Capra ibex Linnaeus, 1758
|
publication ID |
https://doi.org/10.1644/830.1 |
|
persistent identifier |
https://treatment.plazi.org/id/03AB87AA-7707-FFAA-D033-B3D4179FFD4A |
|
treatment provided by |
Carolina |
|
scientific name |
Capra ibex Linnaeus, 1758 |
| status |
|
Capra ibex Linnaeus, 1758 View in CoL
Alpine Ibex
[ Capra View in CoL ] Ibex Linnaeus, 1758:68 View in CoL . Type locality ‘‘[I]n Wallesiae praeruptis inaccessis,’’ Alps of Valais, Switzerland.
Capra alpina Girtanner, 1786:224 . Type locality ‘‘Alpes qui s’e´tend du Dauphine´ jusqu’en Styrie [5 Alps that extend from the Dauphine´ to the Styria];’’ substitute for ibex View in CoL .
Aegoceros ibex: Pallas, 1811:224 . Part (see Lydekker 1913:141); name combination.
Ibex alpinus: Gray, 1847:59. Name combination.
Ibex europea Hodgson, 1847:700. Type locality ‘‘Europe.’’
Capra ibex graicus Matschie, 1912:120 . Type locality ‘‘ Valsavaranche ,’’ southwestern Aosta, Graina Alps, Italy.
CONTEXT AND CONTENT. Context as for genus. Capra ibex View in CoL is monotypic.
NOMENCLATURAL NOTES. We used the taxonomy of Grubb (2005), but because all forms in the genus Capra are interfecund, the taxonomy is still debated ( Grubb 2005; Manceau et al. 1999; Schaller 1977; Shackleton 1997). Designation of different species generally is based on differences in morphology, behavior, geographic isolation, and environmental conditions in which the different forms live ( Gauthier et al. 1991). Classifications based solely on morphological characters are the most controversial and are not always supported by allozyme ( Hartl et al. 1990, 1992) or mitochondrial DNA ( Manceau et al. 1999) studies. Capra is Latin for a goat, and ibex is Latin for a kind of goat.
DIAGNOSIS
Capra is distinguished from Ovis by the presence of preorbital, inguinal, and pedal glands; absence of facial glands; a potent body odor; beard in males; and presence of a callus on the knee ( Schaller 1977). Total length of adult male C. ibex is 150 cm, height at withers is 93 cm, and body mass is 67–117 kg, reaching a maximum in November before rut and a minimum in April after rut. It is about the same size as C. hircus aegagrus ( 150 cm, 95 cm, 70–80 kg) compared to 171 cm, 110 cm, and 130 kg for C. sibirica ; 170 cm, 115 cm, and 109 kg for C. falconeri ; and 165 cm, 109 cm, and 96 kg for C. caucasica ( Heptner et al. 1988; Schaller 1977).
Capra ibex ( Fig. 1 View Fig ) has short, broad facial features, the same as C. sibirica , whereas C. nubiana , C. walie , C. falconeri , and C. caucasica have narrow features similar to those of C. hircus ( Schaller 1977) . Horns of C. ibex are scimitar shaped with a relatively flat anterior surface broken by prominent transverse ridges, similar to those of C. caucasica , C. sibirica , C. nubiana , and C. walie . In contrast, horns of C. falconeri are twisted in a spiral, and horns of C. pyrenaica are curved out, up, and then back, inward and then up again, and have a sharp posterior keel. Length of horns of C. ibex is 75–98 cm compared with 100–148 cm in C. sibirica , 55–161 cm in C. falconeri , 110–140 cm in C. hircus , and 48–118 cm in C. caucasica ( Schaller 1977) . All species of Capra have light-colored abdomens, dark dorsal stripes from nape to rump, and beards on the chin; females do not have beards. C. ibex is the least colorful of the Capra species ; it has gray-brown pelage, darker on chin, upper portion of throat, and underparts, and blackish below and along anterior surface above ( Harper 1945). C. ibex does not have prominent markings on its forelegs, in contrast to C. nubiana and C. sibirica ( Schaller 1977) .
GENERAL CHARACTERS
Dimorphism among Capra ibex is striking; sexes mainly differ in body mass and horn dimensions ( Couturier 1962; Giacometti et al. 1997; Michallet et al. 1996; Nievergelt 1966, 1978; Ratti 1981; Ratti and Habermehl 1977). In Graubu¨ nden, Swiss Alps, in October, the gutted weight of adult females was about 50% of that of adult males ( 26– 36 kg, n 5 1,281 versus 59–80 kg, n 5 357). In the same colony, differences in length of body and shoulder height between sexes are about 22% ( 121–141 cm versus 149– 171 cm and 73–84 versus 90–101 cm) and for thoracic circumference, about 30% (76–88 versus 100–114 cm — Giacometti et al. 1997).
Populations of different origins have slightly different body sizes. Males older than 9 years in Gran Paradiso National Park, Italy, have a body length of 160.8 6 5.0 cm (mean 6 SD), shoulder height of 97.6 6 1.9 cm, and thoracic circumference of 107.2 6 6.4 cm compared to 159.1 6 1.6 cm, 95.4 6 0.6 cm, and 106.4 6 1.1 cm, respectively, for males of similar age in Graubünden, Switzerland. Females older than 5 years from Gran Paradiso National Park have a body length of 134.8 6 3.7 cm, shoulder height of 73.2 6 2.4 cm, and thoracic circumference of 80.8 6 2.6 cm compared to 131.9 6 2.4 cm, 78.6 6 0.7 cm, and 82.2 6 2.6 cm in Graubünden ( Giacometti et al. 1997). Males and females weigh more in autumn and reach their minimum mass at the end of winter. Females in Graubu¨ nden reach the maximum mass (dressed weight) in October (29.2 6 2.5 kg, n 5 196), which declines during winter and spring by about 33% to a minimum in May (19.6 6 2.8 kg, n 5 15). Body mass of male C. ibex in Gran Paradiso National Park in 1987–1996 was 99.0 6 5.4 kg ( n 5 27) in September–November but 78.9 6 8.3 kg ( n 5 16) in May–June, equal to a 20.2% loss in body mass during winter ( Giacometti et al. 1997).
Horns of C. ibex are scimitar-shaped, oval-shaped crosssectionally, with well-defined frontal surfaces broken by prominent transverse ridges ( Fig. 2 View Fig ). Horn cores are isosceles triangular with a narrow frontal surface ( Schaller 1977). Maximum length of horns in males is 69–98 cm and in females is 18–35 cm ( Giacometti et al. 1997). Because horns of C. ibex grow through life, age can be determined by counting horn annuli ( Couturier 1962; Nievergelt 1966; Ratti and Habermehl 1977). Horn growth of C. ibex differs between regions. Horn growth varies among Swiss populations because of different nutrition ( Nievergelt 1966). Horn growth and dimensions are influenced by ambient condition such as weather, food availability, and population density ( Giacometti et al. 2002b; Michallet et al. 1994, 1996; Nievergelt 1978). Horn growth in C. ibex from the lower Engadin Valley in the eastern Canton Graubu¨ nden, Switzerland, was significantly greater than in the upper Engadin Valley; horn growth was correlated with favorable ambient temperatures and plant phenology in spring in both regions ( Giacometti et al. 2002b).
Cranial lengths ( Fig. 2 View Fig ) of C. ibex $4 years old are 255– 2 2 288 mm ( X 5 270, n 5 47) for males and 217–246 mm ( X 5 231.6, n 5 29) for females ( Couturier 1962). Maximum 2
width of the skull is 120–159 mm ( X 5 141, n 5 76) for 2
males and 116–131 mm ( X 5 124, n 5 35) for females. 2
Mandible length is 73–103 mm ( X 5 89.8, n 5 83) for males 2
and 68–79 mm ( X 5 74, n 5 35) for females ( Couturier 1962).
DISTRIBUTION
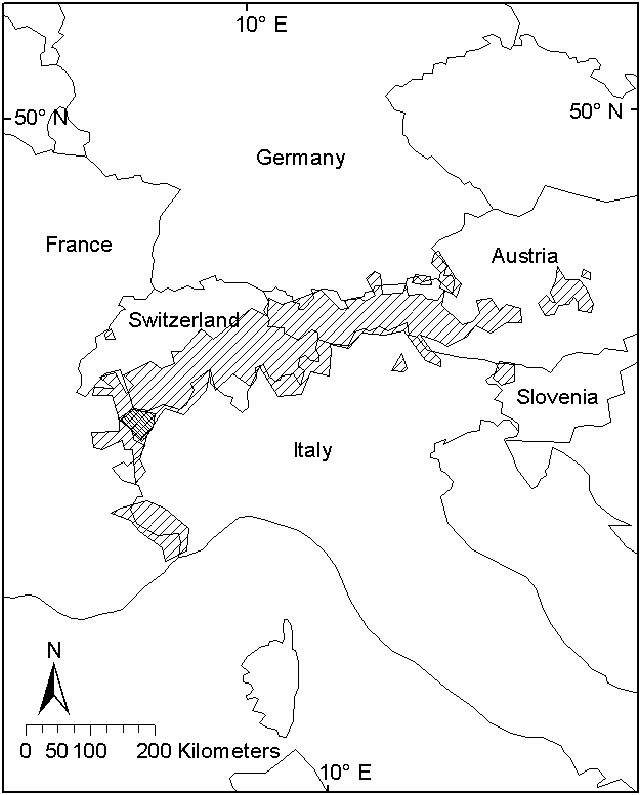
Capra ibex previously occupied the Alpine Range in central Europe but was reduced in numbers and distribution to only Gran Paradiso National Park in the northwestern Italian Alps. C. ibex has now been reestablished in the Alps of Austria, France, Germany, Italy, Slovenia, and Switzerland at elevations of 1,600–3,200 m ( Fig. 3 View Fig ) .
period (Wu¨ rm— Koby 1958). C. ibex is more common in Pleistocene deposits than in Mesolithic and Neolithic deposits (Chaix and Desse 1994). Pleistocene fossils have been found at elevations of 1,500 m in the Alps to, 300 m near the Mediterranean coast in France and northern Italy in steep, rocky habitats ( Boule 1906; Chaix and Desse 1994). Fossil specimens show that the present-day C. ibex is smaller than pre-Holocene forms; extant males have similar dimensions to fossil females of the Wu¨ rm period ( Koby 1958). Metacarpals of fossil specimens of the Würm period measured 146.7 6 9.8 mm (mean 6 SD; n 5 185) compared to 134.8 6 4.7 mm ( n 5 26) for males and 126.3 6 4.1 mm ( n 5 15) for females from an extant French population (Chaix and Desse 1994).
FOSSIL RECORD
Capra and Ovis represent the extreme development of species that once resembled Himalayan goral ( Naemorhedus goral — Schaller 1977). The Oiceros of the Miocene and early Pliocene found in China, Kenya, and Slovenia resembled the goral in size and horn shape ( Gentry 1978; McKenna and Bell 1997; Pilgrim 1947). By the late Miocene, the genus Tossunnoria , with features intermediate between those of goral and goats, appeared in China ( Pilgrim 1947; Wang et al. 2007). The genus is known from late Pliocene deposits in northern Africa (McKenna and Bell 1997). Fossils of Capra camburgensis date to 1.2 million years ago at the end of the Riss glacial period in Germany ( Toepfer 1934). This caprid shared characteristics with C. ibex and C. pyrenaica , which probably evolved from this common ancestor ( Manceau et al. 1999).
Few paleontological data are available for Capra , because their preferred mountainous habitats are not favorable to fossil preservation ( Simpson 1945). C. ibex from the Alps and C. pyrenaica from the Pyrenees are found more regularly in deposits from the start of the last glacial
FORM AND FUNCTION
Pelage length of Capra ibex varies seasonally from short during summer to a longer wooly pelage in winter ( Novak 1999). It is shed once a year in the spring, leaving mainly coarse and sparse outer hair. Summer pelage is thin, made up of short hair without an undercoat. Autumn molt begins at the end of September when a thick undercoat begins to grow. Winter pelage is maintained until April–May, when spring molt starts. The thick wooly undercoat falls out in large tufts, and C. ibex rubs against rocks, trees, and bushes to remove the patches of hair (Bassano 1992).
Dental formula is i 0/3, c 0/1, p 3/3, m 3/3, total 32 ( Schaller 1977). Horns of both male and female C. ibex grow through life and because horn growth stops in winter each year’s growth is defined by a border on the horn ( Nievergelt 1966; Ratti and Habermehl 1977). Horns grow an average of 80 mm /year until 5.5 years in males and then a linear decline occurs as age increases. In males.10 years old, horns grow, 40 mm /year, similar to the decline of horn growth of C. sibirica and C. pyrenaica ( Fandos 1995; Schaller 1977). In contrast to other species of Capra , the highest rate of horn growth in C. ibex occurs in the 2nd year of life ( Giacometti et al. 1997). C. ibex has a callus on the carpal joint of front legs to climb steep inclines ( Schaller 1977).
ONTOGENY AND REPRODUCTION
Ontogeny.— Sexual maturity is reached at 1.5 years in both sexes ( Couturier 1962). Age of primiparity in females can be delayed depending on the population density ( Gaillard et al. 2000). In high-density populations, females give birth at 3–4 years of age, but in newly established populations, the age of the 1st parturition is 2 years ( Loison et al. 2002; Martinot et al. 1983; Michallet et al. 1994). In a recently established population in the Belledonne-Sept-Laux Reserve (Ise`re, France), 43% of females were breeding at 2 years of age and 87% of females $3 years of age were breeding every year ( Loison et al. 2002).
Depending on location, males reach full body size at 8.5–10.5 years of age and females at 4.5–5.5 years of age (Bru¨ llhardt and Lu¨ ps 1984; Giacometti et al. 1997; Ratti 1981; Zu¨ mbach and Lu¨ ps 1987), but body mass and thoracic circumference of males increase slowly until 11.5 years of age. Body mass increases by 9%/year until 8.5 years of age and slows to 1.2%/per year afterward. After the age of 11.5 years, males start to lose mass. Thoracic circumference increases at 5.8%/year until 10.5 years of age and slows to 0.8%/year until the maximum is reached at 11.5 years of age ( Giacometti et al. 1997). Females reach adult dimensions at 4.5–5.5 years of age. Both body mass and shoulder height reach their maximum in this age range, but body length and thoracic circumference increase until 9.5–10.5 years of age. Body length increases at 7.3%/year until 4.5 years and slows to 0.8%/year until it reaches a maximum at 10.5 years of age. Similarly, thoracic circumference slows to a 0.3%/year increase after 4.5 years of age until the maximum values are reached ( Giacometti et al. 1997). Body mass in females only starts to decrease at 18.5 years of age ( Giacometti et al. 1997).
Reproduction.— In captivity, female Capra ibex are seasonally polyestrous, with about 20 days between cycles and 1 month apart (Stu¨ we and Grodinsky 1987). They reproduce from 1 to 15 years of age and give birth to an average of 0.78 offspring/year. Twins are born about 20% of the time, and the sex ratio at birth is unbiased (Stüwe and Grodinsky 1987). Gestation is 167 days 6 3 SD in captivity and in the wild ( Couturier 1962). In the wild, the birth rate is lower than in captivity and appears to be negatively related to population density. In Gran Paradiso National Park, the mean birth rate index, based on 20 years of censuses, is 0.42 6 0.1 (mean 6 SD; range: 0.2–0.5). In Gran Paradiso National Park, lowest birth rates are found in years with heavy winter snowfall (B. Bassano, in litt.). The 6-week breeding season starts in December but length can vary geographically depending on weather ( Couturier 1962).
ECOLOGY
Population characteristics.— Predation is negligible in populations of Capra ibex ; for example, most individuals die of old age, starvation, or disease in Gran Paradiso National Park ( Bassano et al. 1992). Population sizes of C. ibex in Gran Paradiso National Park are negatively affected by density, snow depth, and interactions between the 2 ( Jacobson et al. 2004). Avalanches cause direct mortality of all age classes ( Nievergelt 1966). Snow depth has a relatively stronger effect on the survival of.10- to 12-yearold C. ibex ( Toïgo et al. 2007) .
Survival rate is high and constant for both sexes and over time in the Alps close to Grenoble, France (0.97 6 0.011 SD — Toïgo et al. 1997) and high and constant for all ages in females in Ise`re, France (0.98 6 0.013— Loison et al. 2002). Survival of C. ibex in Belledonne-Sept-Laux-Reserve, France, is affected by age ( Toïgo et al. 2007). All yearlings of both sexes survived to 2 years of age. Survival of prime-aged (2–8 years) females was 0.99 6 0.01 but declined with increasing age thereafter (0.89 6 0.04). Male survival was similar to that of females (0.98 6 0.01) between 2 and 8 years of age and also decreased later in life under low-quality forage conditions (0.85 6 0.03) but remained high under high-quality forage conditions (0.97 6 0.03). Males of C. ibex in captivity live to 22.3 years ( Jones 1982) and up to 16 years in the wild ( Grzimek 1990; Toïgo et al. 2007), and females live up to 19 years in the wild ( Toïgo et al. 2007). Growing long horns does not constrain longevity ( Bergeron et al. 2008).
Space use.— Capra ibex is sexually segregated ( Grignolio et al. 2004, 2007; Pedrotti 1995; Villaret et al. 1997). Adult males and females use different habitats for most of the year ( Grignolio et al. 2003; Peracino et al. 1989). Females are more dependent on steep terrain throughout the year than are males ( Francisci et al. 1985; Grignolio et al. 2004). Males move more and select different habitats depending on the time of the year and their physiological needs ( Francisci et al. 1985; Grignolio et al. 2004). In spring, males move to low-elevation meadows where the snow melts 1st and grass is green ( Grignolio et al. 2003; Nievergelt 1966; Pedrotti 1995; Peracino et al. 1989) and during summer, they move to highelevation alpine meadows ( Grignolio et al. 2003; Mascellani 1997). During winter, both sexes actively select rocky steep slopes with minimal snow accumulation ( Grignolio et al. 2003, 2004, 2007; Nievergelt 1966; Pedrotti 1995; Schaller 1977; Tosi et al. 1986). Habitat selection by C. ibex is influenced by the gradient and extension of slopes. Slopes of 30–45 u are used, particularly during winter. Typically, slopes are interrupted by small caves and overhangs that provide shelter ( Couturier 1962; Hoffmann and Nievergelt 1972; Nievergelt 1966; Pedrotti 1995; Tosi et al. 1986; von Elsner Schack 1982; Wiersema 1983, 1984).
In winter, C. ibex occurs at elevations of 1,800 –3,000 m ( Couturier 1962; Parrini et al. 2003; Wiersema 1983). Observed differences in use of various elevations between localities are mostly due to differences in availability of preferred habitats by elevation. In Gran Paradiso National Park, C. ibex moves to elevations of 2,100 –2,900 m in spring and 2,300 –3,300 m in summer ( Parrini et al. 2003). In highdensity populations, individuals occur over a wide range of elevations in summer and can be found as low as 2,000 m (B. Bassano and V. Peracino, in litt.). Recently translocated populations can live at elevations as low as 1,500 m ( Choisy 1994; Raye 1994; Terrier et al. 1994; von Elsner Shack 1982), if steep cliffs are available ( Choisy 1994). In Belledonne- Sept-Laux in the French Alps, C. ibex uses relatively low elevations in spring ( 1,748 m 6 250 SD in April) and the highest elevations in summer (2,358 6 183 m in July– August—M. Lembke et al., in litt.).
Home-range sizes of C. ibex are larger in translocated populations than in the surviving original population of Gran Paradiso National Park, Italy, where space use is highly traditional ( Parrini et al. 2003). Home ranges are 737 ha 6 116 SD for males in Gran Paradiso National Park ( Parrini et al. 2003) but may vary from 2,200 to 2,800 ha in translocated populations in the Italian and French Alps ( Michallet et al. 1994; Pedrotti 1995; Terrier et al. 1994; Tron et al. 1994). Despite variation in annual home-range sizes, patterns of seasonal range use are similar in the native and translocated populations. Home ranges are larger in summer and autumn, smaller in spring, and smallest in winter ( Parrini et al. 2003; Terrier et al. 1994). In Gran Paradiso National Park, seasonal ranges of 12 radiocollared males were 300–500 ha in summer, 250–400 ha in autumn, 100–200 ha in spring, and 50–180 ha in winter. Females use smaller home ranges than do males, and their space use is more restricted in the native population in Italy compared with reintroduced populations ( Grignolio et al. 2004; Michallet et al. 1994; Pedrotti 1995; Terrier et al. 1994; Terrier and Rossi 1994; Tron et al. 1994). Mean annual home-range size of 14 radiocollared females in Gran Paradiso National Park was 186.2 ha 6 71.7 SD; mean seasonal ranges were 20.2 6 11.1 ha in winter and 135.7 6 42.1 ha in summer, with autumn and spring ranges in between ( Grignolio et al. 2004).
Capra ibex usually avoids large woodland areas (Ba¨chler 1935; Baumann 1949; Nievergelt 1966; Pedrotti 1995; Rauch 1937), but adult males in high-density populations can be found in larch and mixed larch–spruce woodlands when snow is scarce ( Bassano et al. 1992; Grignolio et al. 2003). In Albris-Swiss National Park, which has a high-density population, C. ibex uses forests for most of the year (Buchli and Abderhalden 1999). In Hochlantschstock, about 30 km from Graz, Austria, C. ibex uses coniferous and broadleaf forests for the entire winter ( Kofler 1981).
Areas with precipitation. 1,500 mm /year appear to be unfavorable for establishment of C. ibex populations; optimal precipitation is 100–1,000 mm /year (von Elsner Schack 1982). Snow depth limits C. ibex ( Schaller 1977) ; areas with heavy snow cover are avoided ( Couturier 1962; Hoffmann and Nievergelt 1972; Nievergelt 1966) by selecting the rockiest and steepest areas in winter ( Peracino et al. 1989; Toïgo et al. 1995), where food resources are available but often in limited quantity and quality ( Toïgo et al. 1995). Males and females tend to move little when snow is deep and use smaller home ranges in years with heavy snowfall compared with years free of snow ( Grignolio et al. 2004; Parrini et al. 2003).
Diet.— Capra ibex is mainly a grazer, with 82–94% of its diet being herbaceous ( Tataruch et al. 1991). Graminaceae make up 60% of the diet, dicotyledons 38%, and woody species 2% ( Houte De Lange 1978). Favored grass genera include Agrostis , Avena , Calamagrostis , Festuca , Phleum , Poa , Sesleria , and Trisetum . Other families of plants eaten by C. ibex are Cyperaceae ( Carex , Schoenus , and Kobresia ), Papillonaceae, Ranunculaceae ( Pulsatilla alpina , Thalictrum foetidum , and Ranunculus glacialis ), Poligonaceae ( Oxiria digyna, Rumex scutatus , and Polygonum viviparum ), Leguminosae ( Trifolium , Anthyllis , Lotus , Astragalus , and Oxitropis ), Umbelliferae, and Compositae ( Achillea , Aster , Erigeron , Tanacetum , Carduus , Carlina , and Cirisium). In winter and spring, C. ibex also feeds on branches and leaves of shrubs such as Alnus viridis , Corylus avellana , Juniperus communis , Rhododendrum ferrugineum , and Salix erbacea ; mosses and lichens; and needles and bark of young Abies alba , Larix decidua , Picea excelsa , Pinus mugo , and P. cembra ( Couturier 1962; Peracino 1995a, 1995b).
Diseases and parasites.— Capra ibex is subject to a variety of zoonotic infections caused by agents such as Parapoxivirus ovis , Pasteurella , Mycoplasma conjunctivae , Dichelobacter nodosus , Brucella abortus , B. melitensis , and Sarcoptes scabei var. caprae , mostly transmitted from domestic ungulates. Lethal diseases include contagious ecthyma (sore mouth) caused by a poxvirus; pasteurellosis, a respiratory disease caused by the bacteria Pasteurella ; foot rot (infectious pododermatitis), hoof infections caused by the anaerobic bacteria Fusobacterium necrophorum and Bacteroides melaninogenicus ; and sarcoptic mange ( Lanfranchi et al. 1991; Montagut et al. 1981; Rossi et al. 1988). Infectious keratoconjunctivitis from M. conjunctivae is a highly contagious ocular infection characterized by inflammation of the conjunctiva and cornea that leads to opacity and perforation of the cornea; mortality following infectious keratoconjunctivitis outbreaks occasionally can reach 30% ( Giacometti et al. 2002a). Contagious brucellosis caused by B. melitensis is usually sporadic in wild ruminants in Europe, but it has been diagnosed in C. ibex from Gran Paradiso National Park ( Ferroglio et al. 1998).
BEHAVIOR
Grouping behavior.— Capra ibex is gregarious, but sexual and spatial segregation occurs seasonally between sexes ( Couturier 1962; Francisci et al. 1985; Gauthier et al. 1992; Grignolio et al. 2007; Rucksthu¨ l and Neuhaus 2001). Four types of groups can be distinguished: adult males, young individuals (2–3 years old), females with kids and yearlings, and mixed groups ( Toïgo et al. 1995; Villaret and Bon 1995). Groups of 2- to 3-year-olds are particularly common at the beginning of summer; these individuals are displaced by mothers about to give birth. In contrast, yearlings were observed with females throughout the year in the Bargy Massif in the northern French Alps (Villaret and Bon 1995). Groups of females with kids can be observed year-round but are more frequent during summer ( Gauthier et al. 1992; Mascellani 1997; Peracino et al. 1989; Toïgo et al. 1995; Villaret and Bon 1995). Adult males and females only aggregate during rut (December–January). After April– May, sexes segregate completely and mostly same-sex herds are observed ( Gauthier et al. 1992; Pedrotti 1995; Peracino et al. 1989; Toïgo et al. 1995).
In the Bargy Massif in the northern French Alps, males.9 years old and females of all ages formed separate groups outside rut, whereas younger males segregated from females gradually over several years (Villaret and Bon 1995). Males.4 years old and females were rarely observed together during summer, but 30% of males 2–3 years of age remained in female groups (Villaret and Bon 1995).
For both sexes, largest groups are formed in late spring– summer (June–July— Gauthier et al. 1992; Pedrotti 1995; Peracino et al. 1989). Aggregation in males decreases in autumn (October–November) and reaches the lowest level during rut to early spring (December–March— Gauthier et al. 1992; Pedrotti 1995; Peracino et al. 1989) when males aggregate again from their separate wintering areas ( Gauthier et al. 1992; Parrini et al. 2003). Sexual segregation is influenced by population density; in low-density, recently translocated populations, mixed-sex groups are more common than in established high-density populations ( Couturier 1962).
Male C. ibex have a diverse behavioral repertoire of dominance and subordination displays similar to other species of Capra ( Aeschbacher 1978; Schaller 1977), characterized by high frequency of direct ritualized agonistic interactions and a lack of fighting among.2 individuals ( Gauthier et al. 1992). The social system of males of C. ibex is based on absolute rank order, where individuals follow a linear hierarchy based on memory of past encounters in cohesive social units in small populations ( Nievergelt 1966) and on horn length in mobile and large populations where encounters are commonly between strangers ( Schaller 1977).
Male C. ibex display 2 types of agonistic behavior, common to all Capra : direct and indirect aggression. Direct aggression occurs when 1 individual makes body contact with another in the form of a butt with the blunt parts of the horns or tips, or by jumping on its hind legs in front of an opponent and coming down with a downward thrust of the horns ( Fig. 4 View Fig ). This can end by showing an intention to clash or with a real clash. The clash is the most conspicuous behavior among male Capra . It requires synchronization of both individuals and is most common in C. ibex between individuals in the same age class ( Couturier 1962; Nievergelt 1966). All forms of aggressive behavior mainly occur outside rut ( Nievergelt 1966). Indirect aggression behaviors include lateral displays such as an animal holding its head up to enhance its appearance or walking parallel with horns tipped toward each other. Two C. ibex run shoulder to shoulder if they are of equal rank, but the subordinate takes the lead if they are not ( Walther 1961).
Reproductive behavior.— Aeschbacher (1978) described the ethogram of courtship displays of Capra ibex in captivity in Wildpark Langenberg (Zu¨ rich, Switzerland) and in the wild in Val Trupchun (Oberengadin, Switzerland). The most common displays of males are low stretch, twist, and front kick.
The 1st signs of rut are changes in the composition of male herds, which separate into smaller groups and move to areas occupied by females ( Peracino et al. 1989). During rut, males tend to associate with each other regardless of age (Villaret and Bon 1995). Rut is divided in 2 phases ( Aeschbacher 1978). The 1st phase is the collective heat phase when males interact with females in small groups: males approach females in a low stretch and stand at a distance proportional to their rank order ( Fig. 5 View Fig ). Males then alternate low stretch with normal standing and other courtship displays ( Aeschbacher 1978). The 2nd phase of rut is the individual heat phase. When a female comes in estrus, a dominant male detaches himself from the other males and gets close to the female; he generally stays in a low stretch by the female, while threatening any approaching males with his horns. Just before copulation, the female starts to move her tail, and the displays of the tending male become more intense. After copulation, the male joins other males in the collective heat phase again ( Aeschbacher 1978). Young and low-ranked male C. ibex use dynamic courtship tactics that end in running mounts ( Aeschbacher 1978).
Snow influences courtship behavior. When snow depth is high in Gran Paradiso National Park, Italy, the number of male courtship events is reduced, dynamic courtships are avoided, and intensity of courtship (number of low stretches per courtship time) decreases compared with rut in years with scarce or no snow depth (I. Rossi et al., in litt.).
Miscellaneous behavior.— In the Belledonne-Sept-Laux Reserve, France, both sexes and 1 year olds spent 39–44% of the time foraging in spring ( Toïgo 1999). Lactating females rested less than the other segments of the populations (32% versus 47–56% of the time— Toïgo 1999). In June–July, adult males spent only 6–11% of daytime feeding compared to 26– 33% of adult females, 20–33% of yearling males, and 34–47% population that survived extinction and subsequent translocations (Stüwe and Scribner 1989). All living C. ibex originated from an unspecified low number of individuals (may have been,100 and unlikely.200–300) that survived at the end of the 19th century in Gran Paradiso National Park, Italy ( Girtanner 1878; Passerin d’Entre¨ves 2000). All Swiss populations originated from about 80 individuals captured in Gran Paradiso National Park and transferred to wildlife parks in St. Gallen and Interlaken ( Maudet et al. 2002; Randi et al. 1990; Stu¨ we and Scribner 1989).
The important bottlenecks explain the low genetic variability of the extant populations of C. ibex . Stüwe and Scribner (1989) studied heterozygosity, allelic frequency, and population differentiation in 57 individuals from 9 Swiss populations. Average heterozygosity of C. ibex from the Swiss populations was lower (Ĥ 5 0.007) than values reported for 10 other species of Artiodactyla (Ĥ 5 0.011 – 0.053 — Baccus et al. 1983), and genetic similarity among populations was high ( S 5 0.992). The monophyly of C. ibex is confirmed by studies on the Y-chromosome sequence ( Pidancier et al. 2006) and by allozyme studies ( Hartl et al. 1992).
of yearling females; males spent more time lying (58–61%), standing (19–33%), and walking (1.0–1.9%) than females (lying 55–61%, standing 9–14%, and walking 0.4–0.9%— Neuhaus and Rucksthül 2002). In the Gran Paradiso National Park, from May to September, adult male ibex spent on average 51% of daytime feeding, 38% lying, 5% standing, 2% walking, and 4% in other activities (Aulet et al. 2009). Both temperature and solar radiation had a negative effect on time spent foraging at midday and in the evening (Aublet et al. 2009).
GENETICS
Capra ibex has a diploid number (2n) of 60 chromosomes (Schmitt and Ulbrich 1968). The genetic history of C. ibex passed through a series of population bottlenecks, linked to the severe population decline of the only
CONSERVATION
Capra ibex formerly ranged through France, Italy, Switzerland, Lichtenstein, Bavaria, Austria, and Slovenia, but was gradually exterminated in all but 1 locality in Italy. From the beginning of the 1500s, overexploitation and poaching led to a steady decline in numbers of C. ibex in the European Alps. In Switzerland, Germany, and France all populations of C. ibex were extinct in the 18th century ( Couturier 1962; Giacometti 1991). By the 19th century, C. ibex disappeared from Austria and the northeastern Italian Alps and only survived in the area of the Gran Paradiso Massif in the western Italian Alps ( Couturier 1962).
Gran Paradiso in Italy was established by Vittorio Emanuele II as a royal hunting reserve in 1854. Because of protection from poaching, the population increased to about 3,020 by 1914. From this stock, populations were established 1st in Switzerland and in 1922, Gran Paradiso was established as a National Park to protect C. ibex (Passerin d’Entre¨ves 2000). Between 1906 and 1942, #52 C. ibex from the Italian population arrived at Peter and Paul Wildlife Park in St. Gallen and 47 at Wildlife Park Harder in Interlaken to start the 1st successful captive-breeding programs (Stu¨ we and Nievergelt 1991). Between 1911 and 1948, 10 populations were established in Switzerland from these captive stocks. Individual C. ibex from these populations were released in other parts of the European Alps, such as France ( Ratti 1981). The individuals released as founders of new populations in the Italian Alps were captured in the original Gran Paradiso population to reduce the negative impact of successive genetic bottlenecks (Stu¨ we and Niever- gelt 1991). By 1976, translocations increased the number of extant populations of C. ibex in the European Alps to at least 104 ( Graf 1977).
Since then, ranges of many populations of C. ibex have expanded naturally and begun to overlap. By 2008, C. ibex occurred in the French, Italian, Swiss and Austrian Alps, Lichtenstein, Bavaria, and Slovenia. The number of C. ibex in the European Alps is.20,000 individuals (Stüwe and Nievergelt 1991); it is not listed as an endangered species (International Union for Conservation of Nature 2008) or protected under the Convention on International Trade in Endangered Species of Wild Fauna and Flora (2008). C. ibex can be hunted in Switzerland, Austria, Lichtenstein, and Slovenia ( Hoffmann 1991).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Capra ibex Linnaeus, 1758
| Parrini, Francesca, Cain, James W. & Krausman, Paul R. 2009 |
Capra ibex graicus
| MATSCHIE, V. P 1912: 120 |
Aegoceros ibex :
| LYDEKKER, F 1913: 141 |
| PALLAS, P 1811: 224 |
Capra alpina
| GIRTANNER, C 1786: 224 |
Capra
| LINNAEUS, C 1758: 68 |