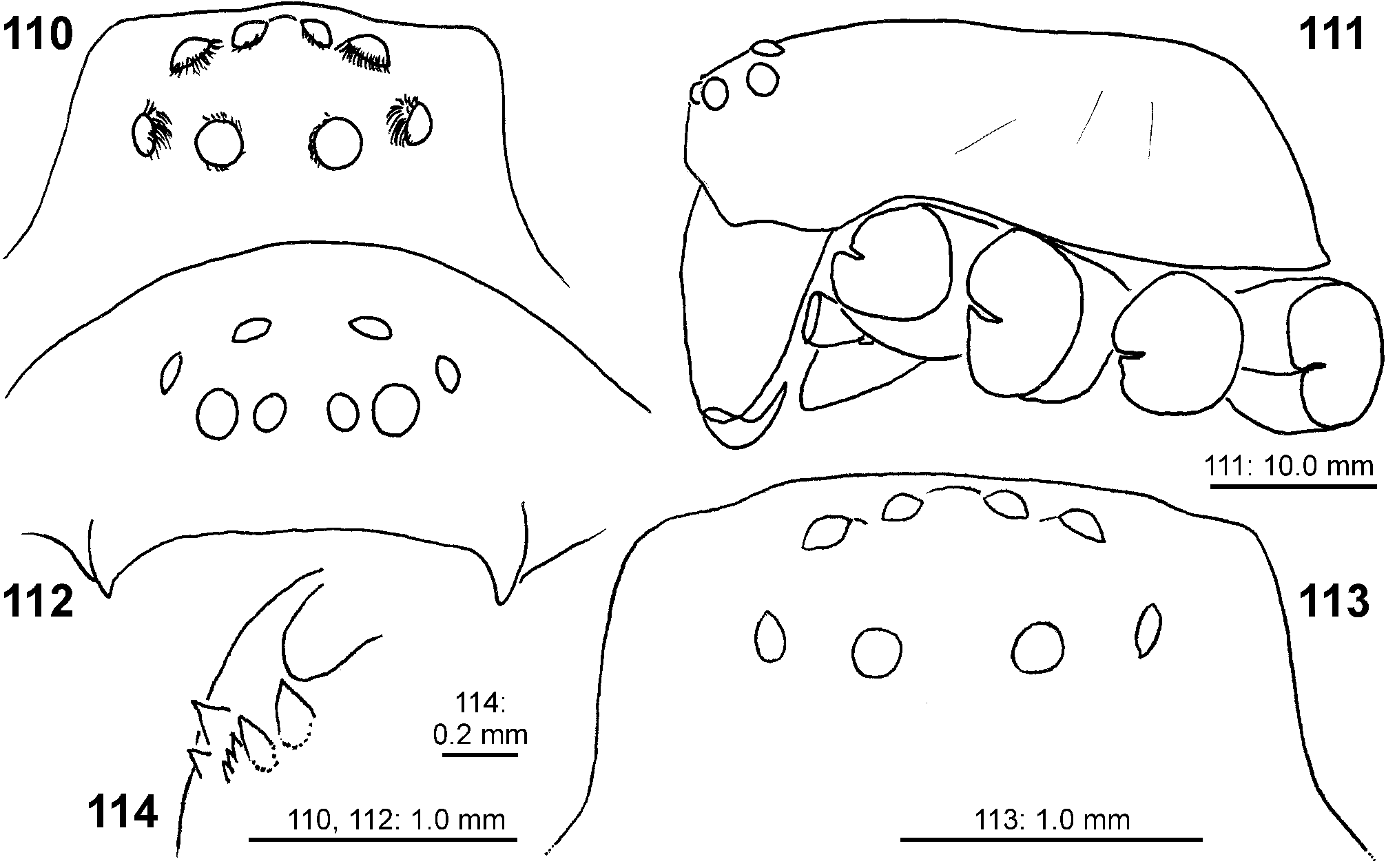
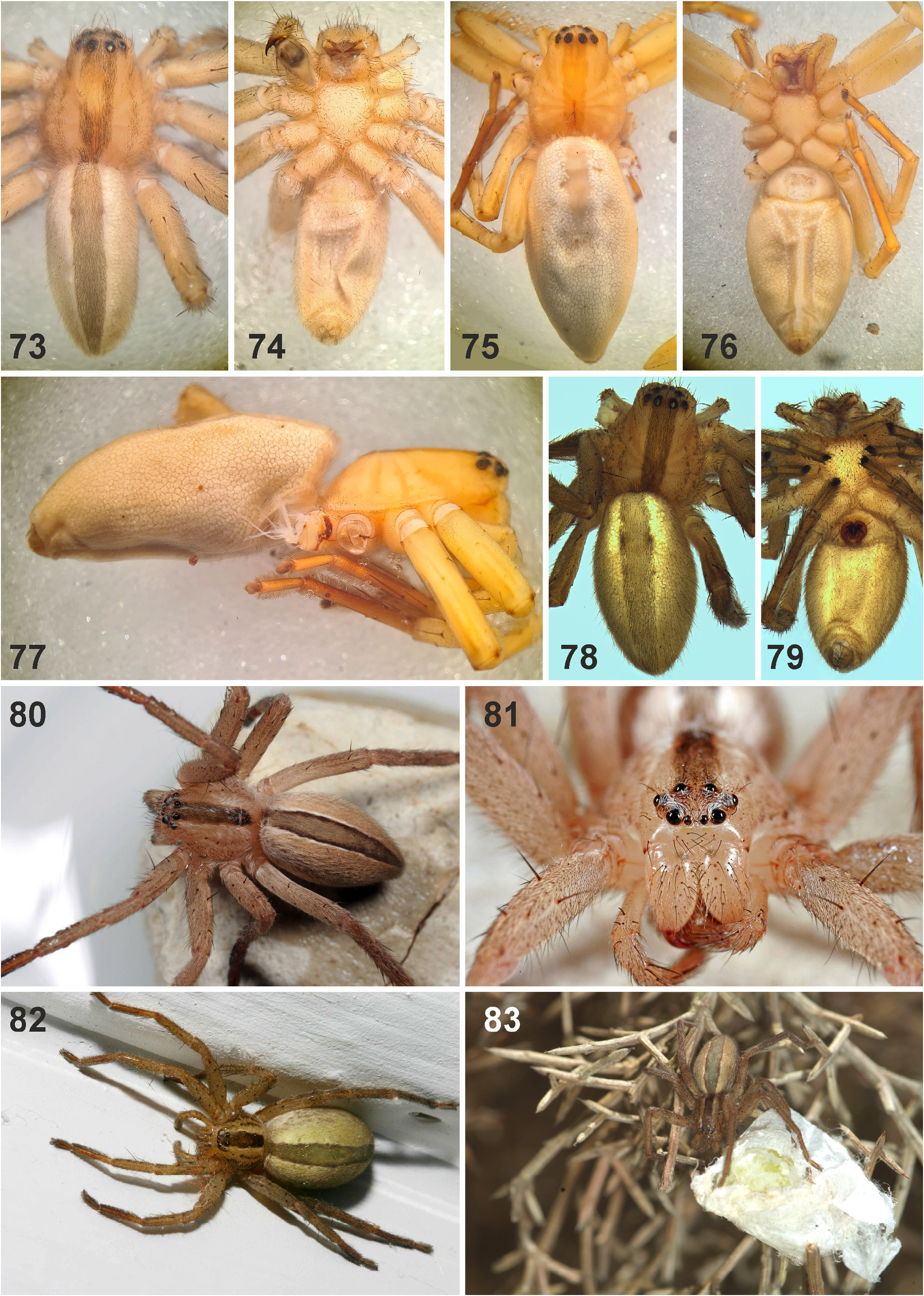
Description. Small to median-sized spiders (TL males 5.1–9.8, females 8.2–17.7). PS longer than wide, fovea distinct, longitudinal, located in posterior half. Head region between 0.5 times (males) and 0.65 times (females) the width of the prosoma. Striae distinct in preserved specimens, not so in live spiders. Prosoma in lateral view with straight outline dorsally until fovea, then steeply sloping almost in a straight line to posterior margin. Eight eyes arranged in two rows, anterior row narrower and recurved, posterior row procurved ( Figs 89 View FIGURES 84–89 , 100–101 View FIGURES 90–101 , 110–113 View FIGURES 110–114 ). AME smallest, ALE largest. Eyes surrounded by white setae (easily rubbed off in preserved material), typical of diurnal spiders. Sternum slightly longer than wide; labium wider than long; gnathocoxae longer than wide. Chelicerae with 2 promarginal, 3–5, rarely up to 7 retromarginal denticles, and 5–10 retromarginal escort setae on the base of the fang. Leg formula: II-IV-I-III or IV-II-I-III. Ta I –IV and MT I–III with sparse scopulae (in MT except for very proximal part); MT IV ventrally with distal patch and a double row of stronger setae, very sparse scopulae present in most distal part. Trilobate membrane with median hook and lateral projections equally strongly developed ( Figs 88 View FIGURES 84–89 , 96 View FIGURES 90–101 ). Leg claws with 16–20 secondary teeth, arranged comb-like with primary tooth only slightly longer in males and distinctly longer in females, and female palpal claw with ca. 9 straight teeth and distinctly longer primary tooth ( Figs 86–87 View FIGURES 84–89 , 97–99 View FIGURES 90–101 ). OS clearly longer than wide, posterior end slightly pointed.
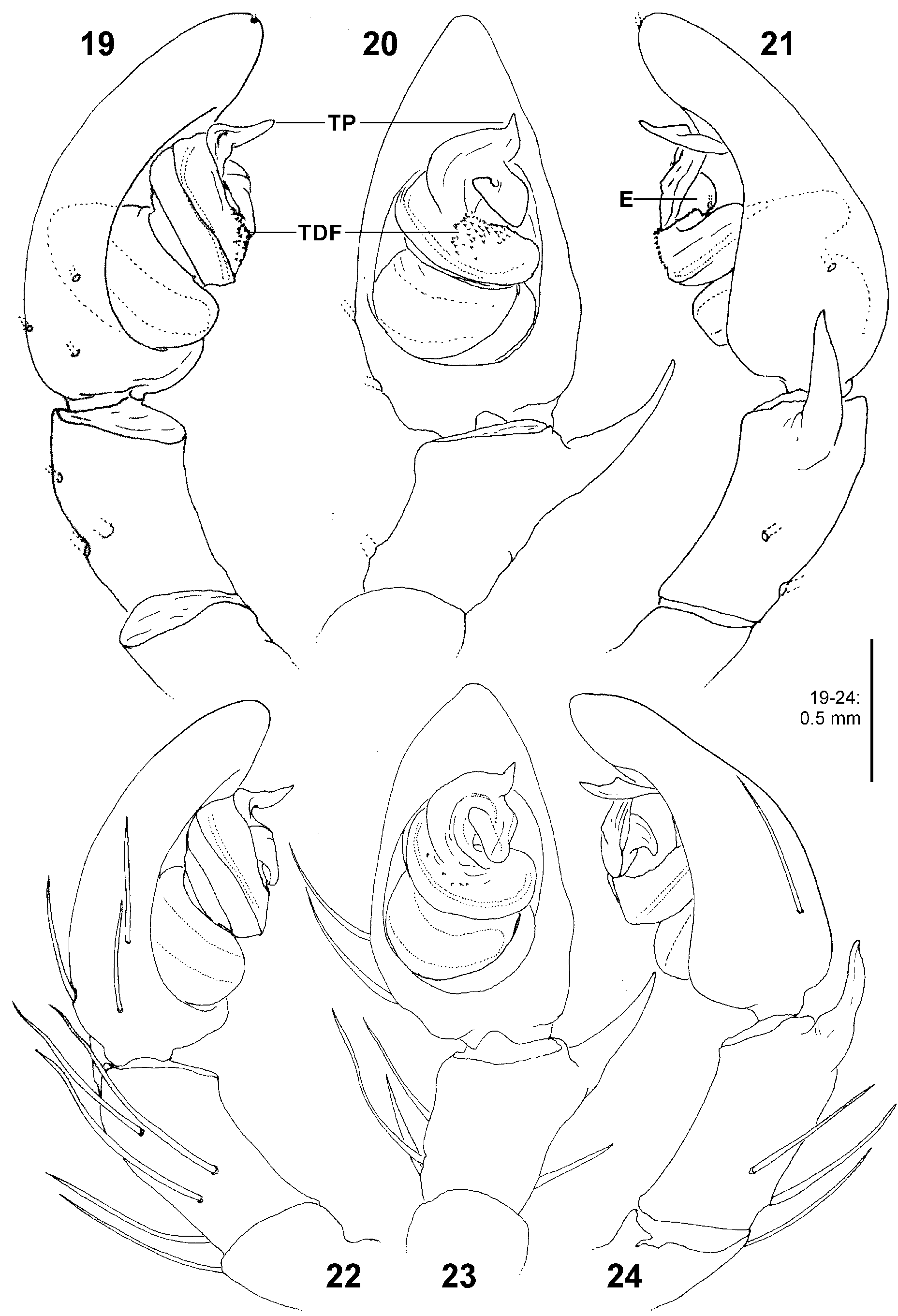
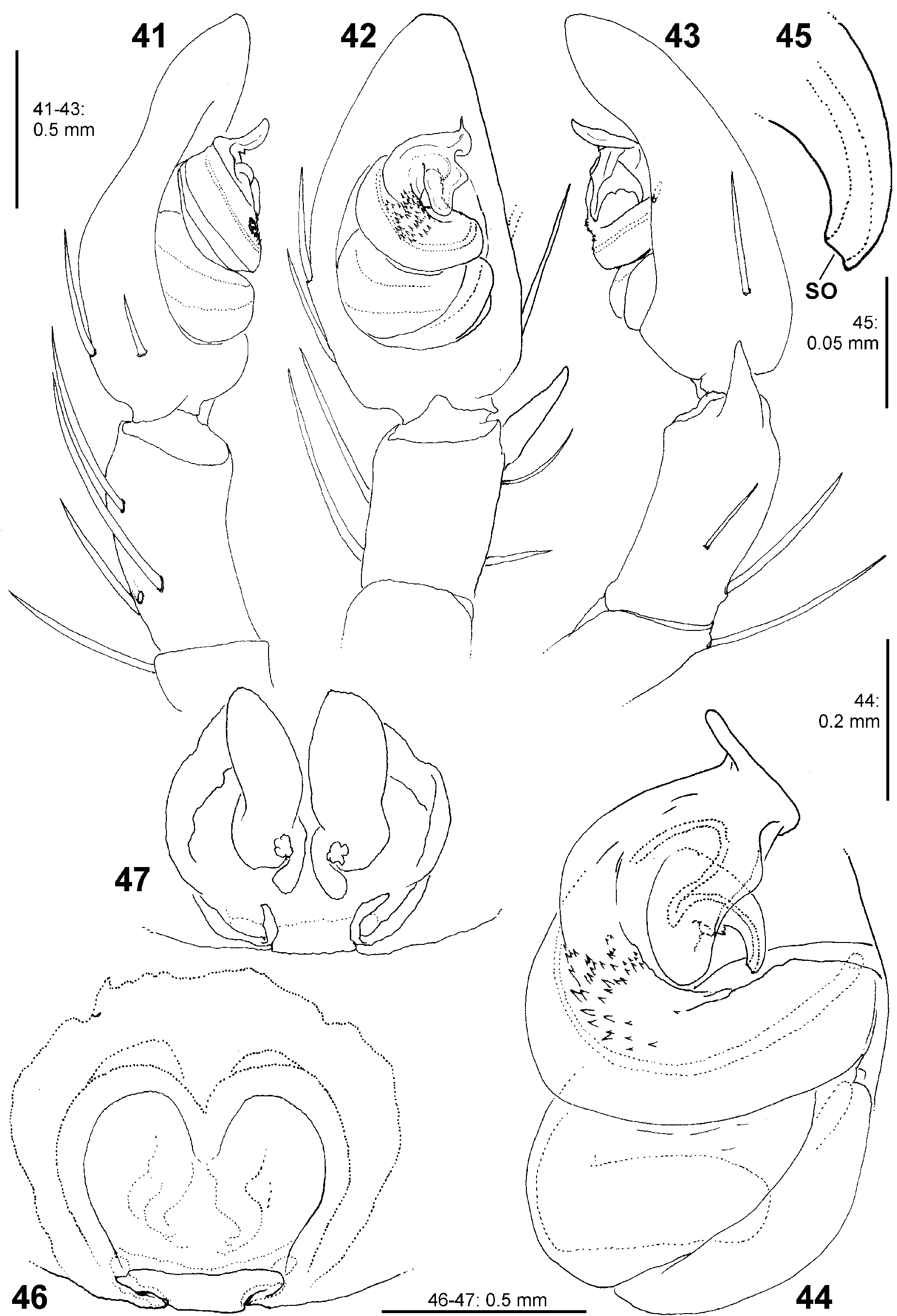
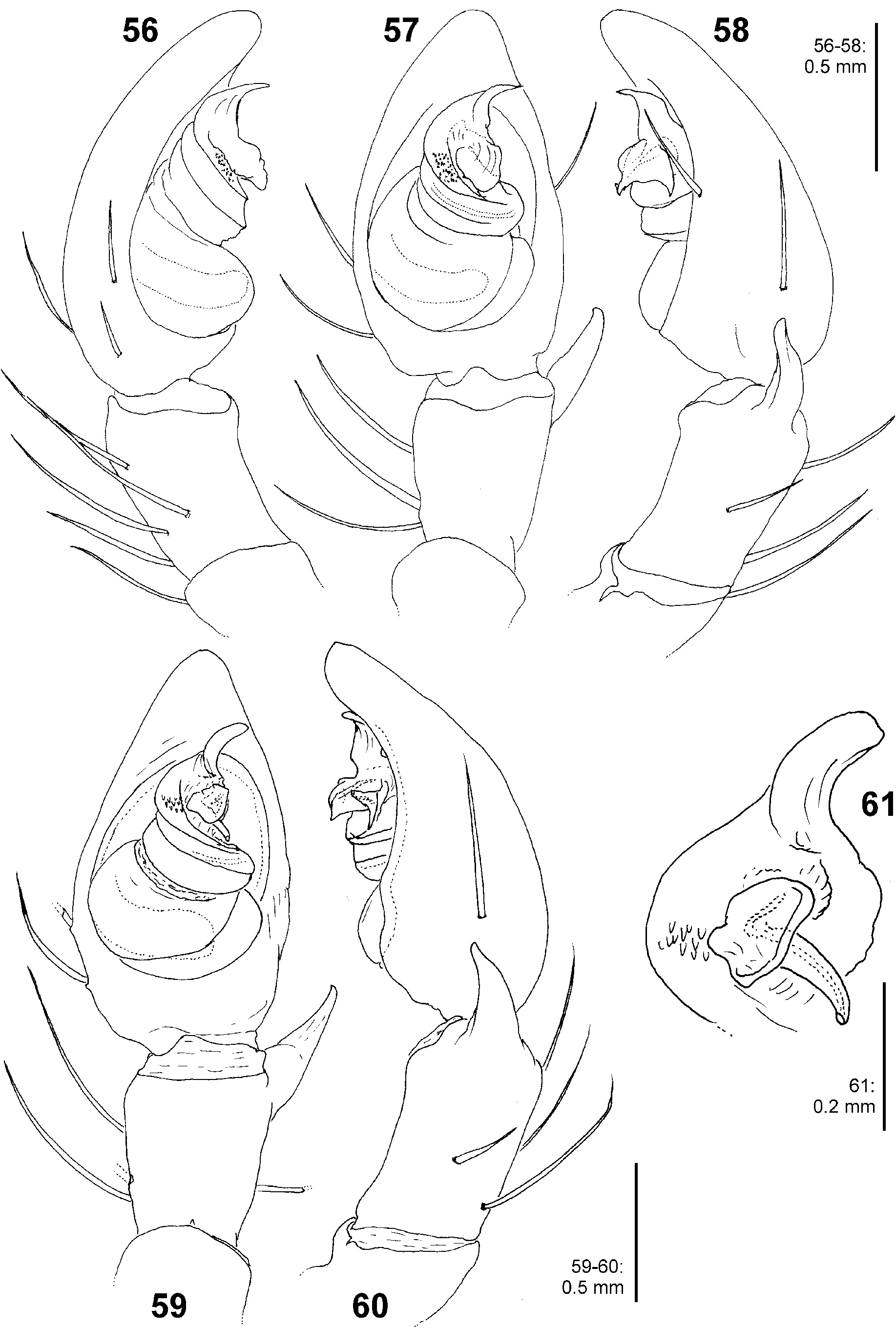
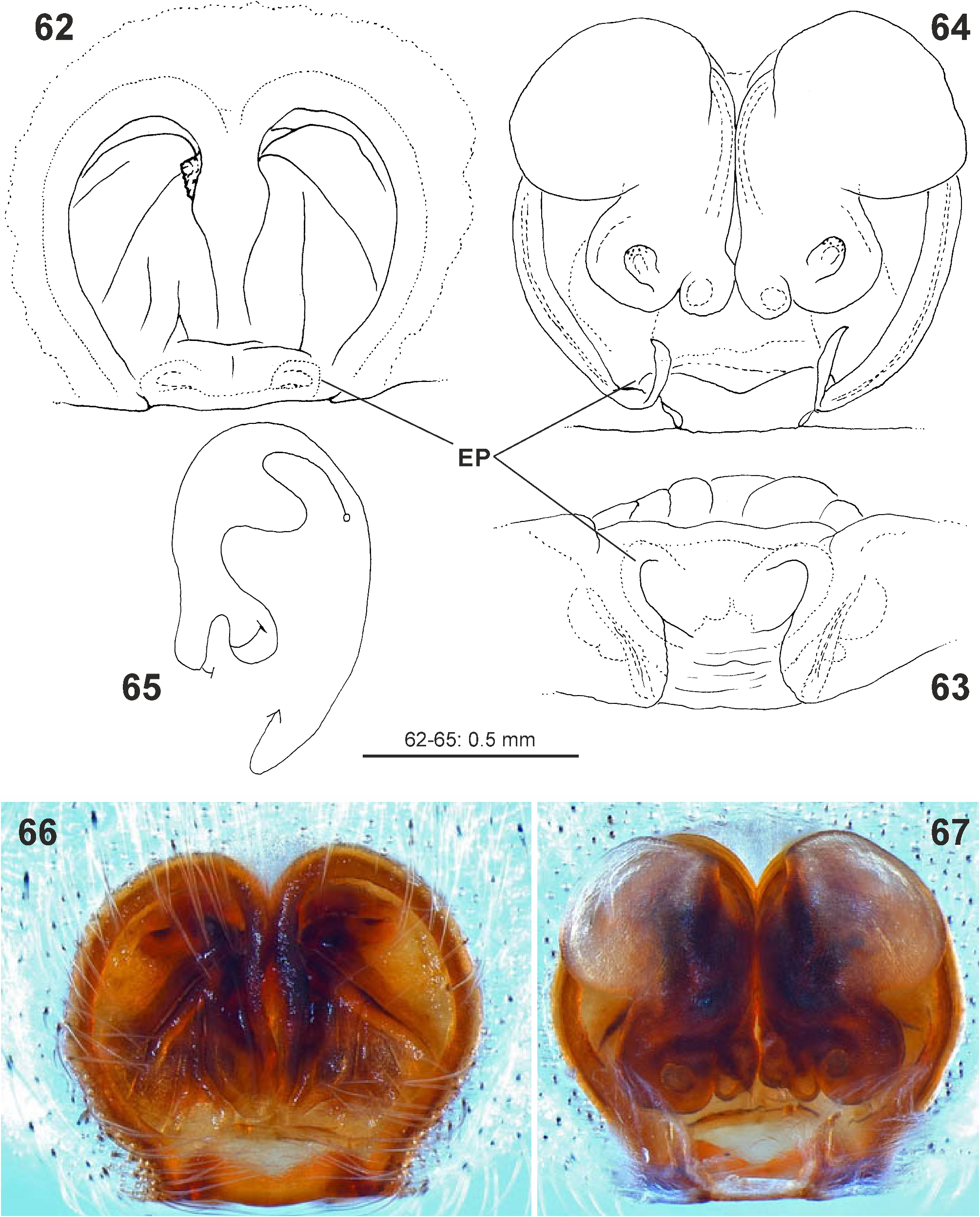
Palp. The palpal tibia is always shorter than the cymbium and has a simple, pointed, distally arising RTA. The cymbium is characterised by the presence of spines prolaterally and retrolaterally of the alveolus, which are reduced in the majority of Sparassidae . The alveolus is slightly smaller than known from other Sparassidae and is situated relatively centrally within the cymbium. The subtegulum is well visible as a broad ring situated retrolaterally from the tegulum and prolaterally converging in ventral view. The tegulum is twisted with a broad proximal part carrying the broad fundus of the spermophor and a narrowing distal part with a ventral field of denticles terminating in a flat and pointed tegular prong. A conductor is absent. The embolic division is connected to the tegulum by a membranous part near the prong and contains a plate with a second field of denticles and the claw-shaped embolus ( Figs 4–6 View FIGURES 4–6 , 19–24 View FIGURES 19–24 , 41–43 View FIGURES 41–47 , 56–61 View FIGURES 56–61 , 84–85 View FIGURES 84–89 , 104–108 View FIGURES 104–109 ).
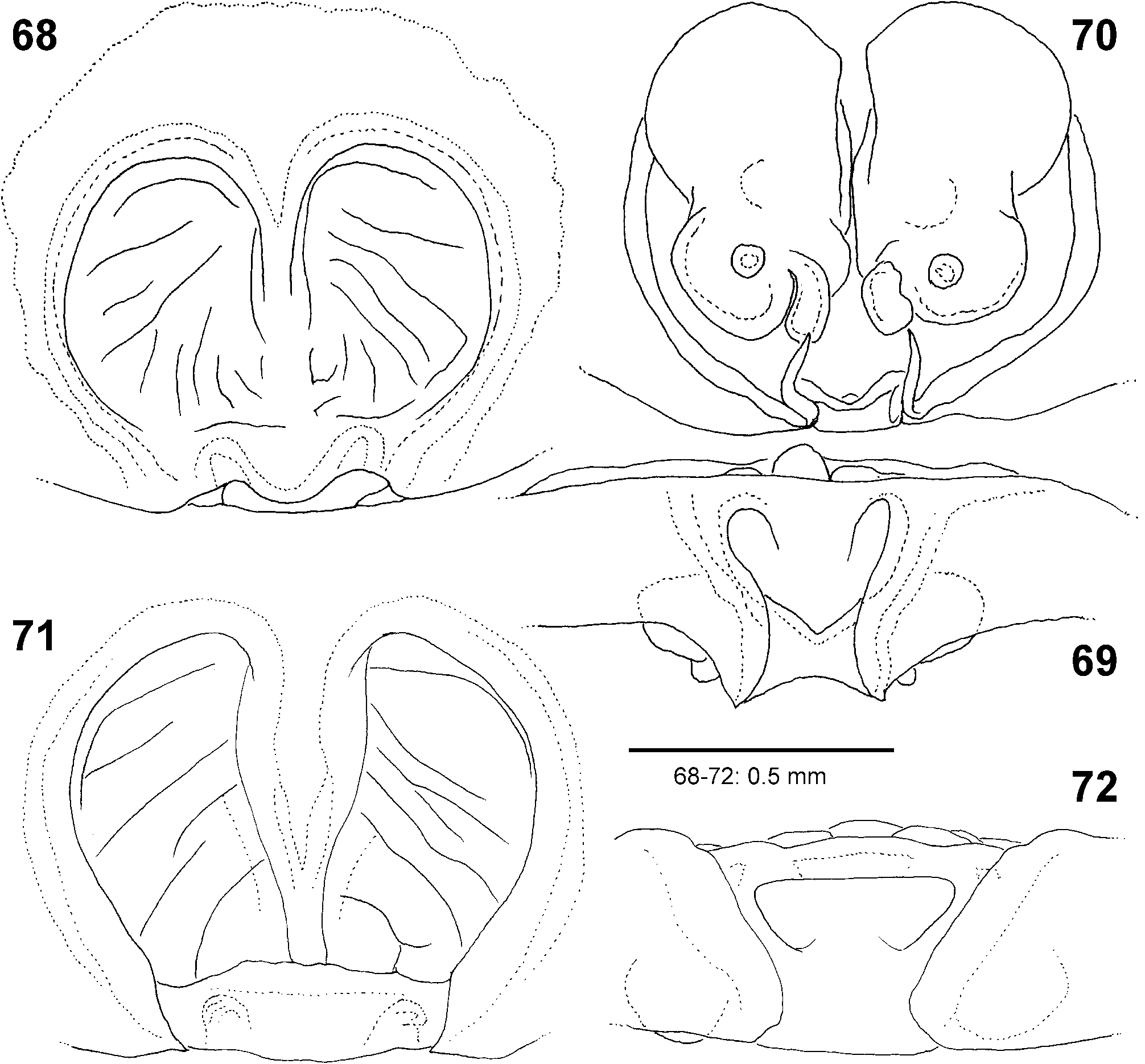
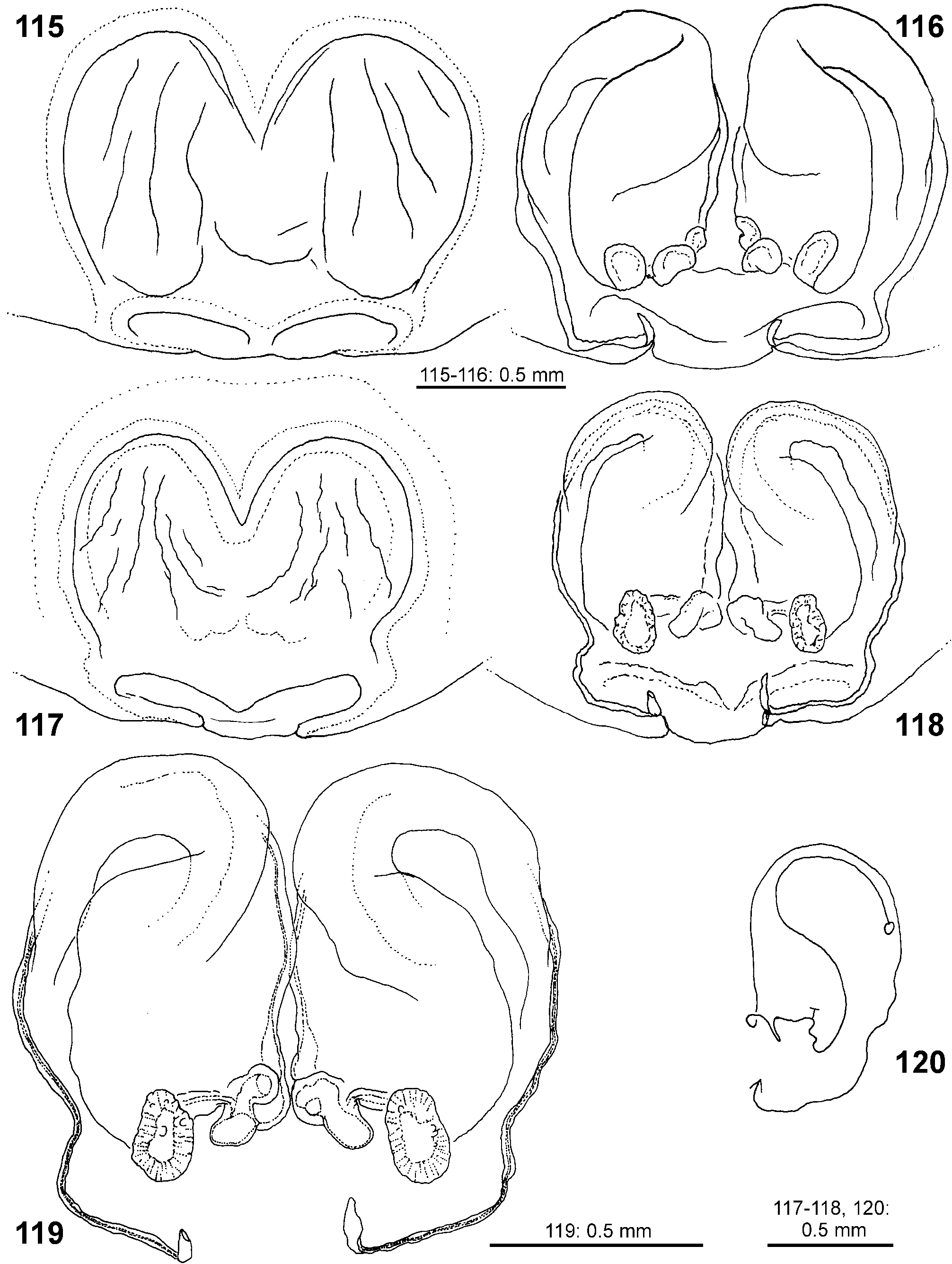
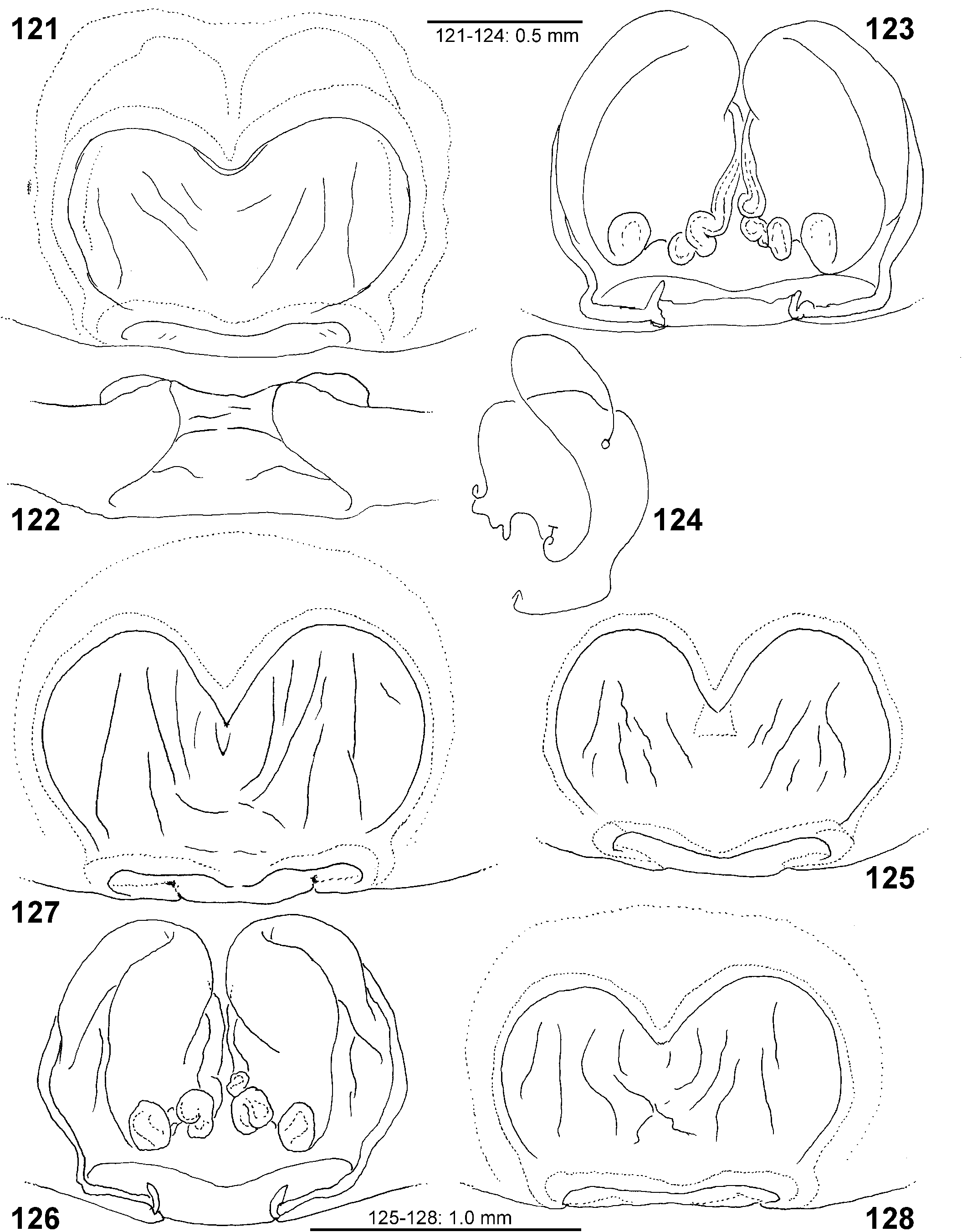
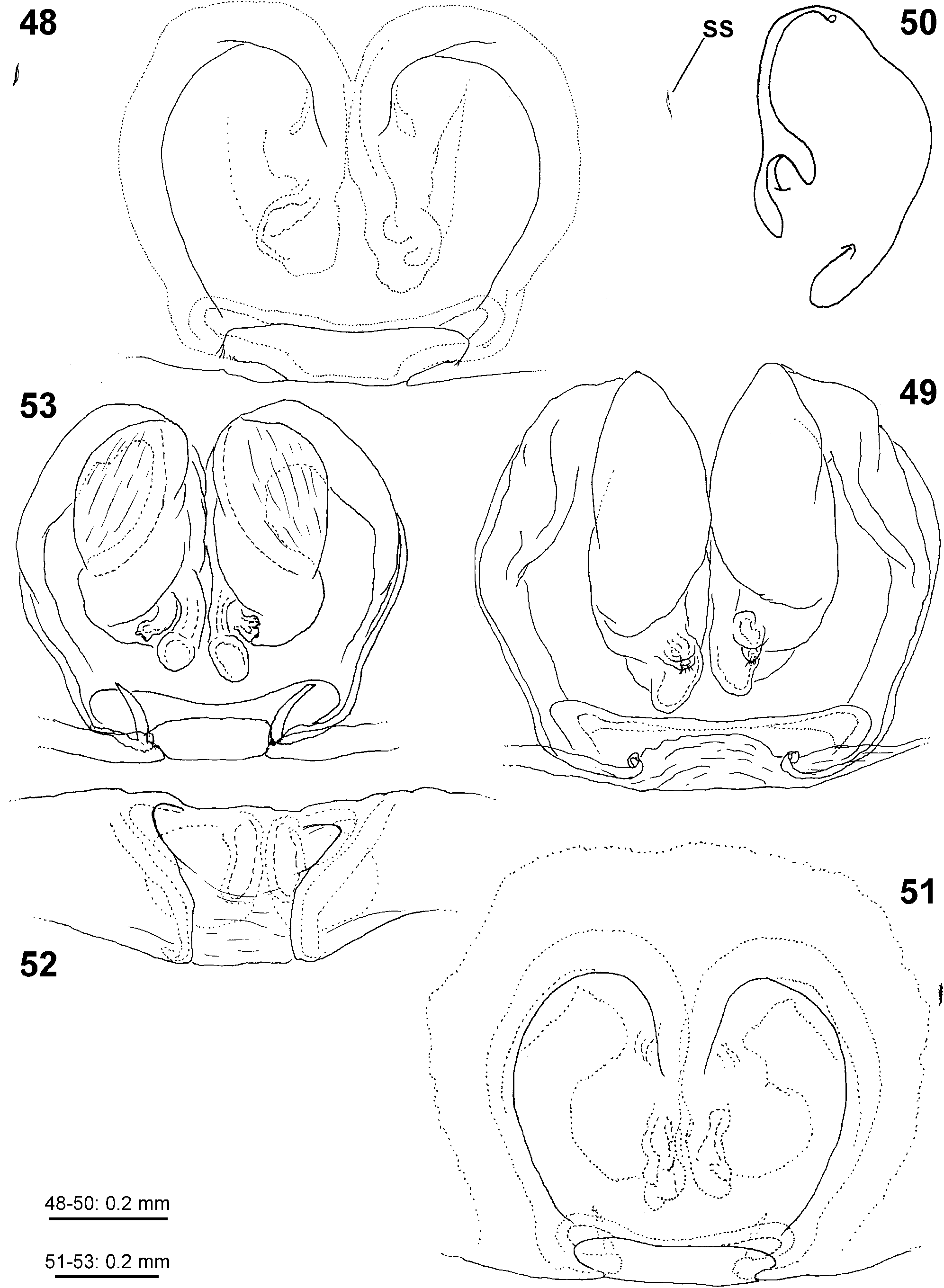
Female copulatory organ. The epigynal field is only weakly sclerotised and not always clearly visible. It is roundish and lacks anterior bands. Slit sensilla are more distant from the field than in other Sparassidae and therefore not always observable due to the dissection method. The median septum is strongly sclerotised and has radial grooves in most species and a few transversal grooves in some species. In continuation of the septum there are two epigynal pockets posteriorly, sometimes visible in ventral view, best seen in posterior view. The copulatory openings are slit-like and located laterally to the septum. The IDS begins with a membranous part running from laterally to antero-medially, where it widens into a dorsal pouch, which merges into a narrower sclerotised part with median windings, with a glandular appendage on each side. Fertilisation ducts run from postero-medially first anteriorly, then laterally ventral to the copulatory ducts in a semicircle to the posterior margin ( Figs 7–12 View FIGURES 7–12 , 25–32 View FIGURES 25–28 View FIGURES 29–32 , 39 View FIGURES 39–40 , 46–51 View FIGURES 41–47 View FIGURES 48–53 , 62–72 View FIGURES 62–67 View FIGURES 68–72 , 90–95 View FIGURES 90–101 , 115–128 View FIGURES 115–120 View FIGURES 121–128 ).
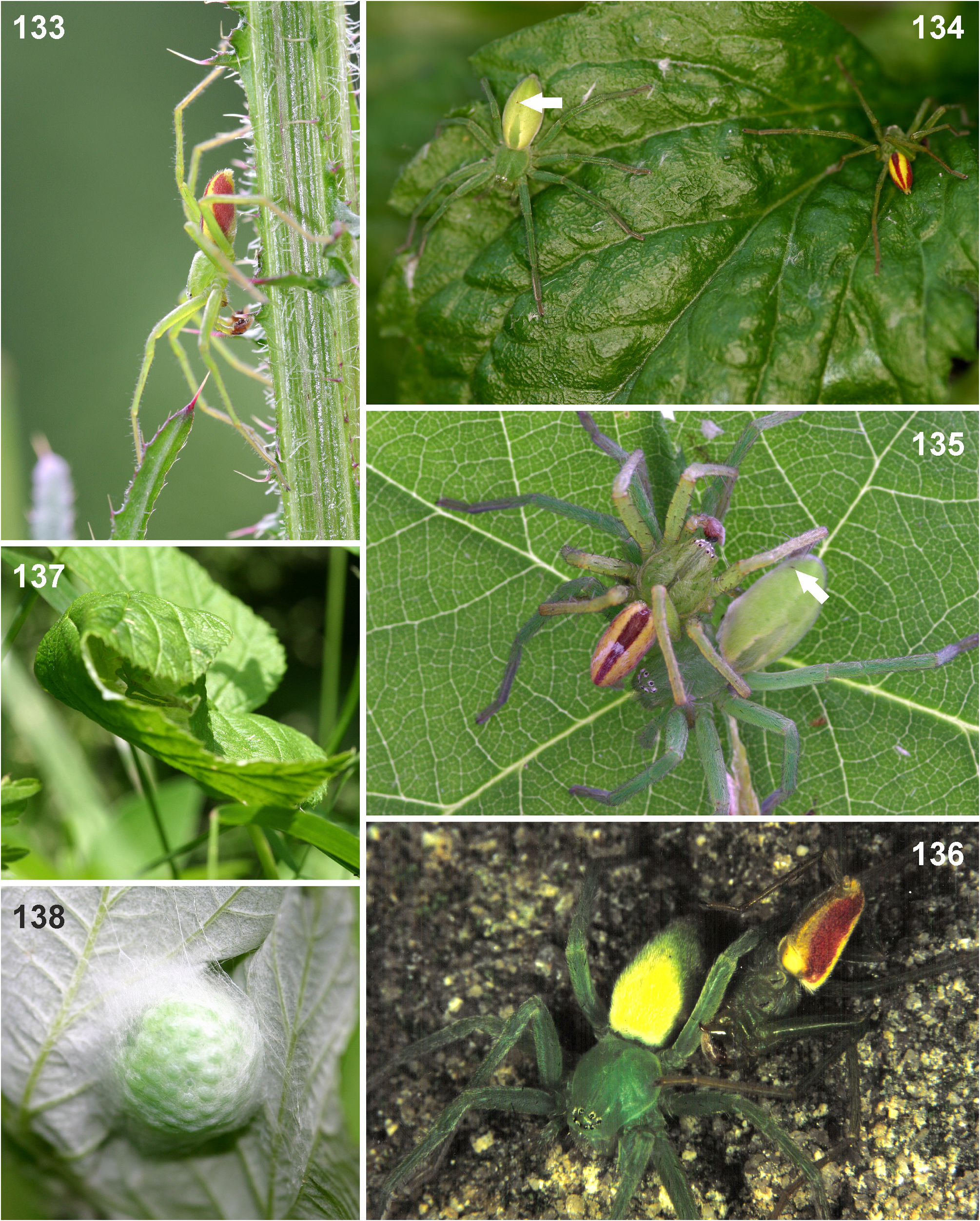
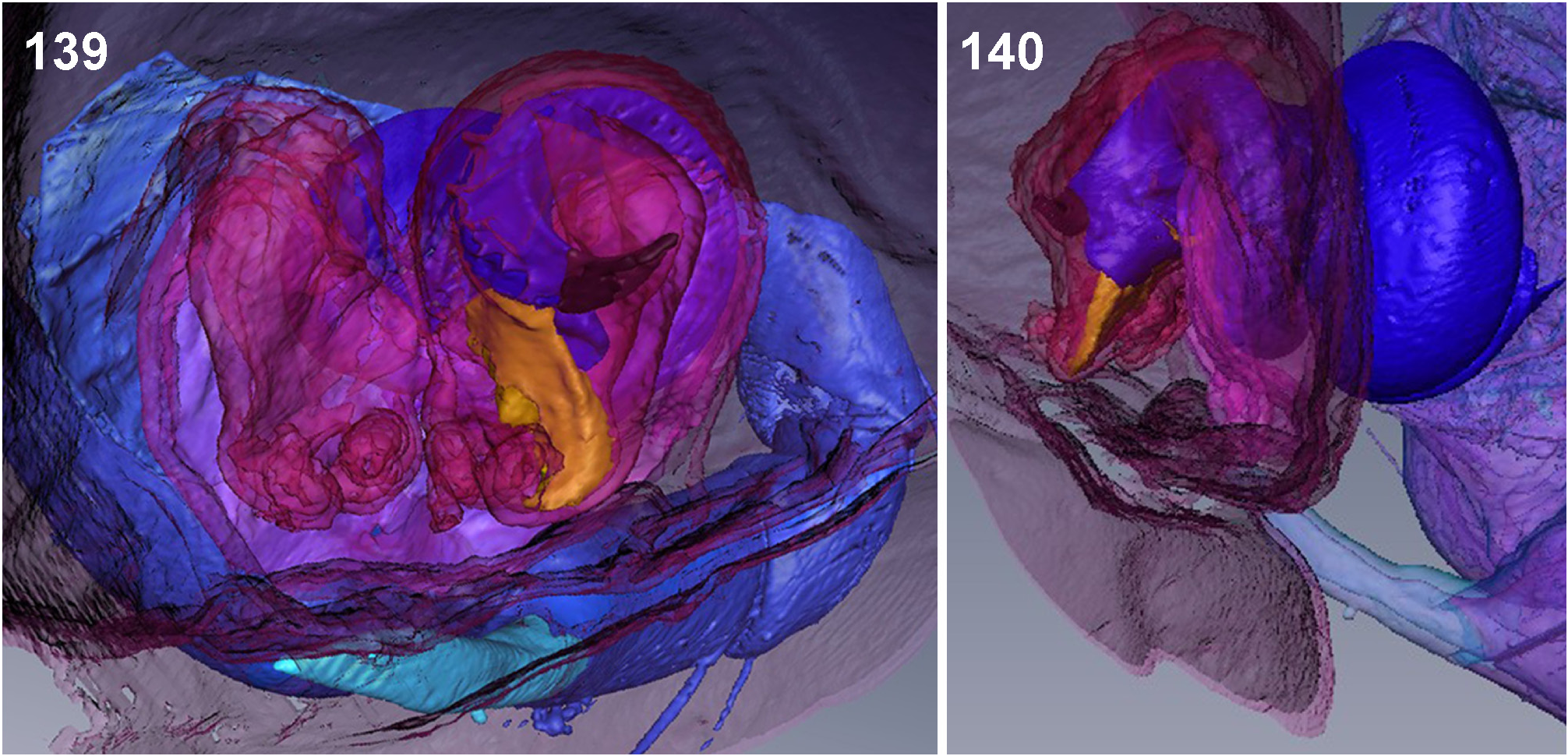
Reproductive behaviour. The mating and copulatory behaviour of M. virescens was described by Menge (1875), Bristowe (1926) and Nielsen (1932a, b). After detecting a female and a short courtship scene, the male mounts the female in the opposite direction and alternately inserts its genital bulbs into the female’s copulatory opening ( Fig. 135 View FIGURES 133–138 ). It will direct its body towards the right side of the female’s body and insert its right bulb and its left bulb respectively on the left side of the female’s body. The right bulb is inserted into the right half and the left bulb into the left half of the female’s copulatory organ. µCT scans ( Jäger & Michalik, unpublished) show that the male spider first anchors its RTA in one of the epigynal pockets. By expanding the basal haematodocha, the tegular prong is inserted into the slit-like copulatory opening and the entire distal part of the tegulum is inserted into the membranous pouch of the female. This greatly dilates the opening and deforms the median septum. The embolic division is then expanded and the tip of the embolus is in its final position close to the glandular appendages ( Figs 139–140 View FIGURES 139–140 ).
The whole copulation process can take several hours ( Menge 1875; Jäger, personal observations). During this time, the male may bite the female ( Fig. 136 View FIGURES 133–138 ), causing injuries, which later become scars ( Figs 134–135 View FIGURES 133–138 : arrows). In some extreme cases, the female was reported to have died ( Menge 1875). A thorough analysis of all scars found was carried out and will be reported in a separate paper with other species of the spider family Sparassidae ( Jäger, in preparation). Most scars were found on one female with 50 scars.
The female will build a retreat by weaving leaves together and will lay eggs inside ( Fig. 137 View FIGURES 133–138 ). The green eggs are covered within this retreat with only a thin layer of silk ( Fig. 138 View FIGURES 133–138 ) and are guarded by the female until the spiderlings hatch.
Colouration. Yellowish (preserved material) or green to brown (live spiders). Appendages uniformly coloured, covered with dark setae (spotted in M. aljibica Urones, 2004 ). Dorsal PS with lateral bands of dark spots and dark median stripe or longitudinal patch, the latter continuing onto the dorsal OS in most species (except for M. virescens ). Ventral PS and OS uniformly coloured. It may be that the colour depends on the colour of the vegetation in each latitudinal zone, i.e. the spiders are green where the vegetation is greenish at the peak of the spiders’ life cycle and brown in the more southern parts of the distribution range. Juveniles are coloured differently than adults ( Figs 129–132 View FIGURES 129–132 ). Body and appendages with variable coverage of dark setae (easily rubbed off in preserved specimens).
Species included. Micrommata aljibica , M. biggi spec. nov., M. diesenhoff spec. nov., M. formosa , M. ligurina , M. virescens .
Notes. “ Micrommata ” darlingi from Zimbabwe is not congeneric with M. virescens and, therefore, not considered here.
Systematic relationships. Member of the family Sparassidae without exact systematic position ( Jäger 1998; Moradmand et al. 2014; Tong et al. 2019; Gorneau et al. 2022). Micrommata was always recovered as separate clade from so-called Sparassinae (i.e. Olios and other genera with 2 promarginal cheliceral teeth, no intermarginal denticles), no sister group could be found so far. If Micrommata proves to be in a clade for its own, this has to be called Sparassinae and the other clade containing above mentioned genera would have to be renamed.
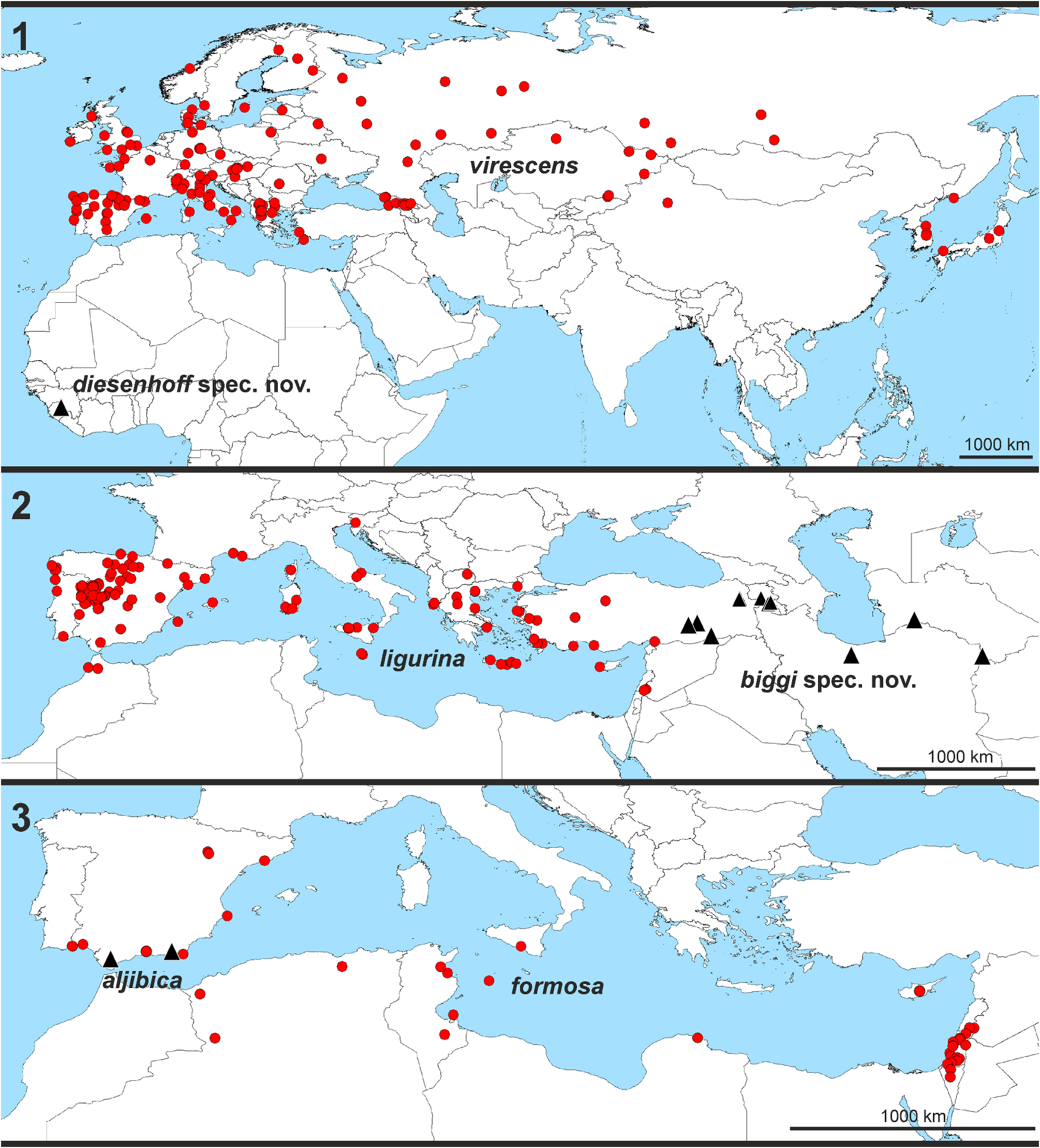
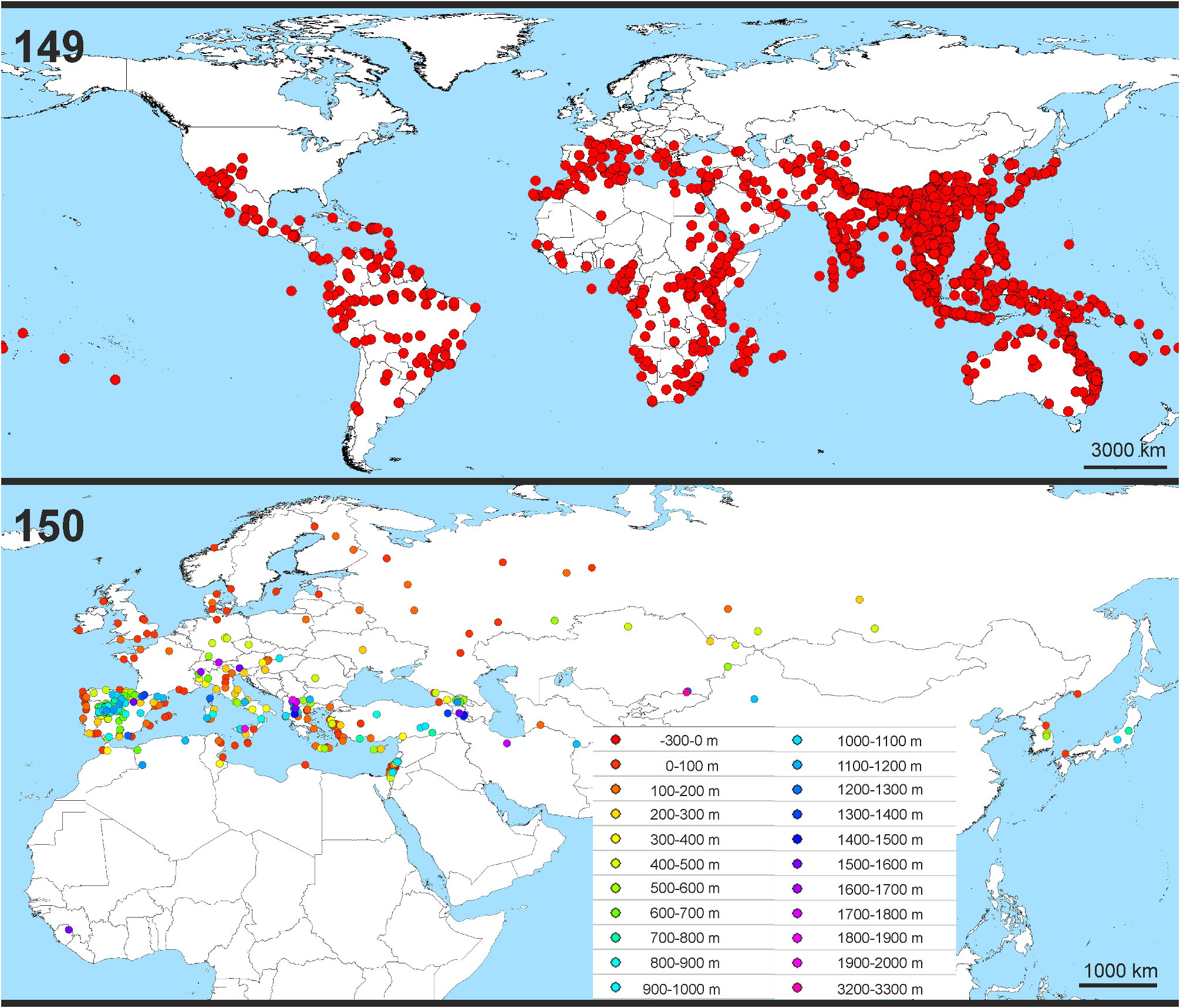
Distribution. Palearctic (without the very northern parts in Europe and Asia), Sierra Leone ( Figs 1–3 View FIGURES 1–3 , 150 View FIGURES 149–150 ).
Identification key to Micrommata species
1 Males.............................................................................................. 2
- Females (those specimens of M. aljibica View in CoL known not fully sclerotised, therefore the respective parts of this key should be treated with caution)......................................................................................... 7
2 Tip of tegular prong retrolaterad in ventral view ( Figs 4 View FIGURES 4–6 , 57, 59 View FIGURES 56–61 )................................................ 3
- Tip of tegular prong distad in ventral view ( Figs 20, 23 View FIGURES 19–24 , 42 View FIGURES 41–47 , 84 View FIGURES 84–89 , 105, 107 View FIGURES 104–109 )......................................... 4
3 Tip of tegular prong long, extending distally beyond alveolus ( Figs 57, 59 View FIGURES 56–61 ); spiders with distinct dark median band on DS and OS ( Figs 73, 75, 78, 81–83 View FIGURES 73–83 )........................................................................ formosa View in CoL
- Tip of tegular prong very short, not extending distally beyond alveolus ( Fig. 4 View FIGURES 4–6 ); spiders spotted, without distinct median band ( Figs 13–18 View FIGURES 13–18 ).................................................................................... aljibica View in CoL
4 Distal margin of tegular prong with incision close to tip in ventral view ( Fig. 84 View FIGURES 84–89 ); fovea with dark elongate patch ( Fig. 102 View FIGURES 102–103 )................................................................................................ ligurina View in CoL
- Distal margin of tegular prong without incision close to tip in ventral view ( Figs 20, 23 View FIGURES 19–24 , 42 View FIGURES 41–47 , 105, 107 View FIGURES 104–109 ); fovea without such patch, in some cases with median band ( Figs 35 View FIGURES 33–38 , 54 View FIGURES 54–55 , 135 View FIGURES 133–138 )........................................................... 5
5 Tip of embolus visible in ventral view, tip of tegular prong roughly as long as the prong’s distal width, tegular loop without or with only few denticles ( Figs 105, 107 View FIGURES 104–109 ).............................................................. virescens View in CoL
- Tip of embolus visible in retrolateral view, tip of tegular prong shorter than the prong’s distal width, tegular loop with several to many distinct denticles ( Figs 20–21, 23–24 View FIGURES 19–24 , 42–43, 44 View FIGURES 41–47 )..................................................... 6
6 RTA long, i.e. apical tip of RTA reaching tegulum in retrolateral view ( Figs 21, 24 View FIGURES 19–24 ).............................. biggi
- RTA short, i.e. apical tip of RTA not reaching tegulum in retrolateral view ( Fig. 43 View FIGURES 41–47 ).......................... diesenhoff
7 Epigynal MS without radial grooves ( Figs 7, 10 View FIGURES 7–12 , 46 View FIGURES 41–47 , 48, 51 View FIGURES 48–53 )................................................... 8
- Epigynal MS with radial grooves ( Figs 25 View FIGURES 25–28 , 29–31 View FIGURES 29–32 , 39 View FIGURES 39–40 , 62, 66 View FIGURES 62–67 , 68, 71 View FIGURES 68–72 , 90, 92 View FIGURES 90–101 )...................................... 9
8 Antero-median ends of epigynal furrows as much separated as lateral lobes at the posterior epigynal margin ( Figs 7, 10 View FIGURES 7–12 ), IDS with posterior part distinctly bulging laterally and a distinct lateral incision between anterior and posterior part ( Figs 8, 11 View FIGURES 7–12 )................................................................................................ aljibica View in CoL
- Antero-median ends of epigynal furrows distinctly closer than lateral lobes at the posterior epigynal margin ( Figs 46 View FIGURES 41–47 , 48, 51 View FIGURES 48–53 ), IDS with anterior and posterior part in one longitudinal line, without lateral bulge or incision ( Figs 47–48, 51 View FIGURES 41–47 View FIGURES 48–53 ).... diesenhoff
9 Antero-dorsal membranous part of IDS broadly roundish ( Figs 64 View FIGURES 62–67 , 70 View FIGURES 68–72 )...................................... formosa View in CoL
- Antero-dorsal membranous part of IDS tapering or at least not widened ( Figs 27 View FIGURES 25–28 , 32 View FIGURES 29–32 , 91, 94 View FIGURES 90–101 , 116, 118–119 View FIGURES 115–120 , 123, 126 View FIGURES 121–128 )..... 10
10 Antero-ventral, narrow part of IDS freely visible in dorsal view ( Figs 116, 118–119 View FIGURES 115–120 , 123, 126 View FIGURES 121–128 ).................. virescens View in CoL
- Antero-ventral, narrow part of IDS covered by dorsal, broad part of IDS in dorsal view ( Figs 27 View FIGURES 25–28 , 32 View FIGURES 29–32 , 91, 94 View FIGURES 90–101 )............ 11
11 Anterior incision of MS V-shaped and with straight and clearly delimited margins, broad dorsal part of copulatory duct with median constriction, anterior ends diverging ( Figs 90, 92 View FIGURES 90–101 ), fovea marked distinctly with dark longitudinal patch ( Fig. 102 View FIGURES 102–103 )... ligurina View in CoL
- Anterior incision of MS indistinctly delimited, broad dorsal part of copulatory duct without median constriction, anterior ends close together ( Figs 25 View FIGURES 25–28 , 29–31 View FIGURES 29–32 ), fovea without such patch, but with narrow band with slight waist in front of fovea ( Figs 35, 38 View FIGURES 33–38 ).............................................................................................. biggi
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Sparassinae |