Paraphlebia Selys in Hagen, 1861
|
publication ID |
https://doi.org/10.11646/zootaxa.5089.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:E12F2B20-A84A-48E2-9C77-B281F1BFC62E |
|
DOI |
https://doi.org/10.5281/zenodo.7603009 |
|
persistent identifier |
https://treatment.plazi.org/id/039887EB-3022-FFD9-FF73-FC1BF0CEF887 |
|
treatment provided by |
Plazi |
|
scientific name |
Paraphlebia Selys in Hagen, 1861 |
| status |
|
Paraphlebia Selys in Hagen, 1861 View in CoL
Paraphlebia Selys 1860: 435 View in CoL ( nomen nudum); Hagen [Selys in Hagen] 1861: 71 ( Paraphlebia zoe View in CoL type species, by monotypy); Selys 1862: 8–9 (genus description); Felder in Felder et al. 1864: Tab. 83, fig. 6 (junior homonym); Brauer 1868: 361 (Agrionina key); Scudder 1882: 233 (cat.); Selys 1886: 33 (key); Kirby 1890: 122 (cat.); Calvert 1901: 59 (addition of generic characters); Higgins 1901: 136 (gizzard formulae); Calvert 1902: 31 (comparison with Thaumatoneura McLachlan, 1897 View in CoL ); Calvert 1903: 133–134 (discussion on mimicry with Palaemnema Selys View in CoL ); Calvert 1908: 461, 467 (distribution and biogeographic affinities); Cockerell 1908: 70 (comparison with Megalagrion umbratum (Scudder, 1890) and Trichocnemis aliena (Scudder, 1892) ; Calvert 1913: 260–261, 263 (legion Podagrion key, relationship with Phenacolestes Cockerell, 1908 ); Tillyard 1917: 284 (transferred to Megapodagrioninae); Munz 1919: 28 (fig. 69; Megapodagrionidae View in CoL key); Kennedy 1925: 303 (comparison with megapodagrionids); Beatty & Beatty 1968: 807 (first mention of males colour and behaviour dimorphism); Paulson 1982: 251 (distribution); Bridges (1994: III.38; cat.); González-Soriano & Novelo-Gutiérrez 2007: 113 (distribution in Mexico); Fogarty et al. 2008 (support as sister group of Thaumatoneura View in CoL ); Novelo-Gutiérrez 2008: 29 (larva description); Kalkman et al. 2010: 123 (discussion on larval characters and comparison with megapodagrionids); González-Soriano & Paulson 2011: 303 (discussion on endemism in Chiapas); Dijkstra et al. 2013: 20 (transferred to Thaumatoneuridae View in CoL ); Hämäläinen 2016: 38 (cat.); Cuevas-Yañez et al. 2015: 517 (conservation status of P. zoe View in CoL , P. hyalina View in CoL and P. quinta View in CoL ).
Paraphleoia Hagen 1861, Davies & Tobin 1984: 42 (typo or misprint in cat.).
Note on the authorship of Paraphlebia
Selys (1860) first mentioned Paraphlebia zoe Selys in a note comparing its coloration with that of Palaemnema paulina Drury, 1773 . However, he did so before the completion of the work where he originally intended to describe this species ( Selys 1862) making P. zoe Selys, 1860 a nomen nudum for not complying with the provisions of Art. 12 in the International Code of Zoological Nomenclature ( ICZN 1999). Hagen (1861) introduced Paraphlebia zoe Selys linking the name for the first time to a specimen from Mexico which was deposited in a collection (“Collection of Selys Longchamps”), and providing a (very succinct) diagnosis. This publication year was mainanied in some consequent works with various authorships given as: P. zoe to Hagen, 1861 ( Kirby 1890), P. zoe to Selys in Hagen, 1861 ( Calvert 1901), P. zoe to Hagen, 1861 ( Davies & Tobin 1984). Garrison (1991) discussed the authorship of Paraphlebia and P. zoe and decided that they were not “… adequately described, much less diagnosed.” Therefore, he considered them as nomina nuda, which made P. zoe Selys, 1862 the available name. From this point onwards, the authorship of the genus was attributed in different ways, e.g., Paraphlebia Hagen, 1861 ( Bridges 1994) ; Paraphlebia Selys in Hagen, 1861 ( Garrison et al. 2010; Hämäläinen 2016); Paraphlebia Selys, 1861 ( Dijkstra et al. 2013).
The authorship was revised in the present study. Paraphlebia zoe Selys in Hagen, 1861 was found to be the available name (for the reasons explained above) and therefore, Paraphlebia Selys in Hagen, 1861 should be considered the valid name by monotypy.
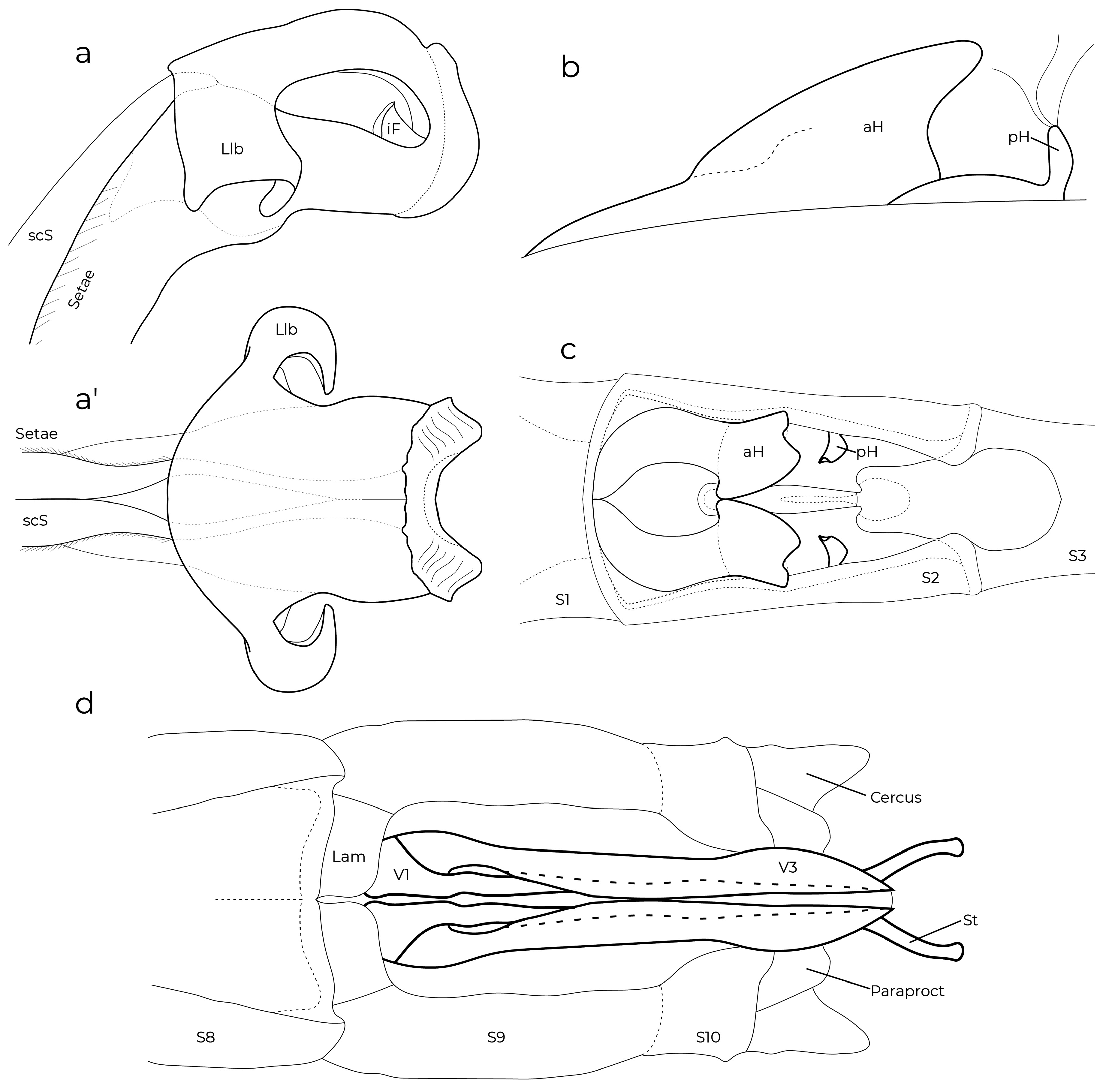
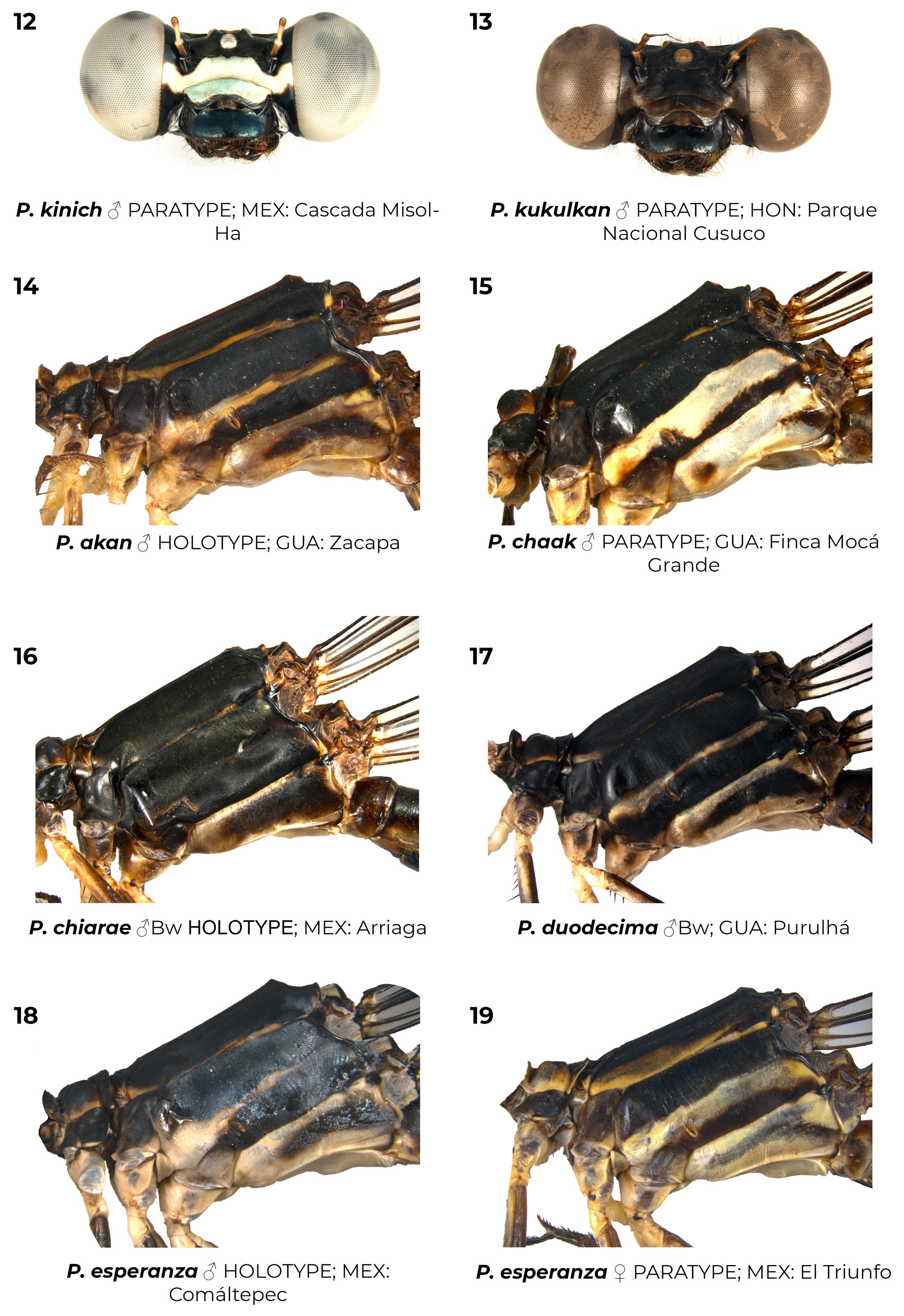
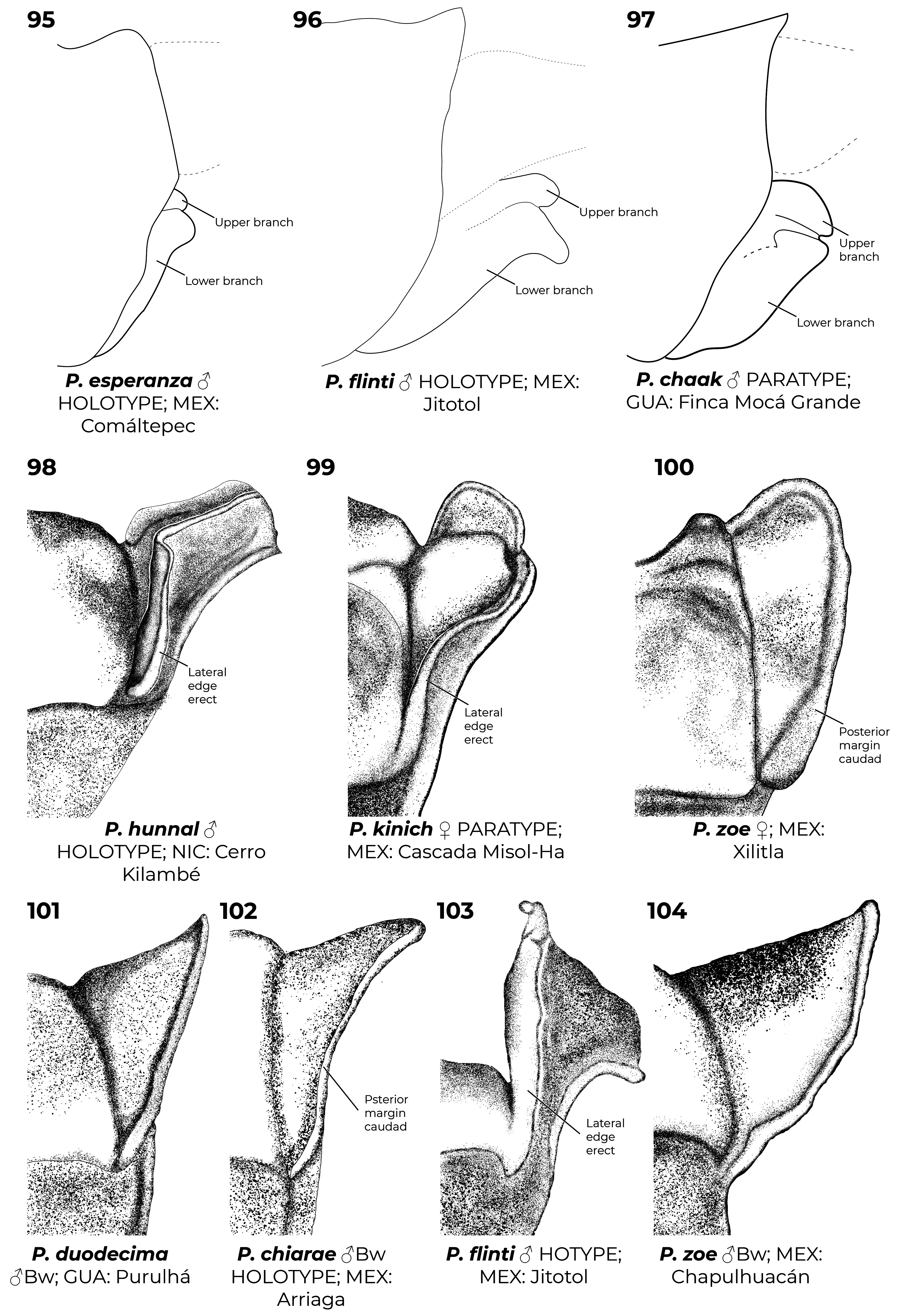
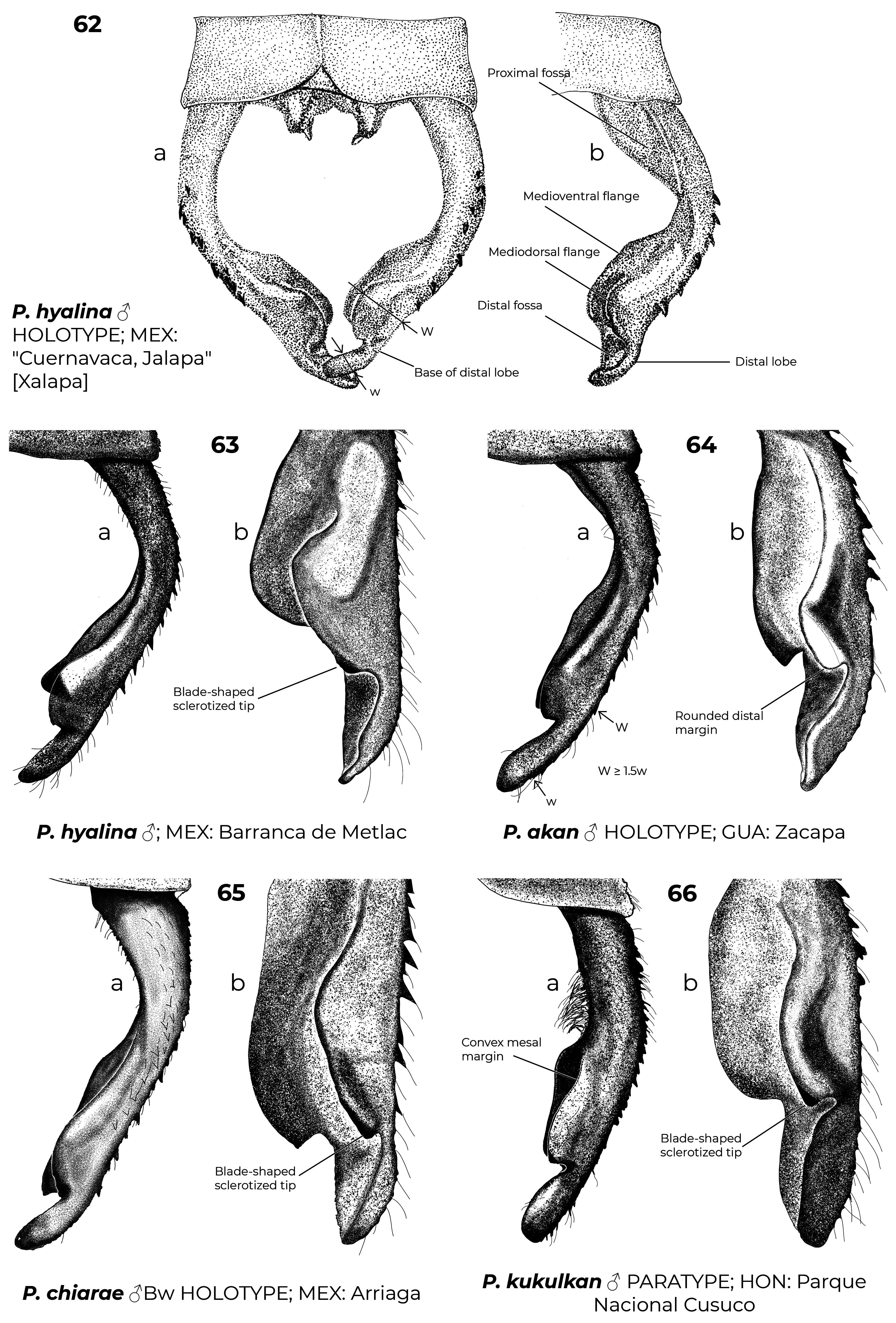
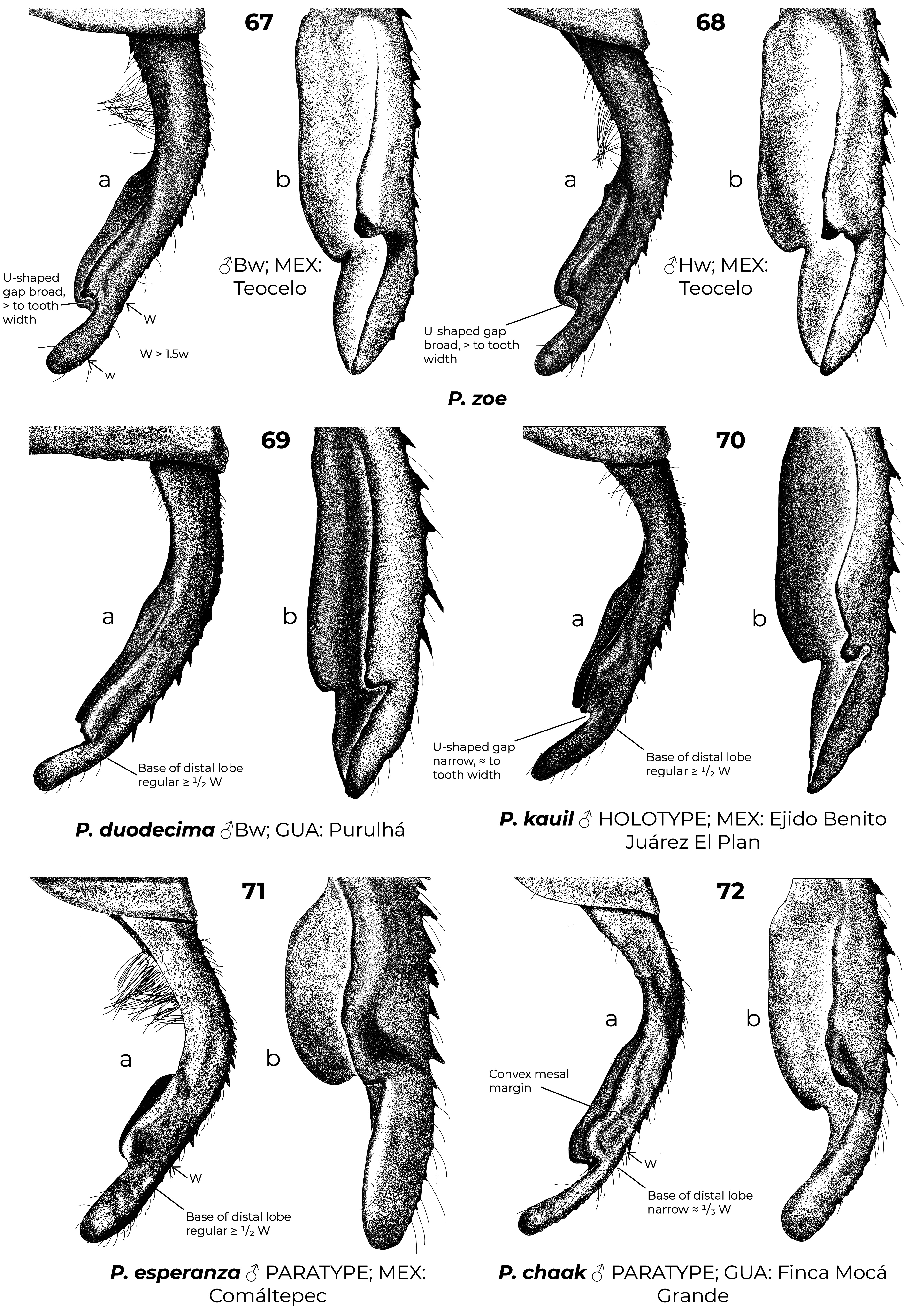
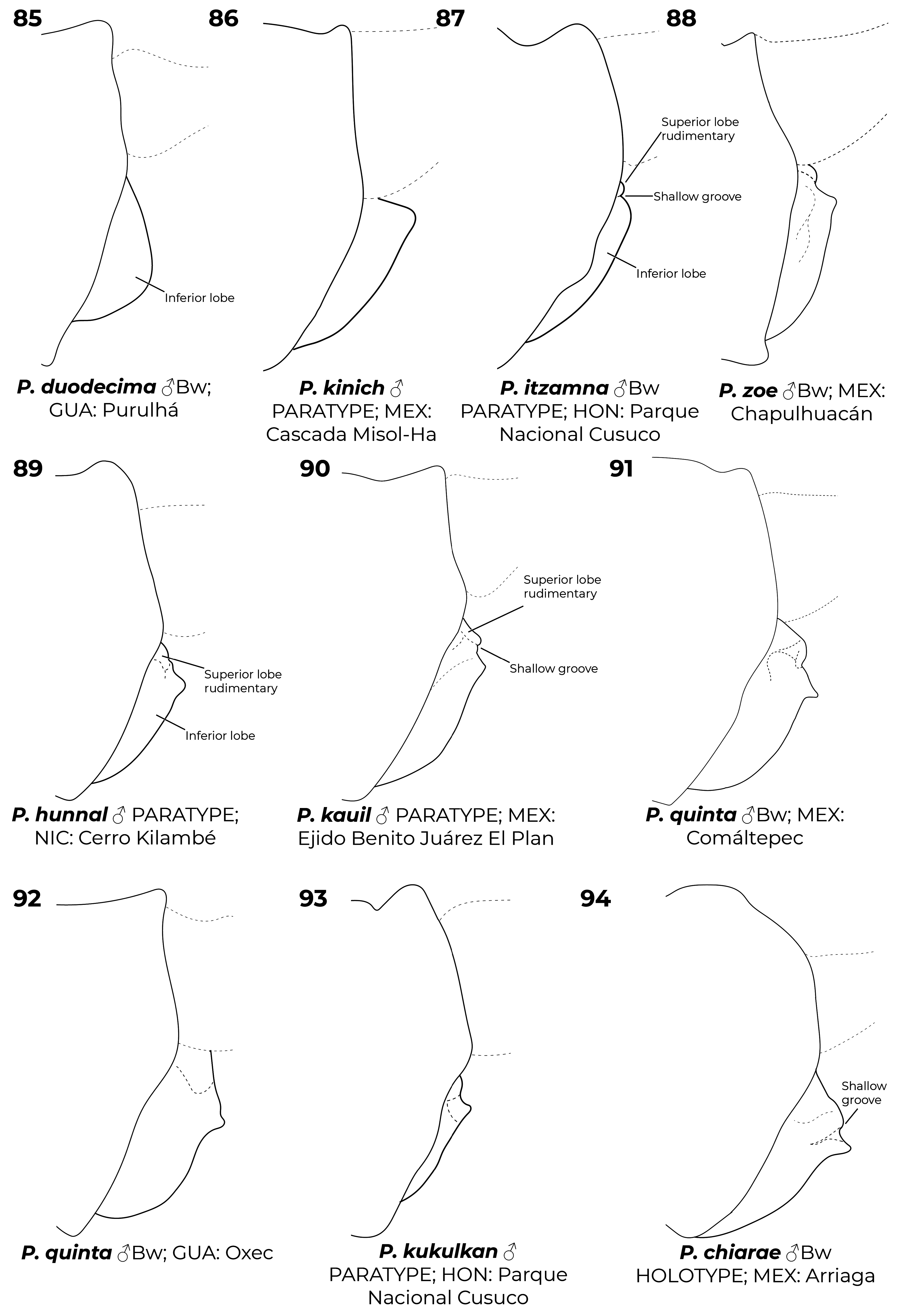
Diagnostic (modified from Garrison et al. 2010): Abdomen medium to large size ( 34.9–48.3 mm). Colouration highly variable: body dark brown to black with metallic green reflections with pale areas blue ( Figs. 6–7 View FIGURES 2–11 , 26 View FIGURES 20–27 , 152 View FIGURES 152–154 , 160–167 View FIGURES 158–160 View FIGURES 161–163 View FIGURES 164–166 View FIGURES 167–169 ), yellowish (e.g., Fig. 22 View FIGURES 20–27 ) or cream (e.g., postclypeus in Figs. 170–171 View FIGURES 170–171 ); in preserved specimens the blue colouration can fade and appear cream or yellowish ( Figs. 2–5 View FIGURES 2–11 , 15 View FIGURES 12–19 , 29 View FIGURES 28–34 ). Head: frons rounded, location of most posterior point of head at level of eyes. Thorax: prothorax posterior margin entire ( Fig. 110 View FIGURES 105–116 ), with a medial bump ( Fig. 108 View FIGURES 105–116 ) or deeply cleft ( Fig. 116 View FIGURES 105–116 ); with ( Figs. 107–108 View FIGURES 105–116 , 121–122 View FIGURES 117–128 ) or without ( Figs. 110–120 View FIGURES 105–116 View FIGURES 117–128 ) laterally or posterolaterally directed lobes, subquadrate ( Figs. 123 View FIGURES 117–128 ) or with two corniform dorsolateral projections ( Figs. 103 View FIGURES 95–104 , 105–106 View FIGURES 105–116 ); pterothorax second line complete ( Figs. 14–15, 19–20 View FIGURES 12–19 View FIGURES 20–27 ) or reduced ( Figs. 16, 18 View FIGURES 12–19 , 23 View FIGURES 20–27 ); thorax ( Fig. 18 View FIGURES 12–19 ) and dorsum of S9–10 and cerci can become pruinose in mature individuals ( Figs. 34–35 View FIGURES 28–34 View FIGURES 35–43 , 152–157 View FIGURES 152–154 View FIGURES 155–157 , 161 View FIGURES 161–163 , 164, 166–169 View FIGURES 164–166 View FIGURES 167–169 , 171 View FIGURES 170–171 ); female mesostigmal plate with rounded ( Fig. 138 View FIGURES 138–143 ) or angulated ( Fig. 139 View FIGURES 138–143 ) depressions mesad to mesostigmal lobe. Wings: hyaline, often with amber tint ( Figs. 38, 41–44, 46–48 View FIGURES 35–43 View FIGURES 44–51 , 52–53, 55 View FIGURES52–61 ), with the tip slightly smoky ( Fig. 158 View FIGURES 158–160 , 170 View FIGURES 170–171 ♀ –171), or with an apical black tip with metallic blue reflections ( Figs. 39–40 View FIGURES 35–43 , 45, 49–51 View FIGURES 44–51 , 54 View FIGURES52–61 , 153 View FIGURES 152–154 , 164– 167 View FIGURES 164–166 View FIGURES 167–169 , 170 View FIGURES 170–171 ♂) in P. zoe preceded by a milky-white patch ( Figs. 54 View FIGURES52–61 , 170 View FIGURES 170–171 ); no accessory crossveins basal to CuP, 1 or rarely 2 distal to it, shared with Thaumatoneura ; CuP closer to antenodal 1 than to 2; petiolation ending well beyond CuP for a distance as long as CuP or longer; vein descending from subnodus proximal to first post-quadrangular Vx to slightly distal to second post-quadrangular Vx; RP 3 slightly proximal to subnodus to slightly distal to subnodus; IR2 arising distal to subnodus; two or more supplementary sectors between IR1 and RP2, one supplementary sector between Rp2 and IR2 and two between IR2 and RP3, field between CuA and posterior margin with ( Figs. 57, 59 View FIGURES52–61 ) or without supplementary veins ( Figs. 56, 58, 60–61 View FIGURES52–61 ) and with one ( Fig. 60 View FIGURES52–61 ) to two ( Figs. 56, 58 View FIGURES52–61 ) supplementary sectors or lacking supplementary sector nor vein ( Fig. 61 View FIGURES52–61 ); pterostigma as long as four or more underlying cells, with proximal margin subequal to or slightly shorter than distal margin; legs with hind femora reaching mid-length of S2 or shorter. Abdomen: genital ligula lacking paired flagella on distal segment ( Fig. 1a, d View FIGURE 1 ); S1 dark ( Fig. 16 View FIGURES 12–19 ) or with lateral pale markings ( Figs. 35–37 View FIGURES 35–43 ); S2 with a pale ventrolateral horizontal lines ( Figs. 33–37 View FIGURES 28–34 View FIGURES 35–43 ); S3–7 with pale basal spots or rings ( Figs. 34–37 View FIGURES 28–34 View FIGURES 35–43 ); S8 black (figs 33–35) or with a pale basal spot ( Figs. 36–37 View FIGURES 35–43 ); S8–10 pruinose on dorsum in males ( Figs. 152–154 View FIGURES 152–154 ), the pruinescence can be absent in young individuals ( Fig. 168 View FIGURES 167–169 ), females with variable pale markings ( Figs. 129–137 View FIGURES 129–137 ); dorsum of male S10 approximately flat. Cerci ( Figs. 62–80 View FIGURES 62–66 View FIGURES 67–72 View FIGURES 73–78 View FIGURES 79–84 ): forcipate, in dorsal view the first third of their length straight and slightly laterad, then bending inwards; from 30-80% two medial flanges and on the distal 0.2–0.35 a distal lobe; mediodorsal flange can be well ( Figs. 62–72 View FIGURES 62–66 View FIGURES 67–72 ) or poorly developed ( Figs. 73–80 View FIGURES 73–78 View FIGURES 79–84 ) and in dorsal view its mesal margin smoothly curved ( Figs. 63–65 View FIGURES 62–66 , 70 View FIGURES 67–72 ), nearly straight ( Figs. 67–69, 71 View FIGURES 67–72 , 73–79 View FIGURES 73–78 View FIGURES 79–84 ), or convex ( Figs. 66 View FIGURES 62–66 , 72 View FIGURES 67–72 , 80 View FIGURES 79–84 ); distal margin of mediodorsal flange variously shaped and armed with a sclerotized tooth ( Figs. 67–71 View FIGURES 67–72 , 73–80 View FIGURES 73–78 View FIGURES 79–84 ), a blade ( Figs. 63, 65–66 View FIGURES 62–66 , 72 View FIGURES 67–72 ), or neither ( Fig. 64 View FIGURES 62–66 ); paraprocts rudimentary, with a single lobe ( Figs. 83–86 View FIGURES 79–84 View FIGURES 85–94 ), a poorly developed superior lobe delimited by a shallow transverse groove ( Figs. 87–94 View FIGURES 85–94 ), or well-developed superior and inferior branches ( Figs. 95–97 View FIGURES 95–104 ); females with ovipositor surpassing posterior margin of S10 and going beyond the posterior margin of the cerci ( Figs. 131–137 View FIGURES 129–137 ) or not ( Figs. 129–130 View FIGURES 129–137 ); valves of ovipositor as in Fig. 1d View FIGURE 1 .
Key to males
1. Mediodorsal flange of cerci well-developed ( Figs. 62–72 View FIGURES 62–66 View FIGURES 67–72 ), its maximum width at least 1.5 times width of distal lobe...... 2
1’. Mediodorsal flange of cerci poorly developed ( Figs. 73–80 View FIGURES 73–78 View FIGURES 79–84 ), its maximum width less than 1.5 times width of distal lobe … 10
2(1). FW with vein descending from subnodus always closer to first post-quadrangular Vx than to second ( Figs. 57–58 View FIGURES52–61 ); posterior margin of posterior lobe of prothorax semi-circular, at most with small medial convex section or notch and slight concavity towards lateral edges ( Figs. 110–120 View FIGURES 105–116 View FIGURES 117–128 )..................................................................... 3
2’. FW with vein descending from subnodus always closer to second post-quadrangular Vx than to first ( Figs. 42 View FIGURES 35–43 , 56 View FIGURES52–61 ); posterior margin of posterior lobe of prothorax concave towards lateral edges, seemingly trifoliate, clearly armed with digitiform projections ( Fig. 108 View FIGURES 105–116 )....................................................................... P. esperanza
3(2). In dorsal view width at base of distal lobe of cerci one-half or more of maximum width of mediodorsal flange Figs. 62–71 View FIGURES 62–66 View FIGURES 67–72 ). ................................................................................................... 4
3’. In dorsal view, base of distal lobe of cerci narrow, about one-third of maximum width of mediodorsal flange ( Fig. 72 View FIGURES 67–72 )................................................................................................... P. chaak
4(3). Mediodorsal flange of cerci nearly straight or smoothly curved, in dorsal view its widest point closer to tip than base ( Figs. 62–65 View FIGURES 62–66 , 67–70 View FIGURES 67–72 ); lateral edges of posterior lobe of prothorax variously shaped but never angled........................ 5
4’. Mediodorsal flange of cerci convex, in dorsal view its widest point not surpassing half its length ( Fig. 66 View FIGURES 62–66 ); lateral edges of posterior lobe of prothorax angulated ( Fig. 112 View FIGURES 105–116 ).................................................... P. kukulkan
5(4). Distal margin of mediodorsal flange of cerci blade-shaped and with sclerotized tip or tooth ( Figs. 63, 65 View FIGURES 62–66 , 67–70 View FIGURES 67–72 )......... 6
5’. Distal margin of mediodorsal flange of cerci smoothly rounded, never blade-shaped and with sclerotized tip or tooth ( Figs. 64 View FIGURES 62–66 )........................................................................................... P. akan
6(5). Paraprocts well-developed, clearly forked at apex, armed with well-defined upper and lower branches ( Fig. 81–82 View FIGURES 79–84 )................................................................................................... P. hyalina View in CoL
6’. Paraprocts rudimentary, never armed with well-defined upper and lower branches, at most with ill-defined superior lobe ( Figs. 85–94 View FIGURES 85–94 )............................................................................................. 7
7(6’). Posterior lobe of prothorax subequal or wider than middle lobe, lateral edges at same height or laterad to notopleural suture; lateral edges of posterior lobe of prothorax straight or forming acute angle at junction with middle lobe ( Fig. 109–111 View FIGURES 105–116 ); superior lobe of paraprocts rudimentary, only recognizable by a transverse groove ( Fig. 87–94 View FIGURES 85–94 ); in lateral view inferior lobe of paraprocts with an acute projection ( Fig. 88–94 View FIGURES 85–94 )..................................................................... 8
7’. Posterior lobe of prothorax narrower than middle lobe, lateral edges clearly mesad to notopleural suture; lateral edges of posterior lobe of prothorax forming obtuse angle at junction with middle lobe ( Fig. 119 View FIGURES 117–128 ); superior lobe of paraprocts completely absent ( Figs. 85–86 View FIGURES 85–94 ), inferior lobe in lateral view smoothly rounded ( Fig. 85 View FIGURES 85–94 )........................... P. duodecima View in CoL
8(7). Distal margin of mediodorsal flange of cerci armed with ventrally or postero-ventrally directed sclerotized tooth ( Fig. 67–68, 70 View FIGURES 67–72 )................................................................................................. 9
8’. Distal margin of mediodorsal flange of cerci blade-shaped with sclerotized tip, wider than long ( Fig. 65 View FIGURES 62–66 )....... P. chiarae
9(8). In dorsal view gap between distal tooth of mediodorsal flange of cerci and distal lobe broad, clearly larger than tooth ( Fig. 67–68 View FIGURES 67–72 )......................................................................................... P. zoe View in CoL
9’. In dorsal view gap between distal tooth of mediodorsal flange of cerci and distal lobe narrow and “U” shaped, subequal to size of the tooth ( Fig. 70 View FIGURES 67–72 )............................................................................ P. kauil
10(1’). In lateral view lateral edges of posterior lobe of prothorax erect, clearly extending dorsad ( Figs. 98–99, 103 View FIGURES 95–104 )........... 11
10’. In lateral view lateral edges of posterior lobe of prothorax not erect, extending caudad ( Figs. 100–102, 104 View FIGURES 95–104 )............ 13
11(10). Posterior margin of posterior lobe of prothorax armed with two laterodorsal corniform projections ( Figs. 105–106 View FIGURES 105–116 )...... 12
11’. Posterior margin of posterior lobe of prothorax concave towards lateral edges, seemingly trifoliate ( Fig. 107 View FIGURES 105–116 )..... P. hunnal
12(11). Posterior margin of posterior lobe of prothorax with lateral projections dorsad, in frontal view these projections higher than medial part of posterior margin; paraprocts well-developed, clearly forked at apex, with well-defined upper and lower branches ( Fig. 105 View FIGURES 105–116 )...................................................................................... P. flinti
12’. Posterior margin of posterior lobe of prothorax with lateral projections laterad, in frontal view these projections lower than medial part of posterior lobe; paraprocts rudimentary and never with well-defined upper and lower branches ( Fig. 106 View FIGURES 105–116 )................................................................................................. P. kinich
13(10). Mediodorsal flange of cerci nearly straight, in dorsal view its widest point closer to tip than base ( Figs. 73–78 View FIGURES 73–78 ); inferior lobe of paraprocts, in lateral view with an acute projection ( Fig. 92 View FIGURES 85–94 )............................................ P. quinta View in CoL
13’. Mediodorsal flange of cerci convex, in dorsal view its widest point not surpassing half its length ( Fig. 80 View FIGURES 79–84 ); inferior lobe of paraprocts in lateral view smoothly rounded ( Fig. 87 View FIGURES 85–94 )................................................ P. itzamna
Key to females
1. Ovipositor ending clearly beyond tip of cerci (without stylus) ( Figs. 131–137 View FIGURES 129–137 ).................................... 2
1’. Ovipositor short, at most reaching tip of cerci (without stylus) ( Figs. 129–130 View FIGURES 129–137 ).................................... 8
2(1). Lateral edges of posterior lobe of prothorax erect, extending dorsad in lateral view ( Fig. 99 View FIGURES 95–104 ).................. P. kinich
2’. Lateral edges of posterior lobe of prothorax not erect, extending caudad in lateral view ( Fig. 100 View FIGURES 95–104 )..................... 3
3(2). Posterior lobe of prothorax narrower than middle lobe, lateral edges mesad to notopleural suture ( Figs. 123–125 View FIGURES 117–128 )......... 4
3’. Posterior lobe of prothorax subequal to or wider than middle lobe, lateral edges at same height or laterad to notopleural suture ( Figs. 126–128 View FIGURES 117–128 )...................................................................................... 5
4(3). Posterior margin of posterior lobe of prothorax subquadrate or slightly concave towards lateral edges ( Figs. 123–124 View FIGURES 117–128 ).................................................................................................. P. quinta View in CoL
4’. Posterior margin of posterior lobe of prothorax rounded ( Fig. 125 View FIGURES 117–128 ).................................... P. duodecima View in CoL
5(3’). Mesostigmal plate depression rounded ( Fig. 138 View FIGURES 138–143 )................................................... P. itzamna
5’. Mesostigmal plate depression angulated ( Fig. 139 View FIGURES 138–143 ).......................................................... 6
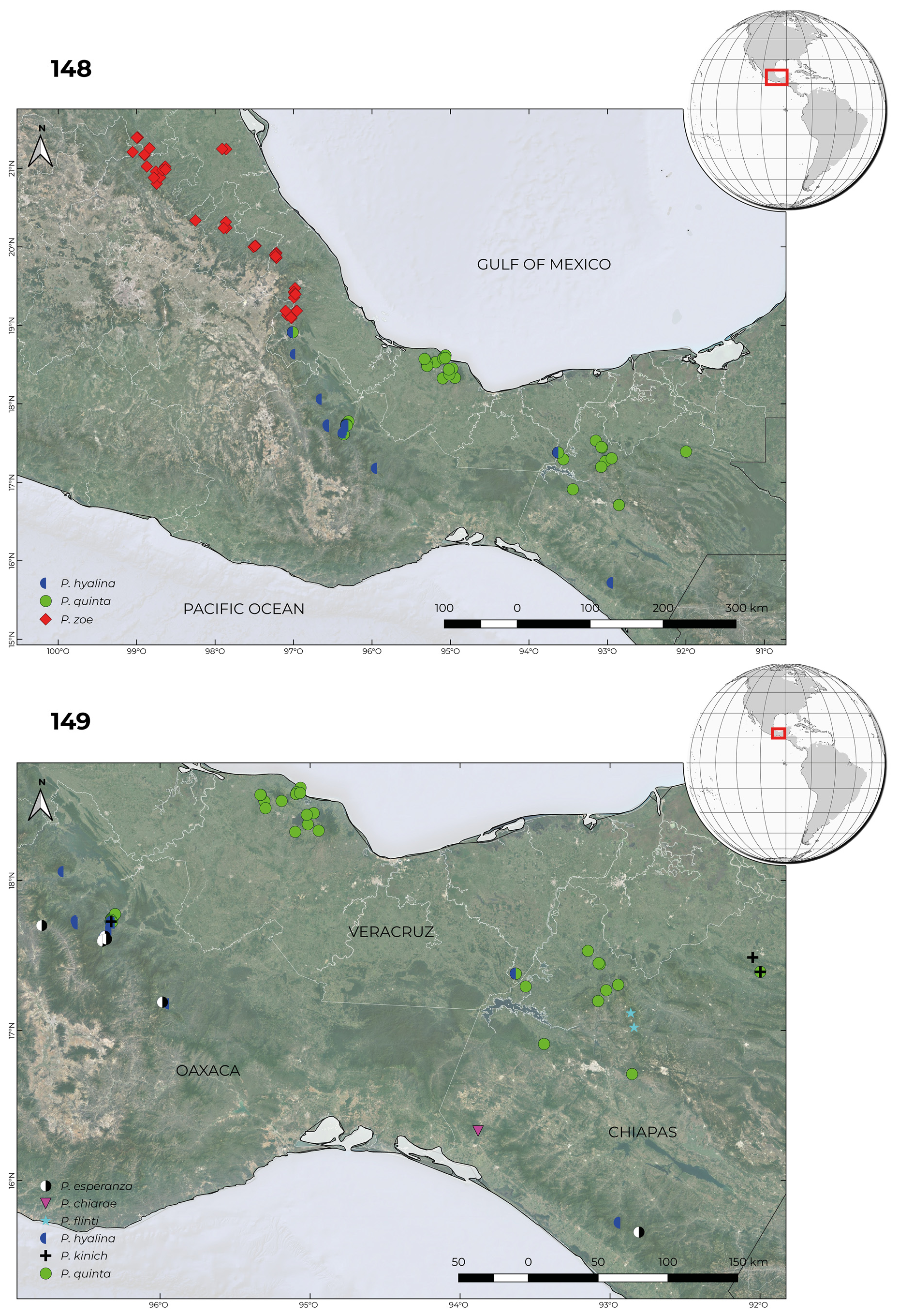
6(5). Metepimeron completely pale; Eastern Chiapas and Guatemala ( Fig. 150 View FIGURES 150–151 ).................................. P. kauil
6’. Metepimeron variously marked but never completely pale; Western Chiapas Sierra Madre and northwest to San Luis Potosí ( Fig. 148 View FIGURES 148–149 )........................................................................................... 7
7(6’) Postclypeus pale colouration cream ( Figs. 170–171 View FIGURES 170–171 ); HW field between CuA and posterior border usually with one extra sector ( Fig. 60 View FIGURES52–61 ); wings with smoky tip colouration usually clearly reaching distal border of Pt ( Figs. 170–171 View FIGURES 170–171 ); Mexico from Coscomatepec, Ver., north to Xilitla, SLP, including Puebla, Hidalgo and Querétaro states ( Fig. 148 View FIGURES 148–149 )............... P. zoe View in CoL
7’. Postclypeus pale colouration pale blue to turquoise ( Fig. 158 View FIGURES 158–160 ); HW field between CuA and posterior border usually without extra sectors ( Fig. 61 View FIGURES52–61 ); wings with or without smoky tip colouration but when present usually not reaching distal border of Pt ( Fig. 158 View FIGURES 158–160 ); Mexico from Metlac, Ver., south to El Triunfo, Chis., including Oaxaca and Tabasco states ( Fig. 148 View FIGURES 148–149 )..................................................................................................... P. hyalina View in CoL
8(1’). FW with vein descending from subnodus always closer to second post-quadrangular Vx than to first ( Fig. 56 View FIGURES52–61 ); in frontal view digitiform projections of posterior lobe of prothorax extending ventrad (as in Fig. 122 View FIGURES 117–128 ).................... P. esperanza
8’. FW with vein descending from subnodus always closer to first post-quadrangular Vx than to second ( Figs. 57–58 View FIGURES52–61 ); in frontal view posterior lobe of prothorax with digitiform projections extending slightly dorsad ( Fig. 121 View FIGURES 117–128 ) or rectangular without digitiform projections................................................................................. 9
9(8’). Posterior lobe concave with well-developed digitiform projections........................................ P. ixchel
9’. Posterior lobe rectangular without digitiform projections............................................. P. kukulkan
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Paraphlebia Selys in Hagen, 1861
| Ortega-Salas, Héctor, González-Soriano, Enrique & Jocque, Merlijn 2022 |
Paraphlebia
| Hamalainen, M. 2016: 38 |
| Cuevas-Yanez, K. & Rivas, M. & Munoz, J. & Cordoba-Aguilar, A. 2015: 517 |
| Dijkstra, K. - D. B. & Bechly, G. & Bybee, S. M. & Dow, R. A. & Dumont, H. J. & Fleck, G. & Garrison, R. W. & Hamalainen, M. & Kalkman, V. J. & Karube, H. & May, M. L. & Orr, A. G. & Paulson, D. R. & Rehn, A. C. & Theischinger, G. & Trueman, J. W. H. & van Tol, J. & von Ellenrieder, N. & Ware, J. L. 2013: 20 |
| Gonzalez-Soriano, E. & Paulson, D. R. 2011: 303 |
| Kalkman, V. J. & Choong, C. Y. & Orr, A. G. & Schutte, K. 2010: 123 |
| Novelo-Gutierrez, R. 2008: 29 |
| Gonzalez-Soriano, E. & Novelo-Gutierrez, R. 2007: 113 |
| Paulson, D. R. 1982: 251 |
| Beatty, G. H. & Beatty, A. F. 1968: 807 |
| Kennedy, C. H. 1925: 303 |
| Munz, P. A. 1919: 28 |
| Tillyard, R. J. 1917: 284 |
| Calvert, P. P. 1913: 260 |
| Calvert, P. P. 1908: 461 |
| Cockerell, T. D. A. 1908: 70 |
| Calvert, P. P. 1903: 133 |
| Calvert, P. P. 1902: 31 |
| Calvert, P. P. 1901: 59 |
| Higgins, H. T. 1901: 136 |
| Kirby, W. F. 1890: 122 |
| Selys, L. M. - E. de 1886: 33 |
| Scudder, S. H. 1882: 233 |
| Brauer, F. 1868: 361 |
| Selys, L. M. - E. de 1862: 8 |
| Selys, L. M. - E. de 1860: 435 |