Acanthonyx petiverii H. Milne Edwards, 1834
|
publication ID |
https://doi.org/10.6620/ZS.2016.55-23 |
|
DOI |
https://doi.org/10.5281/zenodo.8060301 |
|
persistent identifier |
https://treatment.plazi.org/id/0398A53F-FFE7-FF97-6EFE-A6C4FAFEFC00 |
|
treatment provided by |
Valdenar |
|
scientific name |
Acanthonyx petiverii H. Milne Edwards, 1834 |
| status |
|
Acanthonyx petiverii H. Milne Edwards, 1834 View in CoL View at ENA
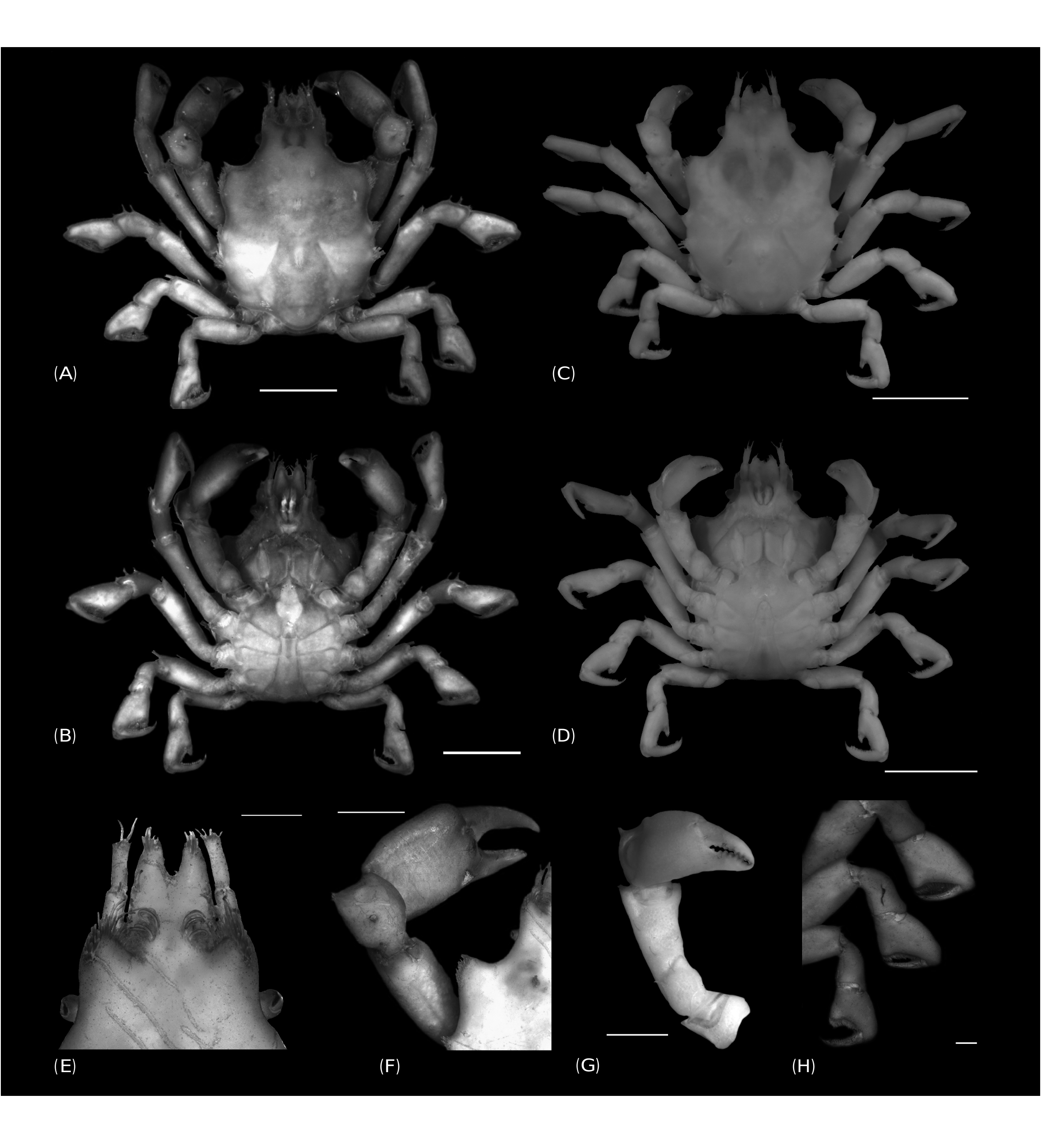
( Fig. 2 View Fig )
Cancer muricatus compressum Petiver, 1712 : plate 20: Fig. 8.
Acanthonyx petiverii H. Milne Edwards, 1834: 343 View in CoL , plate 15: Fig.s 6-8; Bell, 1841: 62; Moreira, 1901: 66; Rathbun, 1925: 142, Fig. 52, plate 44, plate 222: Figs. 1 View Fig -6; Rathbun, 1933: 13, Fig. 11; Garth, 1946: 376, Fig. 4, plate 63; Garth, 1958: 223, plate 25: Fig. 2 View Fig ; Fausto-Filho, 1966: 33; Houvenaghel and Houvenaghel 1974: 143; Abele and Kim, 1986: 43, 495 (key); Hernández-Aguilera et al., 1997: 60, Fig. d, plate 3; Marcano and Bolaños, 2001: 74.
Acanthonyx emarginatus H. Milne Edwards & Lucas, 1843: 9 View in CoL .
Acanthonyx debilis Dana, 1851: 272 View in CoL .
Peltinia scutiformis Dana, 1851: 273 View in CoL .
Acanthonyx simplex Dana, 1852 View in CoL - Emparanza et al., 2007: 534 ( Table 1 View Table 1 ), 535, Fig. 1 View Fig .
Acanthonyx concamerata Kinahan, 1857: 334 View in CoL , plate 14: Fig. 1 View Fig .
Acanthonyx scutiformis View in CoL - Coelho and Torres, 1993: 228; Melo, 1996: 171; Melo, 1998: 455; Melo, 2008: 4; Teixeira et al., 2009: 89.
Acanthonyx dissimulatus Coelho, 1993: 231 View in CoL , Fig. 1 View Fig ; Melo, 1998: 455.
Type locality: The Antilles (H. Milne Edwards 1834; Rathbun 1925; Garth 1958), in the western Atlantic. The holotype of A. petiverii (male with 18 mm) is in the Muséum National d’Histoire Naturelle, Paris, France ( Rathbun 1925; Garth 1958). Examined (see Discussion).
Material examined: UNITED STATES OF AMERICA, Florida, Miami: 1 male (CL 12.57 mm), 22/II/1965, J. Cabrera leg. ( CNCR 1152); MEXICO, Compostela, Nayarit: 1 female (CL 7.7 mm), 11/III/1993, J.L. Villalobos, E. Cadena, M.E. Camacho, F. Álvarez and E. Lira leg. ( CNCR 15302); San Andrés Tuxtla, Veracruz de Ignacio de la Llave: 1 male (CL 4.2 mm), 26/VIII/2007, coll. not available ( CNCR 24952); Veracruz, El Moro: 1 male (CL 9.35 mm), 01/VI/1973, J.A. Rickner leg. ( ULLZ 11303); Veracruz, Punta Delgada: 3 males (CL 4.8 - 5.8 mm), 1 female (CL 4.9 mm), 07/I/2002, D.L. Felder, R. Robles and T. Rodriguez leg. ( ULLZ 6163); COSTA RICA, Isla Murciélago: 2 ovigerous females (CL 7.0 and 8.6 mm), 08/V/1999, coll. not available (UCR 2295-II); PANAMA, Bocas del Toro: 1 male (CL 5.2 mm), 09/VIII/2004, D.L. Felder leg. ( ULLZ 10755); Bocas del Toro, Playa Paunch: 1 male (CL 13.5 mm), 1 ovigerous female (CL 11.4 mm), 05/VIII/2011, F.L. Mantelatto leg. ( CCDB 1063); VENEZUELA, Isla Margarita, Boca Chica: 1 male (CL 8.4 mm), 1 female (CL 10.0 mm), 03/XI/2010, R. Lopez leg. ( CCDB 2428); La Restinga: 1 male (CL 12.4 mm), 1 female (CL 16.4 mm), 25/ VIII/2011, J. Bolaños leg. ( CCDB 3633); BRAZIL, Ceará, Aracati, Retiro Grande Beach: 1 male (CL 14.7 mm), 1 ovigerous female (CL 13.8 mm), 17/ I/1964, A.L. Castro leg. ( MNRJ 4468); Ceará, Mucuripe: 1 female (CL 9.9 mm), 1 juvenile (CL 6.54 mm), 23/IV/1965, coll. not available ( MNRJ 4462); Pernambuco, Recife, Piedade Beach: 2 males (CL 10.3 and 19.2 mm), 1 female (CL 8.52 mm), 1 ovigerous female (CL 15.06 mm), 24/ VI/1986, A.L. Castro, P. Coelho and G. Melo leg. ( MNRJ 4478); Pernambuco, Recife, Boa Viagem Beach: 4 males (CL 7.0 - 17.6 mm), 1 female (CL 9.7 mm), 1 juvenile (CL 5.5 mm), 06/IV/2012, F.L. Mantelatto leg. ( CCDB 3814); Pernambuco, Ipojuca, Serrambi county, Serrambi Beach: 1 male (CL 8.3 mm), 1 juvenile (CL 5.3 mm), 25/XII/2012, F.L. Mantelatto and F.B. Mantelatto leg. ( CCDB 4481); Paraíba, Jacunã, Tambaba Beach: 2 males (CL 6.9 and 17.8 mm), 1 female (CL 9.1 mm), 1 ovigerous female (CL 14.0 mm), 23/II/1995, P.S. Young and C.S. Serejo leg. ( MNRJ 6487); Alagoas, Marechal Deodoro, Francês Beach: 1 ovigerous female (CL 18.7 mm), 16/II/1995, P.S. Young and C.S. Serejo leg. ( MNRJ 6630); Alagoas, Marechal Deodoro, Torto Beach: 1 female (CL 10.7 mm), 16/ II/1995, P.S Young and C.S. Serejo leg. ( MNRJ 6671); Bahia, Lauro de Freitas, Ipitanga Beach: 1 female (CL 13.2 mm), 22/XII/2011, F.L. Carvalho and E.A. Souza-Carvalho leg. ( CCDB 3789); Bahia, Itacaré, Ribeira Beach: 3 males (CL 3.7 - 11.4 mm), 4 females (CL 4.6 - 7.8 mm), 9 juveniles (CL 2.3 - 3.7 mm), 22/II/1994, P.S. Young and M.M. Britto-Pereira leg. ( MNRJ 4507); Bahia, Ilhéus, Morro de Pernambuco, Badusca Beach: 4 males (CL 4.2 - 6.0 mm), 6 females (CL 3.6 - 13.8 mm), 6/XI/2010, F.L. Mantelatto, F. Carvalho and L. Pileggi leg. ( CCDB 2427); Bahia, Ilhéus, Morro de Pernambuco, Badusca Beach: 4 males (CL 7.4 - 11.5 mm), 3 females (CL 6.7 - 9.9 mm), 2 ovigerous females (CL 9.9 and 13.1 mm), 1 juvenile (CL 3.9 mm), 21/I/2011, F. Carvalho and E.A. Souza-Carvalho leg. ( CCDB 3423); Espírito Santo, Guarapari, Castanheiros Beach: 1 ovigerous female (CL 10.1 mm), 30/X/1993, P.S. Young leg. ( MNRJ 4547); Espírito Santo, Vitória, Boi Island: 1 male (CL 20.7 mm), 11/II/1987, Flávio and Iara leg. ( MNRJ 4664); Espírito Santo, Vitória, Tubarão Harbor: 1 male (CL 18.5 mm), 1 ovigerous female (CL 16.6 mm), 10/XI/1987, coll. not available ( MNRJ 4663); Rio de Janeiro, Cabo Frio, Peró Beach: 1 male (CL 9.4 mm), 2 females (CL 6.3 and 7.9), 28/VII/1965, A.L. Castro leg. ( MNRJ 4661); Rio de Janeiro, Niterói, Itaipú Beach: 1 male (CL 19.6 mm), IV/1963, J. Becker leg. ( MNRJ 4471); Rio de Janeiro, Niterói, Itaipú Beach: 1 male (CL 14.4 mm), 1 female (CL 9.6 mm), 3/IV/1992, A. Macedo leg. ( MNRJ 4515); Rio de Janeiro, Rio de Janeiro, Barra de Guaratiba: 1 male (CL 13.1 mm), 30/IV/1953, N. Santos leg. ( MNRJ 4467); Rio de Janeiro, Rio de Janeiro, Flamengo Bay: 1 male (CL 17.9 mm), 06/VIII/1982, R. Leite leg. ( MNRJ 4470); São Paulo, Ubatuba, Grande Beach: 1 male ( 22.6 mm), 04/XI/1987, F.L. Mantelatto leg. ( CCDB 0067); São Paulo, Ubatuba, Itaguá Beach: 2 males (CL 21.3 mm), 2 ovigerous females (15.7 and 16.1 mm), VI/1999, F.L. Mantelatto leg. ( CCDB 0046); São Paulo, Ubatuba, Grande Beach: 1 male (CL 17.6 mm), 1 ovigerous female (CL 15.2 mm), X/2002, F.L. Mantelatto leg. ( CCDB 0760); São Paulo, Ubatuba, Ubatuba Bay: 1 female (CL 12.3 mm), 13/V/2010, F.L. Mantelatto leg. ( CCDB 2436); São Paulo, Ubatuba, Grande Beach: 1 male (CL 24.8 mm), 08/XII/2012, I.C. Leone leg. ( CCDB 3949).
Additional material initially identified as A. dissimulatus . MEXICO, Quintana Roo, La Mancha Rodes: 1 male (CL 15.9 mm), 1 ovigerous female (CL 8.2 mm), 02/VII/2002, coll. not available ( CCDB 2430); BRAZIL, Rio Grande do Norte, Potiguar Basin: 1 ovigerous female (CL 9.9 mm), 23/XI/2003, coll. not available ( DOUFPE 13837); Rio Grande do Norte, Potiguar Basin: 2 males (CL 7.1 and 10.7 mm), 23/XI/2003, coll. not available ( DOUFPE 13906); Rio Grande do Norte, Potiguar Basin: 1 male (CL 15.4 mm), 21/XI/2003, coll. not available ( DOUFPE 13920); Rio Grande do Norte, Potiguar Basin: 1 female (CL 8.6 mm), 2 ovigerous females (CL 10.5 and 13.5 mm), 21/XI/2003, coll. not available ( DOUFPE 13927); Pernambuco, Santo Aleixo Island: 1 female (CL 8.2 mm), 06/ II/2007, coll. not available ( DOUFPE 13523); Pernambuco, Santo Aleixo Island: 1 ovigerous female (CL 11.5 mm), 2 juveniles (CL 2.9 and 3.6 mm), 06/II/2007, coll. not available ( DOUFPE 13524); Bahia, Corumbau, Itacolomis: 3 males (CL 4.9 - 8.0 mm), 16/ II/2000, P.C. Paiva leg. ( MNRJ 16748); Rio de Janeiro, Arraial do Cabo, Anjos Beach: 1 male (CL 24.3 mm), 1 ovigerous female (CL 20.2 mm), 06/IX/2003, C.E.L. Ferreira leg. ( MNRJ 19254); São Paulo, Ubatuba, Itaguá Beach: 2 males (CL 8.8 and 18.7 mm), 1 female (CL 8.5 mm), 2 ovigerous females (CL 17.2 and 18.5 mm), 1 juvenile (CL 9.3 mm), XII/1995, coll. not available ( CCDB 103); São Paulo, Ubatuba, Grande Beach: 1 male (CL 11.7 mm), 04/V/2004, F.L. Mantelatto leg. ( CCDB 1421).
Additional material initially identified as A. scutiformis . BRAZIL, Rio de Janeiro, Angra do Reis: 1 male (CL 13.1 mm), 22/V/1966, coll. not available ( MZUSP 2781); 1 ovigerous female (CL 15.6 mm), 21/V/1966, coll. not available ( MZUSP 2782).
Diagnosis: Rostrum short, deflexed, bifid; gastric region protuberant; hepatic region with lateral lobes curved forwards and upwards; propodus of chelipeds gaping in adult male; ambulatory pereopods subchelate.
Description: Carapace elongate (almost elliptical), subpentagonal, smooth; tubercles in gastric, sometimes absent; cardiac and intestinal region, with no apparent pattern; lateral margins nearly parallel or parallel, with 3 setiferous teeth, 1 on margin of hepatic region, 2 on branchial region, sometimes absent; antennae visible on either side of rostrum. Rostrum short, deflexed, bifid; extremity with minute spine and 1 tuft of setae, setae filling entire sinus of rostrum; long and fine setae distributed laterally on dorsal margin; row of hooked setae on each side of dorsal surface adjacent to preorbital tooth. Orbits absent; orbital region with lateral angles obtuse; preorbital lobes not pointed, elevated, curved forwards and inwards. Eyes visible from above carapace, small, mobile, with short and thick setae on dorsal surface, sometimes absent. Preorbital teeth elevated, not pointed, presence of setae with variable length and thickness along lateral margin. Postorbital teeth absent. Hepatic region usually with almost rectangular angles in dorsal view, lateral margins curved forwards and upwards, with setae of variable length and thickness along margins. Gastric region slightly elevated, with 3 small setiferous tubercles distributed in a triangle, 2 on protogastric and 1 on mesogastric region. Cardiac region not evident; obsolete tubercle, with tuft of setae of variable length and thickness. Branchial region with 2 small teeth not pointed, with short and long setae. Intestinal region smooth or 2 lateral obsolete tubercles, with 3 tufts of setae, a central and 2 lateral tufts. Basal article of antenna with base wider than extremity; following 2 articles subcylindrical, attaining end of rostrum; distal end of second article with long setae; third article with tufts of short and long setae on inner margin; flagellum slender, with setae, sometimes absent. Chelipeds less strong, short, smaller than first pair of ambulatory pereopods in females and males up to 8 mm of carapace length; chelipeds short, considerably enlarged in adult males (carapace length more than 12 mm). Distal end of ischium with 1-5 thin setae on ventral surface. Merus with 3 setiferous lobes at the distal end (1 central and 2 lateral lobes); 1-2 spines on the proximal dorsal surface; a row of 1-3 thin setae on ventral surface. Carpus with setiferous external crest, sometimes smooth; 3 setiferous tubercles (1 proximal, 1 distal, 1 central); row of 7 thin setae along inner margin. Propodus enlarged in lateral margins and compressed in upper margin, less enlarged and compressed in females; fingers gaping extremity in males, almost entirely closed in females; fixed finger smooth in larger males, dentate on outer surface and smooth on inner surface in other males and females, with tufts of setae near distal end. Dactylus dentate on outer surface and smooth on inner surface, with tufts of setae near distal end. Ambulatory pereopods subchelate in larger specimens, not subchelate in smaller specimens; posteriorly decreasing in size. Merus with 3 lobes in distal end of first and second pereopods, 1 or 2 long and stout setae in the central lobe; 1or 2 setae on dorsal surface and sometimes on ventral surface. Merus of third and fourth pairs with less pronounced distal lobes, with setae on extremity, sometimes smooth. Carpus of first, second and third pairs with a setae on distal inner margin, 1 or 2 on median of dorsal surface, 1 setae on distal outer margin; fourth pair smooth; subtriangular in last 2 ambulatory pereopods. Propodus of ambulatory pereopods compressed, subtriangular, with rounded end where dactylus articulas, forming structure with dactylus; distal margin lined with setae. Subchela more pronounced from fourth to first pair. Dactylus with 2 rows of minute spines intercalated with fine setae on ventral surface; end smooth, not pointed. Anterior thoracic sternum smooth. Fourth and fifth abdominal somites fused in both genders, triangular abdomen in males, almost elliptical in females, rounded in ovigerous females; telson triangular; extremity of male first pleopod with subtriangular lobe with minute spines.
Description of juveniles: Carapace transparent, lobes and spines less pronounced than adults; rostrum not deflexed, bifid and more divergent than in adults, forming an obtuse angle and a pronounced cavity; preorbital lobes not curved forwards and inwards, with long and thick setae between preorbital lobes and between preorbital and hepatic lobes; hepatic region smooth, without rectangular angles in dorsal view, lateral margins not curved forwards and upwards; gastric region without tubercles and setae. Chelipeds not strong, articles with setae distributed arbitrarily; carpus without external crest or less pronounced, without tubercles and sometimes only with setae (row with up to 6 thin setae on inner margin); propodus not enlarged and compressed, fingers entirely closed. Ambulatory pereopods subcylindrical, with setae distributed arbitrarily; propodus with almost rounded end where dactylus articulas, but not subchelate. Abdomen almost triangular, but not so marked and thin as in males.
Coloration: It is variable and depends entirely upon the color of the surrounding macroalgae ( Coelho and Torres 1993). Co-ordinated with its body ornaments, its color allows the crab to be confused with algae and difficult to recognize ( Teixeira et al. 2009). Crabs are typically brown or dark green, with bits of algae, grains of sand and/or debris attached to the body. Fresh specimens can present a dark heart-shaped stain in the gastric region and two round spots in the protogastric region around the area of the tubercles. The tips of the cheliped fingers are white and red, and the hepatic region is orange. There are two darker bands in the region between the preorbital teeth.
Remarks: The species in Brazil cannot be effectively separated with the current accepted suite of morphological characters (H. Milne Edwards 1834; Dana 1852; Rathbun 1925, Coelho and Torres 1993; Melo 1996), and with the male first pleopods proving not to be useful. The male first pleopod of Acanthonyx petiverii ( Garth 1958: plate O, Fig. 3) is similar to that of other species, such as A. minor (cf. Manning and Holthuis 1981: Fig. 64h), and clearly differs from A. depressifrons and A. formosa (cf. Manning and Holthuis 1981: Fig. 62f, Wu et al. 1999: Fig. 3d), which means that this character can be used to recognize some species within the genus but is not informative for the species here analyzed.
The original description of A. petiverii (H. Milne Edwards 1834) was short and general, addressing only features of three morphological characters (orbits, carapace and legs). We redescribed A. petiverii with more details and characters based on samples from distinct localities of its distribution. The specimens of A. petiverii studied herein agree with the original description (H. Milne Edwards 1834) in lateral margin with three teeth, the anterior one bigger than the others; they were similar to those described by Garth (1958) and Hendrickx (1999), except for the size variation (the smallest specimen had a CL of 2.9 mm, and the largest specimen had a CL of 24.8 mm) observed in the present study, which was less than that reported in the original description (approximately 20.0 mm) and greater than that reported by Garth (1958) (CL ranging from 4.5 to 34.6 mm) and Hendrickx (1999) (CL ranging from 3.6 to 11.8 mm).
Similar to the present study, Garth (1958) and Emparanza et al. (2007) observed considerable variation in the presence or absence of tufts of setae and tubercles on the carapace and in the angle of the hepatic lobe, whether acute or obtuse. Both variations in the carapace as well as its color appear to be related to the color of the host algae ( e.g. green, brown, or red) in the region where the crabs live ( Emparanza et al. 2007). The specimens analyzed here have their colors related to the color type of algae that they were taken from, brown specimens occuring on Sargassum sp. and Hypnea sp. ; green specimens living on Padina sp. and Ulva sp. Finally, carapace shape variation seems to be morphological not a consequence of the ornamentation.
Ambulatory legs of specimens analyzed herein were less cheliform in females and in young males compared with adult males, and this had already been noted was an age-related character ( Rathbun 1925). Coelho and Torres (1993) described A. dissimulatus and reviewed A. scutiformis , but they noted that adults of A. petiverii were similar to juveniles and adults of A. scutiformis and A. dissimulatus .
The type locality of A. petiverii is the Antilles (H. Milne Edwards 1834; Rathbun 1925; Garth 1958) but we did not analyze material of this region. Then, we had observed specimens from Costa Rica, Panama and Venezuela aiming to cover some Central America regions, places that are close to the type locality. We examined a possible specimen that correspond to the holotype in the Muséum National d’Histoire Naturelle via recent photos made by the curator, but doubts raised about its origin due to lack of information available in the original label, which lead us uncomfortable and with uncertainties during our analysis. The type locality of A. dissimulatus is in Paraíba State ( Brazil), and the holotype (male, MZUSP 6596) was supposedly deposited in Museu de Zoologia da Universidade de São Paulo ( Coelho and Torres 1993). The type locality of A. scutiformis is Rio de Janeiro State ( Brazil), and its holotype is noted in the literature as non-existent ( Dana 1851; Moreira 1901; Coelho and Torres 1993; Melo 1996). At this stage, we analyzed the original description but not the specimens, although we analyzed material from this locality.
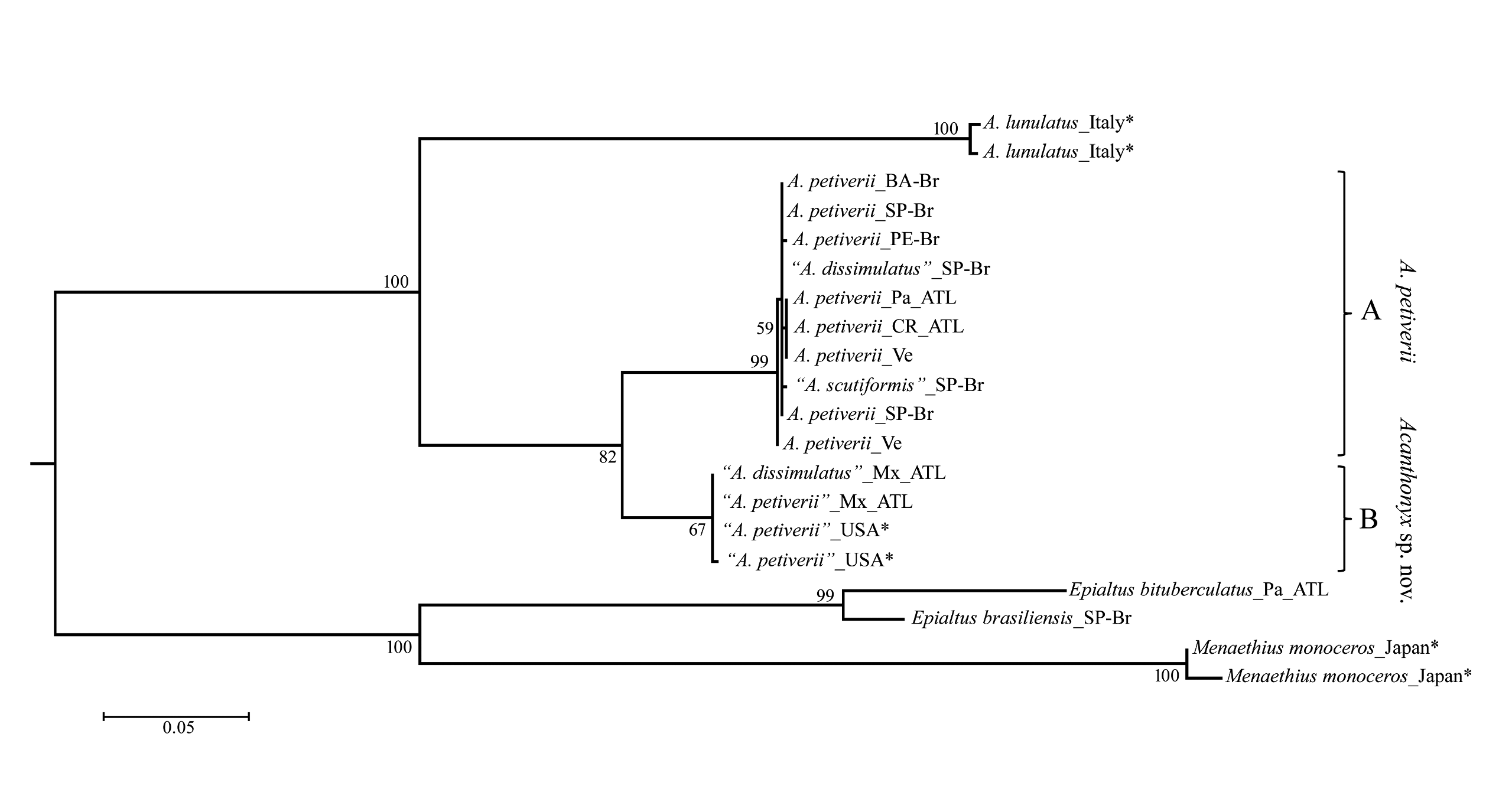
The genus Acanthonyx has a wide distribution with 17 valid species, but its systematics and taxonomy are little studied. Acanthonyx petiverii , A. dissimulatus and A. scutiformis are American species of this genus; however, we did not find a consistent morphological feature to differentiate these species from each other. Thus, we chose s o m e a v a i l a b l e c h a r a c t e r a n d p e r f o r m e d a morphological comparison based on species from distinct geographical distributions described and well supported in the literature ( Table 4 View Table 4 ): A. depressifrons Manning and Holthuis, 1981 and A. minor Manning and Holthuis, 1981 from West Africa ( Manning and Holthuis 1981); A. limbatus A. Milne-Edwards, 1862 and A. euryseroche Griffin and Tranter, 1986 from Indo-West Pacific ( Griffin and Tranter 1986); and A. formosa from Taiwan ( Wu et al. 1999).
We can infer that those combined character that fail to separate A. petiverii , A. dissimulatus and A. scutiformis , can be used to distinguish species from other geographic region, e.g. shape of the rostrum plus male first pleopod can distinguish A. depressifrons from A. minor ( Manning and Holthuis 1981) .
The variation observed in taxonomic relevant characters among specimens of Acanthonyx from Brazil, allow us to question their validity. However, our molecular results suggested a clear division in two groups. Therefore, for specimens from Caribbean and Brazil (clade A) we suggest the synonymization of A. dissimulatus and A. scutiformis with A. petiverii . We validate the name of the last one because its type locality is in the Antilles, and it is a senior subjective synonym of A. dissimulatus and A. scutiformis . Additionally, from North America (clade B), we suggest that there is a different species, here named as Acanthonyx sp. nov. because we cannot find any clear character to diagnose and chose an appropriate name.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Majoidea |
|
Family |
|
|
Genus |
Acanthonyx petiverii H. Milne Edwards, 1834
| Mantelatto, Ana Francisca Tamburus and Fernando Luis 2016 |
Acanthonyx simplex
| Emparanza EJM & Guzman GL & Ng PKL 2007: 534 |
Acanthonyx scutiformis
| Teixeira GM & Fransozo V & Cobo VJ & Hiyodo CM 2009: 89 |
| Melo GAS 2008: 4 |
| Melo GAS 1998: 455 |
| Melo GAS 1996: 171 |
| Coelho PA & Torres MFA 1993: 228 |
Acanthonyx dissimulatus Coelho, 1993: 231
| Melo GAS 1998: 455 |
| Coelho PA & Torres MFA 1993: 231 |
Acanthonyx concamerata
| Kinahan JR 1857: 334 |
Acanthonyx debilis
| Dana JD 1851: 272 |
Peltinia scutiformis
| Dana JD 1851: 273 |
Acanthonyx emarginatus H. Milne Edwards & Lucas, 1843: 9
| Milne Edwards H & Lucas H. 1843: 9 |
Acanthonyx petiverii H. Milne Edwards, 1834: 343
| Marcano J & Bolanos J. 2001: 74 |
| Hernandez-Aguilera JL & Toral-Alamzan RE & Ruiz-Nuno JA 1997: 60 |
| Abele LG & Kim W. 1986: 43 |
| Houvenaghel GT & Houvenaghel N. 1974: 143 |
| Fausto-Filho J. 1966: 33 |
| Garth JS 1958: 223 |
| Garth JS 1946: 376 |
| Rathbun MJ 1933: 13 |
| Rathbun MJ 1925: 142 |
| Moreira C. 1901: 66 |
| Bell T. 1841: 62 |
| Milne Edwards H. 1834: 343 |