Eugnathogobius kabilia ( Herre, 1940 )
|
publication ID |
https://doi.org/10.5281/zenodo.5341767 |
|
publication LSID |
lsid:zoobank.org:pub:D08EA231-8304-49FD-A5F6-CFA37323950F |
|
persistent identifier |
https://treatment.plazi.org/id/03A487B1-FFB5-FF31-FC66-FD161484FB4C |
|
treatment provided by |
Diego |
|
scientific name |
Eugnathogobius kabilia ( Herre, 1940 ) |
| status |
|
Eugnathogobius kabilia ( Herre, 1940) View in CoL
( Figs. 5–10 View Fig View Fig View Fig View Fig View Fig View Fig ; Plate 1A; Tables 3–6, 8)
? Glossogobius mas Hora, 1923: 742–743 View in CoL , Fig. 23 View Fig (Chilka Lake: off Samal Island, Rambha Bay, off Barkul).
Vaimosa kabilia Herre, 1940: 19 View in CoL , Pl. 14 (Kabili River, north Borneo). – Koumans 1953: 388.
Calamiana magnoris Herre, 1945: 80 View in CoL (Calamianes, the Philippines). – Herre 1953: 732; Roberts 1989: 168.
Gnathogobius aliceae Smith, 1945: 523–524 View in CoL , Fig. 104 ( Bangkok). – Suvatti 1981: 203; Kottelat 1989: 19.
Vaimosa rambaiae View in CoL (in part) Smith, 1945: 538–540 ( Bangkok).
Calamiana kabilia View in CoL – Roberts, 1989: 168; Kottelat et al. 1993: 146; Larson 2001: 59–60.
Mugilogobius kabilia View in CoL – Kottelat et al. 1993: 146.
Calamiana aliceae View in CoL – Watson & Horsthemke 1995: 91–92.
Material examined. – MALAYSIA: Holotype of Vaimosa kabilia, CAS 32978, 1(36.5), Kabili River, British North Borneo, Sabah, A. W. Herre, 1936 – 1937 Oriental Expedition, Jan.1937. FMNH 51667, 1(15), East Coast Residency, Sungai Gana, tributary of Little Kretam River, just above Nypa belt, Kinabatangan, North Borneo, Sabah, R. F. Inger, 12 May 1950; NTM S.14302-001, 5(20.5–28.0), Kampong Pangkang Kuap, Sarawak, B. L. Lim, 1 Jan.1969. THAILAND: Holotype of Gnathogobius aliceae, USNM 119604, 1(38), central canal, Bangkok, Thailand, H. M. Smith, 2 May 1931. Paratype of Gnathogobius aliceae, USNM 119605, 1(31), same data as preceding. Paratypes of Vaimosa rambaiae , ex USNM 119647, 2 (27–29), from shallow slough behind Department of Fisheries, Bangkok, N. Pongse on 28 May 1931, preserved 2 Dec.1931. CMK 4789, 2(45–47), aquarium specimens exported by K. Derwanz, 1985; NIFI uncatalogued, 6(26–34), Bangpakong River, Nongnuch. THE PHILIPPINES: Holotype of Calamiana magnoris, CAS 39881, 1(31), Busuanga, Palawan Province, the Philippines, A. W. Herre, 1 Jul.1940. SRI LANKA: SMF 30305, 3(26–33), freshwater, A. Heymer, Dec.1987. NO DEFINITE LOCALITY: AMS I.35823-001, 1(36), Singapore: probable aquarium specimen, I. Benoit, Sorbonne.
Diagnosis. – A relatively large Eugnathogobius with second dorsal rays I,6–8; anal rays I,5–8; pectoral rays 15–18; 15–17 segmented caudal rays (modally 16); longitudinal scales 25–34; TRB 8–12; predorsal scales 10–19; shoulder girdle with smooth low ridge or flange, rarely with fleshy lobes; mouth terminal, large, greatly enlarged in mature males (maxillary sometimes extending past rear edge of preoperculum); each scale on side of body with short dusky bar, two distinct brown stripes extending back from rear of eye and two from eye to snout tip, fins dark, dorsal fins usually distinctly spotted with brown; known from estuarine to fresh waters of Sri Lanka, Thailand, Malaysian Borneo and the Philippines.
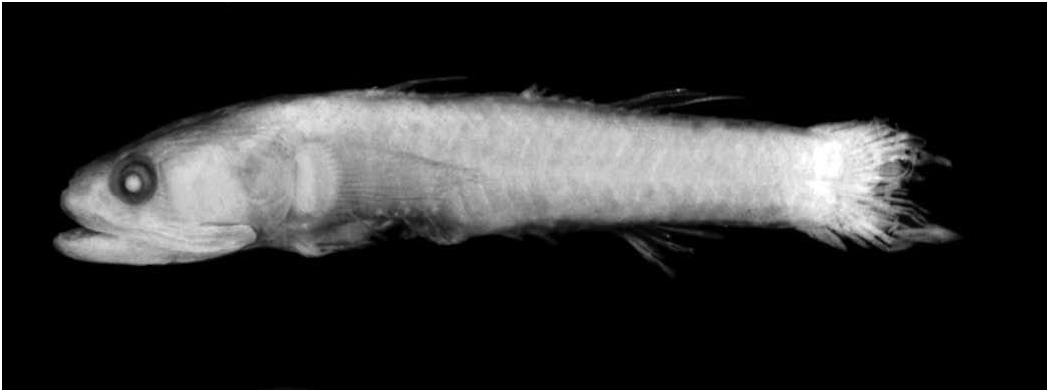
Description. – Based on 23 specimens, 15–47 mm SL. An asterisk indicates counts of holotype of Vaimosa kabilia ( Fig. 5 View Fig ).
First dorsal VI * (in 21), VII (in one); second dorsal I,6–8 (mean I,7*); anal I,5–8 (mean I,7*); pectoral rays 15–18* (mean 17); segmented caudal rays 15–17 (mean 16*); caudal ray pattern 9/6 to 10/7 (modally 9/7*); branched caudal rays 13–15 (modally 15*); unsegmented (procurrent) caudal rays 7/7 to 7/8 (modally 7/7); longitudinal scale count 25–34 (mean 30, 27 in holotype); TRB 8–12 (mean 10, 9 in holotype); predorsal scales 10–19 (mean 14, 11 in holotype); circumpeduncular scales 12*. Gill rakers on outer face of first arch 1+6 to 3+8 (no mode, mean of seven total rakers). Pterygiophore formula 3-12210* (in 11), 3-2311 (in one). Vertebrae 10+16* (in nine); 10+15 (in four). Neural spines of first few vertebra relatively straight, pointed (in five); tips of first three spines somewhat expanded at tips (in four). Two epurals (in eight)*, one in one specimen. Two (in 12*) or three (in one) anal pterygiophores before haemal spine of first caudal vertebra. Anterior tip of preoperculum blunt in males, pointed in females. Metapterygoid broad, forming bridge to quadrate; greatly expanded dorsally and overlapping quadrate in adult males (see Larson 2001: Fig. 61) but separate in females ( Fig. 6 View Fig ) .
Body relatively slender, somewhat cylindrical anteriorly, compressed posteriorly. Body depth at anal fin origin 16.4–21.5% (mean 18.9%) of SL. Head depressed anteriorly, especially in males, always wider than deep, HL 28.1–33.8% (mean 30.8%) of SL. Depth at posterior preopercular margin 44.2–67.1% (mean 57.5%) of HL. Width at posterior preopercular margin 63.2–81.0% (mean 73.6%) of HL, preopercular area tending to be inflated and muscular in males. Mouth large, terminal, slightly oblique, forming angle of about 35–40° with body axis; jaws reaching well past eye to preopercular edge in males and to below mid-eye in females (jaw in large males often reaching past preopercular margin). Lips fleshy, smooth and without fimbriae; lower lip mostly free, fused near tip of jaw. Upper jaw 31.8–86.6% (mean 39.6% in females, 73.9% in males) of HL. Eyes placed dorsolaterally, protruding above dorsal profile in large specimens, eye width 19.4–31.8% (mean 23.9%) of HL. Snout broad, blunt and flattened, 23.2–33.6% (mean 29.1%) of HL; one large male with snout almost square, another with snout almost rectangular (dorsal view). Interorbital broad, flat, 17.9–39.5% (mean 26.6%) of HL; wider in males than females. Caudal peduncle compressed, length 24.2–30.4% (mean 27.1%) of SL. Caudal peduncle depth 11.2–14.4% (mean 12.9%) of SL.
First dorsal fin rounded, rather low, usually third (or second) spine longest; fin not always reaching second dorsal fin origin when depressed, otherwise reaching back to base of first element of second dorsal fin. First dorsal spine always shorter than next two. Second dorsal spine sometimes longest (in males only), spine length 11.1–13.9% (mean 12.7%) of SL. Third dorsal spine length 12.5–15.9% (mean 14.1%) of SL. Second dorsal fin may be taller than first dorsal, especially anteriorly; posteriormost rays barely longer than anteriormost; fin not reaching caudal base when depressed. Anal fin low, posteriormost rays longest, rays falling short of caudal fin base when depressed. Pectoral fin oval, central rays longest, 18.1–26.8% (mean 23.2%) of SL; all rays usually branched. Pelvic fins oval, rays not reaching anus, 14.5–22.2% (mean 19.2%) of SL. Caudal fin moderate in size, oval, rounded posteriorly, 24.5–33.3% (mean 29.6%) of SL.
Chin smooth, without mental fraenum. Anterior nostril in short tube, oriented down and forward over upper lip, preorbital slightly curved to accommodate nostril. Posterior nostril rounded, sometimes with slightly raised rim, placed close to front of eye. Gill opening extending forward to under opercle. Inner edge of pectoral girdle smooth with no ridge or flange (in nine), with low fleshy flange (in eight), or knobs and flaps (in two, including holotype). Gill rakers on outer face of first and second arch short and unspined, rakers longer and more slender near angle of arch; rakers on inner face of first and second arch stubby and with fine spines at tip; inner and outer rakers on other two arches stubby, spiny at tip and equal in size to the first arch inner rakers. Tongue large, broad, concave to rounded at tip. Outer teeth in upper jaw largest, relatively small but sharp and curved, two to three rows of very small sharp teeth behind this row; innermost row of few inward-pointing large teeth may be present. In males, teeth present on anterior half or two-thirds of upper jaw; in females, teeth present either on all or anterior three-quarters of jaw. Lower jaw with teeth mostly across front, with outer row of small pointed curved teeth and two or three inner rows of slightly smaller sharp teeth (inner and outer rows not differing much in size in some specimens); only outermost row of teeth present at side of jaw. Males with teeth restricted to front of lower jaw.
Predorsal scales cycloid, mostly evenly sized, reaching forward to behind eyes; often only one scale anteriormost, in centre of nape close to interorbital space (sometimes this scale and one or two scales near it somewhat enlarged). Operculum with small cycloid scales on upper half to upper third only. Cheek always naked. Pectoral base usually covered with cycloid scales, naked in one specimen. Prepelvic area covered with small cycloid scales. Belly scales cycloid, with area of ctenoid scales anteriorly, close to base of pelvics (ctenoid scales sometimes extending halfway down belly). On side of body, ctenoid scales extending forward in wedge to close behind pectoral base or at least to below first dorsal fin.
Genital papilla in males small and slender, tip slightly expanded (pigmented so that it appears bilobed). Genital papilla in females short, bulbous and round, without lobes at tip.
Head pores absent.
Sensory papillae pattern longitudinal, as in Fig. 7 View Fig . Papillae rows g, x, z, b, d, e and opercular series consisting of small, closely spaced papillae. Papillae rows s, p, a, c, cp and i consisting of widely spaced, relatively large papillae. Three s rows present, each of one papilla. Row e well separated from preopercular margin, running almost vertically, or curving anteriorly or posteriorly. Row d forming curved arch, posteriormost end always pointing downward (not rearward). Two longitudinally oriented f rows, of two papillae each.
Colouration of fresh material. – Herre (1945) gave an account of the freshly preserved holotype of Calamiana magnoris : “...very pale tan, each scale stippled with minute brown dots; a brown stripe runs from the lower margin of the eye across the preopercle and opercle; the dorsals have a few dark spots basally, then a white longitudinal bar, the balance reddish brown; the other fins are all more or less reddish brown”.
Smith (1945) indicated that his type specimens of Gnathogobius aliceae were pale yellow with dull brown to yellowish brown markings; the first dorsal fin was blackish with a white margin, and the second dorsal had reddishbrown spots.
Hans Horsthemke provided several images of captive adults (for example, Plate 1A), from which the following description is taken. Head and body light pearly grey, paler ventrally, with grey-brown edges to scale margins mostly developed on dorsal half of body. Head mostly plain, with scattered brownish blotches over nape, diffuse dark grey streak from front of eye running below nostrils to upper lip, and a narrow irregular dark grey horizontal line just ventral to eye, ending near rear preopercular margin. Iris golden with brown speckling. First dorsal fin fawn with dark grey to blackish mottling, a indistinct black spot in centre of fin; outer third of fin reddish to reddish-orange. Second dorsal fin similar in colour to first, fawn with grey to brownish or blackish mottling, with narrow blue-white outer margin and broad submarginal red band. Anal fin whitish to pale grey, becoming darker toward outer margin and with narrow bright white edge. Pectoral fin whitish to translucent on distal half, basally yellow to pale brownish. Pelvic fins translucent bluish grey. Caudal fin base with grey somewhat square blotches; fin greyish to brownish, rays reddish to brown, darkening toward fin margin, edge of fin narrowly white.
Colouration of preserved material. – Head and body background colour variable, pale fawn to light brown ( Figs. 5 View Fig , 8 View Fig , 9 View Fig ). Top and side of head, back and side of body plain brown or finely speckled with brown; on lower half of side brown spots may be enlarged. Distinct vertical brown line or spot close to margin of each scale on side of body, giving fish a finely reticulate appearance. On upper half of body and nape, irregular brown mottling and blotches present (not always visible). Brown shoulder bar usually present (distinct on holotype); bar beginning above pectoral base and sloping obliquely backward to midline of body behind pectoral fin. At caudal base, three indistinct brown spots forming vague Y-shape.
Dark brown streak running almost horizontally from lower rear edge of eye, across cheek to opercle; opercle brown to blackish (rounded blotches sometimes present), especially on rear half, where blackish pigment usually present. Shorter and less distinct brown streak extending from upper rear edge of eye and running along top of preopercle and opercle, then becoming indistinct. Two short brown streaks extending from front of eye: upper streak from middle of eye curving around top of posterior nostril and running straight to upper lip; lower streak running from front lower edge of eye to just below anterior nostril. Tips of sensory papillae blackish in large male specimen ( Fig. 8 View Fig ); only a few papillae blackishtipped in other specimens. Lips, underside of head and breast plain dusky (lips and folds of branchial membranes may be edged with blackish); dusky underside of head may include pale areas. Belly pale.
Some sexual dichromatism in fin colour present. In males, first dorsal fin plain dusky, with broad translucent whitish margin. In females, first dorsal fin translucent with about three rows of dusky rounded spots over rays and membrane; broad translucent margin also present. Second dorsal fin dusky with broad translucent margin (fin darker in males) and about five irregular rows of dusky spots and streaks (becoming more irregular toward rear of fin) in females, in males only about three rows of spots on lower half of fin visible. Anal fin plain dusky with broad whitish membrane in both sexes. Caudal fin plain dusky grey with very narrow whitish margin in males; fin whitish to translucent in females, with irregular rows of small rounded brownish spots. Pectorals and pelvics light dusky in both sexes; pelvic fraenum whitish.
Distribution. – Specimens are known only from Malaysian Borneo ( Sabah and Sarawak), the Philippines, Thailand and Sri Lanka. The species is rarely collected. The specimen from Singapore is in poor condition, and shows every sign of having died in an aquarium, scales standing out, ulcerations, clumps of fungus), and was very likely purchased from an aquarium dealer and not collected from the wild. The species has never been recorded from Singapore (Larson & Lim 2005).
Ecology. – Apparently restricted to fresh water. Little ecological information is available. One specimen (FMNH 51667) probably came from slightly brackish water: “just above Nypa belt” according to the label ( Nypa grows at upper tidal limits, usually where freshwater infl ow is constant). Smith (1945) kept E. kabilia and Mugilogobius rambaiae alive in a small tank for seven months, fed on mosquito larvae and “entomostracans”.
This species first appeared in the German aquarium trade in the late seventies but most of the specimens were males (Horsthemke, in litt.). Werner (1981) described and illustrated captive behaviour and breeding of this species. He described the fish as strictly benthic and quite secretive; the males constructed nests under flat stones, shovelling sand with their large mouths while pieces of gravel (up to 12 mm diameter) carried out one by one. Males display to each other with mouths wide open, and may push against each other jaw to jaw (Plate 1A); if disturbed by an observer they often switched to locking jaws (as if biting). About 1,000 eggs per batch were laid in the spawning burrow; eggs were elongate (1.5 × 0.5 mm) and hatched after 44–95 hours at 25°C. Larvae were 2.4 mm long and floated up near the water surface until yolk absorbed after 124 hours; they then moved down into the water column. Werner was not able to provide suitable food.
Watson & Horsthemke (1995) compare the behaviour of this species (as Calamiana aliceae ) with that of Awaous flavus Valenciennes (adult males of this species have an enlarged mouth which is gaped extremely widely during territorial displays). They also report that the fish use the mouth “... as a shovel in nest construction ...”. Horsthemke (in litt.) states that males can open the jaws to an almost vertical gape, with the ends of the maxillaries flared out at a considerable angle to the body axis; the males then push against each other with jaws agape.
Remarks. – Glossogobius mas Hora, 1923 , is possibly this species. Hora’s syntypes were deposited at ZSI, but were reported as lost according to the ZSI type register (Barman in litt., 19 Aug.1991). Hora’s description was based on six specimens, four female and two male, about 21–25 mm SL. From the description and drawing, Glossogobius mas could be Eugnathogobius microps , Pseudogobiopsis oligactis , E. kabilia or a valid species. Lateral scale counts are given as 24–26, which is within the range for E. kabilia and E. microps . Freshly collected specimens from Chilka Lake (the type locality) are required to ascertain what Hora may have had.
Calamiana magnoris Herre, 1945 , the type species of the genus Calamiana ( Fig. 9 View Fig ), has priority over Gnathogobius aliceae Smith, 1945 , type species of the genus Gnathogobius ( Fig. 10 View Fig ). The former name was published on the 3.Jun and the latter on the 13 Nov. Herre (1945 d) thought that this species had two vomerine teeth, placed one behind the other. The enlarged, posteriorly directed teeth in the innermost row of the upper jaw (four present in holotype of Calamiana magnoris ) may have been mistaken for vomerine teeth. Herre also noticed the “...3 small fleshy flaps on the inner margin of the shoulder girdle”.
Roberts (1989) compared scalation and colour pattern of Calamiana magnoris and C. kabilia (he had examined the type specimen of C. magnoris but not V. kabilia ) and also with specimens he had collected from the Kapuas River and identified as Calamiana (these specimens were Eugnathogobius paludosus ).
The Thai name for this species is given by Smith (1945) as “pla bu”, a generic name for nearly all small gobies.
| FMNH |
Field Museum of Natural History |
| R |
Departamento de Geologia, Universidad de Chile |
| NTM |
Northern Territory Museum of Arts and Sciences |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| NIFI |
National Inland Fisheries Institute |
| SMF |
Forschungsinstitut und Natur-Museum Senckenberg |
| VI |
Mykotektet, National Veterinary Institute |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Eugnathogobius kabilia ( Herre, 1940 )
| Larson, Helen K. 2009 |
Mugilogobius kabilia
| Kottelat, M 1993: 146 |
Calamiana kabilia
| Larson, H 2001: 59 |
| Kottelat, M 1993: 146 |
| Roberts, T 1989: 168 |
Calamiana magnoris
| Roberts, T 1989: 168 |
| Herre, A 1953: 732 |
| Herre, A 1945: 80 |
Gnathogobius aliceae
| Kottelat, M 1989: 19 |
| Suvatti, C 1981: 203 |
| Smith, H 1945: 524 |
Vaimosa rambaiae
| Smith, H 1945: 538 |
Vaimosa kabilia
| Koumans, F 1953: 388 |
| Herre, A 1940: 19 |
Glossogobius mas
| Hora, S 1923: 743 |