Periphyllus koelreuteriae (Takahashi, 1919)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4585.2.9 |
|
publication LSID |
lsid:zoobank.org:pub:92B6F908-8ED0-460A-A605-419CFFF53D7F |
|
persistent identifier |
https://treatment.plazi.org/id/03A0A74E-FFFC-FF8C-87B0-FB5BFCE6FEC7 |
|
treatment provided by |
Plazi |
|
scientific name |
Periphyllus koelreuteriae (Takahashi, 1919) |
| status |
|
Periphyllus koelreuteriae (Takahashi, 1919) View in CoL
( Figs 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ; Table 1 View TABLE 1 )
Chaitophorinella koelreuteriae Takahashi, 1919a: 275 View in CoL
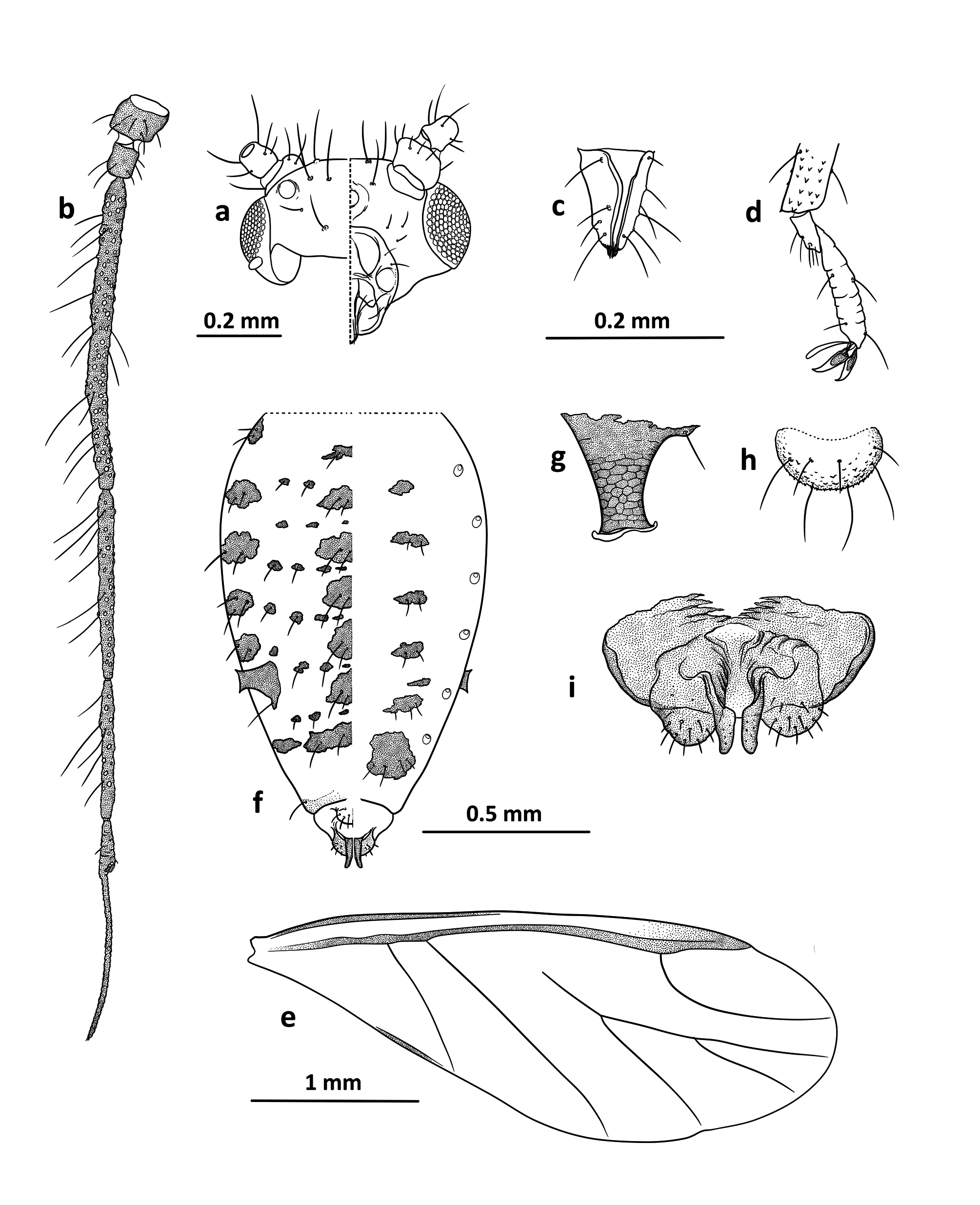
Re-description of apterous viviparous female (based on five specimens) ( Fig. 1 View FIGURE 1 ; Table 1 View TABLE 1 ). Colour. In life not recorded, but colour of apterae in spring generations reported in the literature as variably pigmented (generally dark or green by Essig and Abernathy (1952); black or yellow by Blackman and Eastop (2018)). In mounted specimens: head, legs, sclerites and siphunculi dusky. Antennae dusky, only basal part of ANT III slightly paler. Morphological characters. Body oval ( Fig 1a View FIGURE 1 ). Frons flat. Head with 6–7 pairs of fine, pointed setae, which are 0.08–0.22 mm long. ANT 6-segmented ( Fig. 1b View FIGURE 1 ), long, reaching ABD SEGM III, 0.61–0.64 × BL. ANT IV slightly longer than ANT V; ANT V always shorter than ANT VI; PT 2.72–3.11 × BASE; other antennal ratios: VI:III 0.84–0.93, V:III 0.50–0.51, IV:III 0.52–0.58. ANT I with 6–7 setae, ANT II with 4 setae, ANT III with 16–17 setae, ANT IV with 6–9 setae, ANT V with 2–6 setae, BASE with 2 setae. PT with 2–3 apical setae. ANT setae fine, pointed, up to 0.14 mm long. LS ANT III 3.50–4.50 × BD III. Rostrum reaching middle coxae. ARS with 4 accessory setae ( Fig 1c View FIGURE 1 ), 0.25–0.29 × ANT III and 0.81–0.86 × HT II. Legs setose, with numerous stout, pointed setae, which are 0.04–0.25 mm long. Distal part of hind tibiae with few rows of short spinules; empodial setae spatulate; first tarsal chaetotaxy 5:5:5 ( Fig. 1d View FIGURE 1 ). Abdominal tergites membranous, with large marginal and spinal sclerites, pleural sclerites very small, irregularly placed. 2–3 pale, pointed setae arising from marginal and spinal abdominal tergites, pleural sclerites with 1 setae each. Abdominal setae 0.05–0.34 mm long. Siphunculi truncate with 1–3 rows of reticulations and well developed flange ( Fig. 1e View FIGURE 1 ). Cauda broadly rounded, with 8 setae ( Fig. 1f View FIGURE 1 ).
Description of oviparous female (based on six specimens) ( Fig. 2 View FIGURE 2 ; Table 1 View TABLE 1 ). Colour. In life: unknown, in mounted specimens: head, legs, sclerites and siphunculi dusky. Antennae dusky, 2/3 length of ANT III slightly paler. Morphological characters. Body oval, segments VII–VIII of abdomen strongly elongated ( Fig. 2a View FIGURE 2 ). Frons flat. Head with 6–7 pairs of fine, pointed setae, which are 0.12–0.22 mm long. ANT 6-segmented ( Fig. 2b View FIGURE 2 ), long, reaching abdominal segment III, 0.44–0.48 × BL. ANT IV slightly longer than ANT V; ANT V always shorter than ANT VI; PT 2.2–2.5 × BASE; other antennal ratios: VI:III 0.73–0.75, V:III 0.42–0.51, IV:III 0.46–0.57. ANT I with 3–4 setae, ANT II with 4 setae, ANT III with 11–16 setae, ANT IV with 5–8 setae, ANT V with 3–5 setae, BASE with 2 setae, PT with 2–3 apical setae. ANT setae fine, pointed, up to 0.17 mm long. LS ANT III 4.25 × BD III. Rostrum reaching middle coxae. ARS with 4 accessory setae ( Fig. 2c View FIGURE 2 ), 0.28–0.35 × ANT III and 0.86–0.90 × HT II. Legs setose, with numerous stout, pointed setae, which are 0.06–0.20 mm long. Hind tibiae with numerous (79–85) rounded pseudosensoria, distributed on the whole length of tibiae. Distal part of hind tibiae with few rows of short spinules; empodial setae spatulate; first tarsal chaetotaxy 5:5:5 ( Fig. 2d View FIGURE 2 ). Abdominal tergites membranous, tergites I–VI with large, oval marginal sclerites, clearly visible spinal sclerites and inconspicuous pleural sclerites. Abdominal setae 0.10–0.30 mm long; marginal sclerites with 2–7 setae, spinal sclerites with 2 setae. Siphunculi truncate, with 4–5 rows of reticulations and well developed flange ( Fig. 2e View FIGURE 2 ). Cauda broadly rounded, with 8 setae ( Fig. 2f View FIGURE 2 ).
Description of alate male (based on four specimens) ( Fig. 3 View FIGURE 3 ; Table 1 View TABLE 1 ). Colour. In life: unknown, in mounted specimens: head, antennae, pronotum, sclerites, siphunculi and genitalia dusky. Legs dusky with basal part of femur and middle part of tibia slightly paler. Morphological characters. Body elongated. Frons flat. Head with 7 pairs of long fine, pointed setae, which are 0.15–0.17 mm long ( Fig. 3a View FIGURE 3 ). ANT 6-segmented ( Fig. 3b View FIGURE 3 ), long, reaching abdominal segment III, 0.76–0.82 × BL. ANT IV slightly longer than ANT V; ANT V always shorter than ANT VI; PT 3.63 × BASE; other antennal ratios: VI:III 0.71–0.82, V:III 0.43–0.48, IV:III 0.61–0.64. ANT I with 7–8 setae, ANT II with 3–5 setae, ANT III with 10–19 setae, ANT IV with 8–12 setae, ANT V with 4–6 setae, BASE with 2 setae, PT with 2–3 apical setae. ANT setae fine, pointed, up to 0.13 mm long. LS ANT III 3.25 × BD III. The whole surface of ANT III–V covered by rounded, secondary rhinaria: ANT III with 64–86 rhinaria, ANT IV with 30–42 rhinaria, ANT V with 12–18 rhinaria. Rostrum reaching fore coxae. ARS with 4 accessory setae ( Fig. 3c View FIGURE 3 ), 0.17–0.20 × ANT III and 0.81–0.86 × HT II. Legs setose, with numerous stout, pointed setae, which are 0.05–0.23 mm long. Distal part of hind tibiae with rows of short spinules; empodial setae spatulate; first tarsal chaetotaxy 5:5:5 ( Fig. 3d View FIGURE 3 ). Fore wings with normal venation ( Fig. 3e View FIGURE 3 ). Abdominal tergites membranous, with large fused spinal sclerites, pleural sclerites very small, irregularly placed, marginal sclerites oval. Abdominal setae 0.10–0.35 mm long; marginal sclerites with 2–3 setae, pleural sclerites with 1 setae each, spinal sclerites with 4 setae ( Fig. 3f View FIGURE 3 ). Siphunculi truncate, reticulated, except basal part, with developed flange ( Fig. 3g View FIGURE 3 ). Cauda broadly rounded, with 8 setae ( Fig. 3h View FIGURE 3 ). Genitalia well developed, strongly sclerotized with roundish, lobate parameres, covered by numerous spine-like setae. Basal parts of phallus slightly extended, tongue-shaped, with numerous short spinules ( Fig. 3i View FIGURE 3 ).
Life cycle. P. koelreuteriae is a holocyclic species and its life cycle was studied in detail by Liu et al. (1999a) and Lin et al. (2001b) in Taiwan. Specifically, the sexual generation appeared in winter with eggs as the overwintering form. The oviparous females appeared in the period when the foliage began to fall off the host plants (December to February), whereas the males and eggs were found from mid-January to early February. The highest population density was found in the lower layer and the lowest density in the upper layer of the canopy of the host trees. Both winged and wingless viviparous females were found in the field throughout the year, except from July to September, when the aphid population was extremely low. The aestivating forms occurred from March to June and reached their peak from April to May. These morph were probably present during the whole summer, what is in line with the phenology of host tree and in accordance with the biology of other species of the genus Periphyllus ( Essig & Abernathy 1952; Wieczorek et al. 2017). Such a shift in the life cycle is also typical in the warm, humid subtropical climate in the Ta-Keng area of Taichung in Taiwan, where the aphids were studied. In this area, the highest temperature of the year occurs in July and August, while the lowest temperature occurs in January and February. Numerous experimental studies have shown that the temperature has the greatest effect on the development, longevity and reproduction of the various morphs of this species ( Liu et al. 1999b; Kuo et al. 2001; Lin et al. 2001a, b, 2002; Ding Xu et al. 2015; Jing et al. 2015). By contrast, the life cycle of P. koelreuteriae in the condition of the temperate climate of Tiencin, northeast China (present study) was typical for aphids, with the sexual generation being observed from mid-October to mid-November.
Host plants. Koelreuteria is a small genus of flowering plants of the family Sapindaceae , which is native to southern and eastern Asia. Koelreuteria spp. grows easily in average, dry to medium, well-drained soil in full sun and adapts to a wide range of soils. Moreover, it tolerates drought and many urban air pollutants ( Gilman & Watson 1993). Therefore, is significant the shade trees of the green areas and parks in its native range in China, Korea and Japan and its use as a medicinal plant in China ( Yang et al. 2018). Although the Golden Rain Tree, K. paniculata , is treated as the main host plant for P. koelreuteriae (Holman 2010) , the species is also associated with other representatives of this genus, including K. elegans (Seem.) A.C. Sm. , which is endemic to Taiwan ( Taiwan, Ta- Keng area of Taichung Liu et al. 1999a), or K. bipinnata , which is native to southern China ( Jing et al. 2015). Because P. koelreuteriae seriously affects the growth of its host-plants, not only by causing new leaf deformities and curly leaves, but also by inducing its own leaves to have sooty mould, it is known as one of the most damaging pests of the ornamental trees of this genus ( Ping et al. 2004).
Distribution. China, Chaozhou ( Wu 1935); China, Shanghai ( Ping et al. 2004); China, Changzhou ( Wang et al. 2018); China, Beijing (BMNH coll., London, UK); China, Tiencin (present study); Taiwan, Ta-Keng area of Taichung ( Liu et al. 1999a); South Korea ( Paik 1972); Japan, Tokyo ( Takahashi 1919a); Japan, Tokyo, Morioka, Aomori ( Shinji 1927, 1941) ( Fig. 4 View FIGURE 4 ).
It is highly possible that P. koelreuteriae occurs more widely in countries where Koelreuteria spp. is grown, as it is a significant ornamental plant.
Remarks. Among the 13 species of the East Asian Periphyllus , the sexuales are only known for four of them ( Blackman & Eastop 2018). Therefore, for now, it is impossible to compare and key the oviparous females or males of these species of the genus Periphyllus from the mentioned area. Apterous viviparae of P. koelreuteriae were first found by the authors on the K. paniculata that were growing on the campus of Nankai University, Tiencin, China in July 2010. In October and November 2010, Dr Kai Dang simultaneously collected apterous viviparous females and sexuales from this same tree, which confirmed that the described morphs belong to P. koelreuteriae . In mounted specimens, the spring and autumn generations of apterous viviparous females, are broadly similar in body colouration and sclerotization of dorsal abdominal tergites.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Chaitophorinae |
|
Genus |
Periphyllus koelreuteriae (Takahashi, 1919)
| Junkiert, Łukasz & Wieczorek, Karina 2019 |
Chaitophorinella koelreuteriae
| Takahashi, R. 1919: 275 |