Sciurus anomalus Güldenstädt, 1785
|
publication ID |
https://doi.org/10.1093/mspecies/sew004 |
|
publication LSID |
lsid:zoobank.org:pub:78EC2763-FA0D-45B6-B93A-03E6B29E3F79 |
|
DOI |
https://doi.org/10.5281/zenodo.4589243 |
|
persistent identifier |
https://treatment.plazi.org/id/0396FD65-3F21-FFD8-FF5A-FBFB5E04FD17 |
|
treatment provided by |
Felipe |
|
scientific name |
Sciurus anomalus Güldenstädt, 1785 |
| status |
|
Sciurus anomalus Güldenstädt, 1785 View in CoL
Caucasian Squirrel
Sciurus persicus Erxleben, 1777 . Mistaken application of name following Gmelin (1774:379 —see “Nomenclatural Notes”).
Sciurus anomalus: Güldenstädt, 1785:781 View in CoL . Type locality “ Georgien, Asien;” restricted to the “fortress of Sabeka on the River Sulori, about 25 kilometres S.W. of Kutais, Georgia,” by Chaworth-Musters (1937:560). First use of current name combination.
Sciurus caucasicus = caucasus Pallas, 1811:186. Type locality “frequens est in fagetis et quercetis subalpinis et montanis Caucasi.”
Sciurus syriacus Ehrenberg, 1828 :plate 8. Type locality “ Lebanon.”
Sciurus russatus Wagner, 1843:155 . Type locality “without indication of locality” Ognev 1966:368.
Macroxus syriacus var. pallescens Gray, 1867:285 . Type locality unknown but restricted to “Mountains of Kurdistan, northeast Iraq ” by Harrison (1972:398).
Sciurus historicus Gray, 1867:273 . Type locality “ Syria.”
Sciurus fulvus Blanford, 1875:311 . Type locality “Shiraz, Persia.”
CONTEXT AND CONTENT. Order Rodentia , suborder Sciuromorpha , family Sciuridae , subfamily Sciurinae , tribe Sciurini , subtribe Sciurina , genus Sciurus , subgenus Tenes
( Stroganova 1958; Hoffmann et al. 1993). Three subspecies of S. anomalus are recognized ( Ellerman 1948; Corbet 1978:78):
S. a. anomalus Güldenstädt, 1785:781 . See above (caucasus = caucasicus Pallas , russatus Wagner , and persicus Erxleben are synonyms).
S. a. pallescens Gray, 1867:285. See above ( fulvus Blanford is a synonym).
S. a. syriacus Ehrenberg, 1828 :plate 8. See above ( historicus Gray is a synonym).
NOMENCLATURAL NOTES. The name ‘ persicus ’ was mistakenly assigned by Erxleben (1777) to S. G. Gmelin’s (1774:379) description of Sciurus persicus , which was based on a dormouse, Glis glis (Thorington and Hoffmann 2005; Kryštufek 2010). The name has occasionally been applied to Sciurus anomalus since the initial error (e.g., Pavlinov et al. 1995). The authority is sometimes mistakenly listed as J. F. Gmelin (1788), which references a description of S. anomalus in the 13th edition of Systema Naturae; however, the publication date is actually 1788. The result is that Güldenstädt, 1785 becomes the appropriate author.
Sciurus is from the ancient Greek, skia meaning shadow or shade, and oura for tail ( Thorington et al. 2012); anomalus refers to being irregular or abnormal. Additional common names are golden squirrel ( Paz 1967), Transcaucasian squirrel ( Vereshchagin 1967), Persian squirrel, Georgian squirrel, tritina, tria, tsikvi, tsiku, abkhidkha, kovkasi, skyur, mkrosh, garmzysychan ( Ognev 1966), Syrian squirrel, suriye sincabi, dereek, dirik, kallay, kalle, sincab, sincap, teyin, gasgas ( Osborn 1964), galia ( Masseti 2012), sinjab ( Atallah 1977), and anadolu sincabi (Gavish and Gurnell 1999).
DIAGNOSIS
No naturally sympatric congeners of Sciurus anomalus occur. However, S. vulgaris (Eurasian red squirrel) was introduced into the northern slopes of the greater Caucasus from Altai in 1937 and is sympatric with S. a. anomalus in the southern and central Caucasus ( Stroganova 1958; Vereshchagin 1967). S. anomalus can be distinguished from S. vulgaris by a rustchestnut to buff-yellow, but not white, venter ( Lurz et al. 2005), less prominent (1.0– 1.2 cm) ear tufts (compared to 2.5–3.5 cm for S. vulgaris ) during winter in the northern areas of overlap, the presence of a plantar pad on the posterior soles of the hind feet, 4 (not 6) digital pads on the hind feet ( Wiltafsky 1978), 10 (not 8) mammae, the absence of a reduced P3, and a baculum that is distally curved ( Polyakova 1962; Ognev 1966; Corbet 1978:78; Albayrak and Arslan 2006). It has been suggested, based on a sample of nearly 200 S. anomalus specimens, that 5 digital pads are found on the hind feet and that only 8 mammae occur ( Stroganova 1958; Albayrak and Arslan 2006). Bones of extremities of S. anomalus are more massive and relatively shorter than those of S. vulgaris , which is likely a reflection of the less arboreal habits of S. anomalus ( Polyakova 1962) . Differences between the bones of girdles and extremities of S. anomalus relative to S. vulgaris have been described also ( Polyakova 1962). The scapular body of S. anomalus is more massive, humerus is relatively shorter with a wider head and a more developed lesser tuberosity and epicondyle, and the radius and ulna are shorter and wider with a greater separation between the 2 bones. The manus and pes are relatively shorter and wider, the pelvic girdle contains a widened ilium and an innominate bone that is longer and wider with more ridges and tuberosities, and the long bones of the hindlimbs (femur, tibia, and fibula) are relatively short but thickened ( Polyakova 1962).
Although some authors have questioned the validity of specific status for S. anomalus (Lowe and Gardiner 1983) , specific status has been supported based upon cranial morphology, pelage characters, dentition ( Ognev 1966; Corbet 1978), pectoral and pelvic girdle morphology, and extremity morphology ( Polyakova 1962; Hecht-Markou and Zafiratos 1990). Mitochondrial DNA evidence suggests a polyphyletic Old World origin for Sciurus that is ancient for 3 clades ( S. anomalus alone, the 2 other Old World species, S. lis, Japanese squirrel and S. vulgaris , and the New World Sciurus — Oshida et al. 2009).
GENERAL CHARACTERS
Sciurus anomalus ( Fig. 1 View Fig ) is a small tree squirrel with no obvious sexual dimorphism in size or coloration. Ranges (mm) of external measurements for adults of the 3 subspecies ( S. a. anomalus : n = 61; S. a. pallescens: n = 9; S. a. syriacus : n = 10— Osborn 1964; Ognev 1966; Ondrias 1966; Lewis et al. 1967; Harrison 1972) combined were: total length, 322–358 ( n = 10); length of tail, 130–180 ( n = 20); length of hind foot, 45.8– 60 ( n = 75); and length of ear, 23–31 ( n = 64). Adult body mass ranges 250–410 g ( Ondrias 1966; Hecht-Markou and Zafiratos 1990; Gavish 1993; Hecht-Markou 1994; Albayrak and Arslan 2006).
The dorsum is buffy chestnut-gray in S. a. anomalus to a pale grizzled gray in S. a. pallescens; countershading is evident with a light venter that varies from rust-chestnut to buff-yellow ( Ellerman 1948; Ognev 1966; Gavish 1993). The longest hair on the dorsum of 4 specimens from Iraq was 13 mm, and the maximum length of vibrissae was 38 mm ( Etemad 1978). A pale buff to yellow eye ring is evident ( Ognev 1966; von Lehmann 1966; Gavish 1993). The heavily furred tail ranges from a light yellowbrown in S. a. pallescens to a deep red in some specimens of S. a. anomalus ( Ellerman 1948; Harrison 1972). The dorsal part of the tail in S. anomalus is red and the ventral part is gray-yellow. The hair has 2 or more than 2 colors, usually black-yellow. Typically, tips of the hair are black. Widest part of tail is at base and narrower part at the rounded end (Hecht-Markou 1994). Authors disagree on extent of seasonal differences in pelage coloration. Summer pelage is not much different in coloration than winter pelage; however, venter may take on more of a rusty hue in winter (Vinogradov and Argiropulo 1941; Vinogradov and Gromov 1952; Osborn 1964; Kolosov et al. 1965; Ognev 1966). Dorsal hairs during winter are banded with deep gray near the follicle, with successive bands of black-chestnut, pale whitish buff, and a chestnut-black tip ( Ognev 1966). White may be evident on posterior dorsum ( Vinogradov et al. 1953). Ears in winter may have small ( 10–12 mm) terminal tufts ( Ognev 1966), which develop in interior part of ear (Hecht-Markou 1994) in northern parts of the distribution, and the soles of hind feet become covered sparsely with hair in the Caucasus ( Ognev 1966).
Skull ( Fig. 2 View Fig ) is relatively short but robust with broad and expanded zygomata (Yalçin and Arslan 2010). Braincase is broad, smooth, and depressed posteriorly, whereas rostrum is compressed laterally and frontal region is flattened ( Etemad 1978). Auditory bullae are moderately inflated. Means (mm; range in parenthesis) of cranial characteristics for 17 specimens ( Ognev 1966; Ondrias 1966; Lewis et al. 1967; Harrison 1972) were: condylobasal length, 45.6 (42.4–49.0); basal length, 41.3 (40.9–42.7); length of diastema, 12.8 (11.8–14.2); occipital breadth, 22.3 (21.6–23.1); zygomatic breadth, 28.2 (26.2–31.0); interorbital constriction, 15.6 (14.3–18.1); height of skull per bullae, 19.6 (18.1–21.2); palatine height, 11.9 (11.2–12.3); height of brain case, 17.3 (17.0–17.8); length of maxillary toothrow, 9.1 (8.3–9.5); and length of mandibular toothrow, 8.7 (8.1–9.1). A detailed series of 17 cranial and external measures from western and central Turkey and describing little sex dimorphism are available (Albayrak and Arslan 2006).
DISTRIBUTION
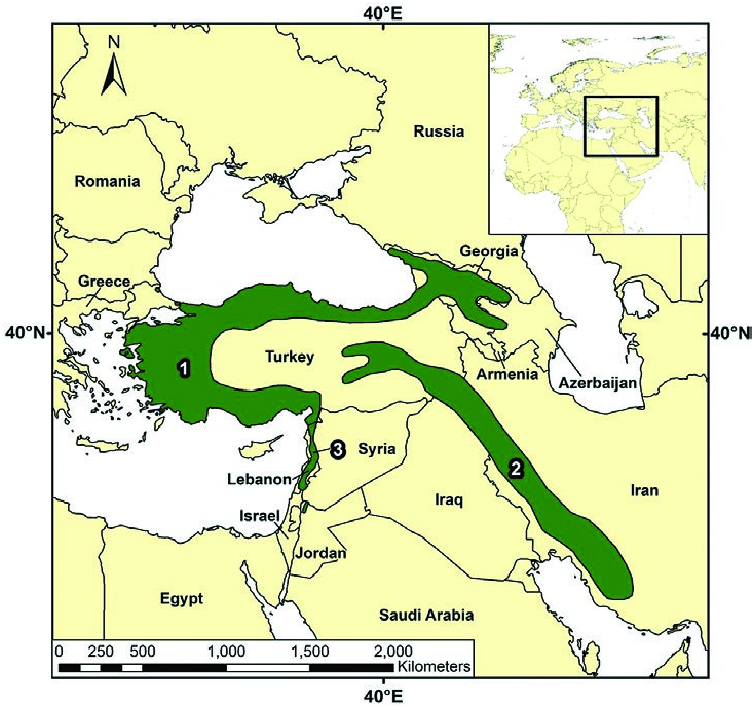
Sciurus anomalus is distributed in southwestern Asia ( Fig. 3 View Fig ). S. a. anomalus is found in Transcaucasia north to the southern slopes of the Caucasian Range to include Georgia, Armenia, and Azerbaijan ( Dahl 1954; Ognev 1966; Vereshchagin 1967; Pavlinov and Rossolimo 1987; Harrison and Bates 1991), nearly all of Turkey ( Misonne 1957; Osborn 1964; Alkan 1965; Yiğit et al. 2006) including the island of Imbros (Gavish and Gurnell 1999; Özkan 1999; Masseti 2005, 2010), the Greek island of Lesbos ( Ondrias 1966; Hecht- Markou 1994, 1999; Masseti 2005, 2010), and possibly northern Iraq ( Hatt 1959; Etemad 1978; Harrison and Bates 1991). S. a. syriacus is known from Lebanon, Syria, Israel, Jordan ( Tristram 1866; Abel 1933:222; Bodenheimer 1935; Lewis et al. 1967; Paz 1967; Ilani 1982, 1986; Ilani and Shalmon 1984; Harrison and Bates 1991), and potentially extreme southern Turkey ( Alkan 1965). S. a. pallescens is found in Iraq and the Zagros Mountains of western Iran and the Fars district of southern Iran ( Harrison 1956; Etemad 1978; Harrison and Bates 1991). Two individuals were released on the European side of the Bosporus ( Osborn 1964; Bertolino 2009); an unknown number of individuals from Syria were introduced into Jordan’s Dibbeen Nature Reserve ( Khoury et al. 2012).
FOSSIL RECORD
Fossils of the earliest Sciurus from the Miocene in Europe and North America are indistinguishable from the present-day genus (Emry and Thorington 1984). The M3 of S. anomalus was recovered at the Tourkobounia-1 locality and estimated to be from the early Villanyian (late Pliocene or early Pleistocene—De Bruijn and van der Meulen 1975) and was common in the middle Paleolithic in southern Turkey ( Demirel et al. 2011) and late Paleolithic in Lebanon ( Hooijer 1961; Kersten 1992). S. anomalus is known in the Levant from the late middle Pleistocene until the dawn of the Holocene ( Tchernov 1988, 1992, 1994, 1997). The species appears to have spread southward during the last postglacial period and subsequently retreated northward away from Israel (except for current relict populations—Gavish 1993) in the late Pleistocene (Boessneck and von den Driesch 1974). Fossils of S. anomalus have been reported at several excavation sites in the Mediterranean maquis and forests in Israel including Kebara at Mt. Carmel from 60,000 years ago, Qafzeh in Galilee from 92,000 years ago, and Oumm Qatafa in the Judean desert from 150,000 years ago (Belmaker and Hovers 2011; Maul et al. 2011). S. anomalus was a widely distributed mesophilous resident of the Transcaucasian region during the Pliocene ( Vereshchagin 1967) but was apparently absent from Anatolia at this time ( Sen 1977). A humerus of S. anomalus was collected in an archaeological excavation from the early Bronze Age at Demircihüyük in Turkey ( von den Driesch 1981).
FORM AND FUNCTION
Form. —Dental formula is i 1/1, c 0/0, p 1/1, m 3/3, total 20. Incisors are orthodont, recurved, compressed laterally, and with an anterior orange pigmentation (Harrison and Bates 1991). The formula for roots of cheek teeth are p2 3/2, m1 3/4, m2 3/4, m3 3/3; the 3rd molar can have a small, underdeveloped 4th root (Hecht-Markou 1994). Vertebral formula is 7 C, 12 T, 7 L, 3 S, and 25 Ca, total 54. The 5th cervical vertebra lacks the dorsal projection from the center of the vertebra. Three of the caudal vertebrae are fused in the adult. Eight of the 12 pairs of ribs are real and connected to sternum (Hecht-Markou 1994). Lengths of limb bones to total body length (given as % of total body length ± SE) for 7 specimens from Azerbaijan ( Polyakova 1962) were: forelimb (47.1 ± 0.55), hindlimb (69.5 ± 0.77), humerus (67.7 ± 0.25), radius (15.0 ± 0.42), manus (16.0 ± 0.22), femur (21.4 ± 0.23), tibia (23.1 ± 0.22), and pes (24.9 ± 0.32). Ratio of the length of forelimb to hindlimb is 0.68:1 ( Polyakova 1962).
Sciurus anomalus has 4 digits on the front feet and 5 on the hind feet (Hecht-Markou 1994; Albayrak and Arslan 2006). Soles of forefeet have 5 in Turkey and the Caucasus ( Ognev 1966; Albayrak and Arslan 2006), 6 ( Ondrias 1966) or 7 pads (Hecht-Markou 1994) in Greece; 5 or 6 pads are found on the plantar soles of the hind feet in Turkey (Albayrak and Arslan 2006). The dark brown claws are relatively short for a tree squirrel ( Ognev 1966), averaging 0.65 cm in length and 0.45 cm in width with slightly rounded tips (Hecht-Markou 1994).
Blood parameters (mean ± SD) for healthy S. anomalus ( 15 females, 15 males—Khazraiinia et al. 2008) were: red blood cell count, 7.18 ± 1.5 × 106 µl; packed cell volume, 46 ± 6%; hemoglobin concentration, 15.5 ± 1.5 g /dl; mean corpuscular volume 52.4 ± 8 fl; mean corpuscular hemoglobin, 22.5 ± 2.7 pg; mean corpuscular hemoglobin concentration, 33 ± 1.7%; white blood cell count, 4.35 ± 1.42 × 103 µl; neutrophil count, 2.4 ± 0.9 × 103 µl; lymphocyte count, 1.8 ± 0.8 × 103 µl; monocyte count, 0.37 ± 0.04 × 103 µl; eosinophil count, 0.37 ± 0.05 × 103 µl; basophil count, 0.37 ± 0.06 × 103 µl; platelet count, 433 ± 15 × 103 µl; and plasma total protein, 7.44 ± 0.46 g /dl. Two types of hemoglobin (Hb A, Hb F) are found in S. anomalus ( Khazraiinia et al. 2008) . Asadi et al. (2008) provide similar medians and ranges for these blood parameters and include additional blood chemistry (means with ranges in parentheses): creatinine, 388.3 µmol/l (356.8–419.9); uric acid, 0.07 mmol/l (0.06–0.08); cholesterol, 5.1 mmol/l (4.7–5.5); triglyceride, 4.21 mmol/l (4.11–4.42); calcium, 1.88 mmol/l (1.78–2.06); magnesium, 0.62 mmol/l (0.50–0.87); inorganic phosphate, 1.76 mmol/l (1.26–2.17); urea nitrogen, 5.58 mmol/l (4.54–7.28); glucose, 5.93 mmol/l (4.94– 6.92); alkaline phosphatase, 18 U/l (14–27); aspartate aminotransferase, 6 U/l (3–28); and amylase, 545.2 U/l (523.5–616.1).
Abdominal organs are covered by the greater omentum ( Sadeghinezhad et al. 2012). Abdominal gastrointestinal tract is 13.2 ± 4.5 SD cm long with a surface area of 617.9 ± 143.3 cm 2 and holds 8.7±9.2g in contents ( Sadeghinezhad et al. 2012). Stomach is unilocular and 4.9± 0.9cm in length. Small intestine is 71.3 ± 3.9cm long and has clear divisions into duodenum, jejunum, and ileum. The distribution pattern of neuronal cell bodies and fibers in the ileum are similar to other mammals ( Sadeghinezhad et al. 2013). The cecum is 3.7± 0.7cm long, situated on the right side of abdominal cavity, and lacks a vermiform appendix. The ascending colon (13.2± 4.5cm long) appears to be the main fermentation chamber and consists of a unique arrangement formed by 2 loops and 2 straight portions. The transverse colon (6.5± 1.5cm long) connects ascending and descending colon on the left side of abdominal cavity with a sigmoid flexure before entering the pelvic cavity ( Sadeghinezhad et al. 2012). Mucus content of small intestine includes both carboxylated and sulfated acidic mucins with few neutral mucins ( Tootian et al. 2013). Kidneys are bean shaped and average 1.7 by 0.8cm ( n = 6— Veshkini et al. 2011).
Phallus is 44 mm long (Albayrak and Arslan 2006); baculum has a wide, flattened, and rounded apex and is distally recurved on the dorsal aspect with a length of 10–15 mm; basal diameter with a mild bifurcation is 2.5–2.6 mm ( Stroganova 1958; Ognev 1966; Hecht-Markou 1999; Albayrak and Arslan 2006). Males that are not reproductively active have testicles that are 15 by 12 mm and retained within the abdomen, whereas the scrotal testes of reproductively active males reach sizes of 30 by 15 mm ( Stroganova 1958). Males with sperm had scrotal testes> 20 by 10 mm in size, enlarged epididymis, and well-developed Cowper’s glands (diameter = 10–15 mm—Stroganova 1958). Two prominent Cowper’s glands are located under the skin around the anus in males (Hecht-Markou 1994).
The os clitoridis is modestly twisted along the 3 mm length with a splayed distal end that possesses a slight tuberosity on the ventral side (Hecht-Markou 1999). Ten pairs of teats are arranged in 1 pectoral pair, 2 abdominal pairs, and 2 inguinal pairs in the female ( Ognev 1966); however, some females have only 8 pairs of teats ( Stroganova 1958; Hecht-Markou 1994; Albayrak and Arslan 2006).
Function. —Adult Sciurus anomalus molts twice each year; the spring molt begins in February through April and the autumn molt occurs in September and October ( Enukidze 1960). Molting is prolonged at elevations> 1,500 m and ceases during reproductive activity; juvenile squirrels molt once in autumn ( Enukidze 1960).
Testes of males demonstrate some annual cycle of recrudescence; however, some males appear to have functional testes that are descended in the scrotum throughout the year ( Stroganova 1958). For females during the breeding season, the vagina increases from 9 by 3 mm to 17 by 6 mm, the uterine horns extend from 35 by 2 mm to 80 by 5 mm, and ovary expands from 3 by 2 mm to 6 by 3 mm ( Stroganova 1958). Nursing females exhibit distended teats surrounded by a hairless ring at the end of the 5- to 6-week period of nursing ( Stroganova 1958) and teats darken following 1st lactation (Albayrak and Arslan 2006).
Vitamin A levels are highest in spring and decrease throughout the summer with adult levels greater than juvenile levels (Enukidze and Nikolaishvili 1980). Pregnant females with resorbed embryos had the highest levels of Vitamin A with reduced levels obvious in squirrels with helminth infections (Enukidze and Nikolaishvili 1980).
ONTOGENY AND REPRODUCTION
Some mortality occurs in utero, for the average number of placental scars is 4, whereas the average number of embryos is 3.5 for each female; embryos are distributed evenly between the uterine horns ( Stroganova 1958). A nearly fully developed fetus was 36 mm long ( Stroganova 1958). Young can mature at 5–6 months of age; females born in winter or early spring can breed in late summer or early autumn ( Stroganova 1958; Kolosov et al. 1965). Tooth wear and sagittal crest increase noticeably between juvenile, subadult, and adult age classes (Albayrak and Arslan 2006).
Breeding can occur throughout the year, although the intensity appears to decline during October and November in the Caucasus ( Stroganova 1958) and peaks inApril to May andAugust to September in Greece (Hecht-Markou 1994). In Transcaucasia, 2–7 young are born, most frequently in late January–early February, April, and mid-July–late August ( Stroganova 1958; Kolosov et al. 1965; Ognev 1966; Hecht-Markou 1994). Young were reported during late April and May in Lebanon and immature squirrels were collected during November and December ( Hatt 1959; Lewis et al. 1967; Harrison and Bates 1991) leading Harrison (1972) to conclude that the breeding season extends from spring into the summer in the Middle East. Females alone care for the young (Hecht-Markou 1994) and become aggressive in maternal defense, to include attacking threats to the litter with claws and teeth (Hecht-Markou 1994).
ECOLOGY
Population characteristics. —Population numbers of Sciurus anomalus can fluctuate considerably, presumably due to variation in food availability ( Kolosov et al. 1965; Vereshchagin 1967); however, numbers appear more stable than those of S. vulgaris ( Stroganova 1958) . Stroganova (1958) reported sighting 0.75–1.25 individuals/km of census transect in a nut orchard, whereas only 0.11 and 0.17 individuals/km were observed in mixed forest. Density was estimated at 1.04– 1.82 individuals/ha in oak-pine mixed forests in Lebanon (Abi- Said et al. 2014).
Habitat loss due to incompatible agriculture, fire, and timber harvest has resulted in population declines in the Caucasus ( Vereshchagin 1967), Jordan ( Amr et al. 2006), and Iraq ( Hatt 1959). Habitat fragmentation is the major threat to the species on Lesbos (Matsinos and Papadopoulou 2004). S. anomalus was detected in an unburned pine forest but not in forest burned 9 years earlier ( Soyumert et al. 2010). Drought also appears to result in population decline ( Amr et al. 2006). Accidental mortality results from individuals entering water tanks from which they cannot escape ( Amr et al. 2006). S. anomalus becomes habituated to vehicle traffic and noise ( Amr et al. 2006). Wild boar ( Sus scrofa), edible dormouse ( Glis glis ), and a variety of birds pilfer winter seed stores of S. anomalus ( Kolosov et al. 1965) . Eurasian lynx ( Lynx lynx ), wildcat ( Felis silvestris ), domestic cat ( Felis silvestris catus ), red fox ( Vulpes vulpes ), gray wolf ( Canis lupus ), European badger ( Meles meles ), beech marten ( Martes foina), European pine marten ( M. martes ), least weasel ( Mustela nivalis ), northern goshawk ( Accipter gentilis ), Eurasian sparrowhawk ( A. nisus ), common buzzard ( Buteo buteo ), longlegged buzzard ( B. rufinus ), red kite ( Milvus milvus ), black kite ( M. migrans ), Eurasian eagle-owl ( Bubo bubo ), and tawny owls ( Stix aluco) are known to prey upon squirrels ( Stroganova 1958; Kolosov et al. 1965; Heptner and Sludskii 1992; De Cupere et al. 2008).
Space use. —In Israel, Sciurus anomalus prefers forest dominated by oaks ( Quercus calliprinos , Q. boissieri ) and pistachio ( Pistacia pataestina —Danford and Alston 1877; Ilani and Shalmon 1984; Gavish 1993) and was abundant in the last century in the wooded parts of the Upper Galilee and the Golan Heights ( Bodenheimer 1935). In Jordan, pure pine forests are preferred over mixed pine-oak ( Pinus-Quercus — Amr et al. 2006). Throughout Lebanon and the Zagros Mountains in Iraq and Iran, open oak forests are also important squirrel habitat ( Hatt 1959; Lewis et al. 1967; Atallah 1977). In Turkey, S. anomalus is found frequently in orchards and forests that are dominated by oak ( Quercus trojana ), juniper ( Juniperus excelsa ), walnut ( Juglans regia ), pine ( Pinus nigra ), willow ( Salix alba ), apples, pears, plums ( Pyrus elaeagnifolia , P. domestica , P. syria , P. malus ), hawthorn ( Crateagus aronia, C. monogyna ), and rose ( Rosa canina — Hoogstraal 1959; Osborn 1964; Albayrak and Arslan 2006). Beech ( Fagus ) forests ≤ 2,000 m in elevation are commonly inhabited in Caucasia but S. anomalus is also found in elm ( Ulmus ), chestnut ( Castanea ), maple ( Acer insigne ), ash ( Fraxinus ), cherry ( Prunus ), pear ( Caucasica ), poplar ( Populus ), alder ( Alnus ), and linden ( Tilia ), whereas pine forests appear to be avoided ( Dahl 1954; Stroganova 1958; Enukidze 1960; Bobrinskii et al. 1965; Kolosov et al. 1965; Ognev 1966). S. anomalus in Caucasia appears restricted to subtropical forest areas and fruit and nut plantations in elevations where snow is uncommon ( Stroganova 1958). On the Greek island of Lesbos, squirrels dwell in lower elevation chestnut ( Castanea sativa ) plantations, olives ( Olea europea ), walnut ( J. regia ), almonds ( Prunus dulcis ), pears ( Pyrus amygdaliformis ) as well as plantations and mixed natural forests of oak ( Q. aegilops , Q. coccifera ) and conifers ( Cupressus sempervirens , P. brutia , P. nigra —Hecht-Markou 1994) upward to the coniferous forest of Mt. Lepetymnos (1,009 m—Hecht-Markou 1994).
Sciurus anomalus will migrate from one habitat to another dependent on the seasonal availability of food (Hecht-Markou 1994). Based upon the frequent resighting of squirrels in the same areas, S. anomalus probably demonstrates strong site fidelity; however, migrations are reported in response to food shortages ( Stroganova 1958).
Sciurus anomalus nests in tree hollows lined with moss and dry leaves ( Stroganova 1958; Enukidze 1960; Ognev 1966; Gavish 1993) and may do so in groups ( Stroganova 1958; Hatt 1959). In Turkey, nests occur in oak ( Q. trojana ), walnut ( J. regia ), juniper ( J. excelsa ), and willow ( S. alba ) cavities (Albayrak and Arslan 2006). Nest cavities in trees are shallow extending to depths of 20–50 cm (Hecht-Markou 1994) and entrances, facing south and southeast ( Enukidze 1960), are located at heights of 5– 14 m. However, nests may also occur under rocks, tree roots, gravestones, and buildings ( Stroganova 1958; Kolosov et al. 1965). S. anomalus changes nests between seasons to eliminate ectoparasites, but it will reuse the nests in subsequent years (Hecht-Markou 1994).
Diet. —Foods are most abundant in spring and summer, with shortages common in winter ( Stroganova 1958). Tree seeds are the primary food; oaks (including Q. calliprinos , Q. infecloria , Q. cerris ) are major food sources throughout the range of Sciurus anomalus ( Stroganova 1958; Ognev 1966; Lewis et al. 1967; Gavish 1993). Fir seeds (species not noted) are taken in Turkey ( Osborn 1964), Persian walnuts ( Julans regia ), chestnuts ( Castanea ), hazelnuts ( Corylus avellana ), beechnuts ( Fagus ), and apricot seeds ( Pruna) are eaten in Caucasia ( Vinogradov et al. 1953; Ognev 1966). Pine ( Pinus pinea , P. brutia ) and cedar ( Cedrus lebani ) seeds are favorite foods in Lebanon ( Lewis et al. 1967; Abi-Said et al. 2014). Nine larders contained 7–62 nuts about 50% of which were chestnuts, 35% walnuts, and 15% hazelnuts ( Stroganova 1958; Kolosov et al. 1965). Buds and other vegetation ( Lewis et al. 1967), a variety of berries including Smilax excelsa ( Stroganova 1958; Ognev 1966), Cornelian cherries ( Stroganova 1958), the seeds and pulp of commercial fruits such as apples, pears, peaches, grapes, and plums ( Stroganova 1958), cereal grains ( Aegilops peregina), and mushrooms ( Polyporus sulphureus — Stroganova 1958; Kolosov et al. 1965; Gavish 1993) can be significant components of the diets of S. anomalus . Insects ( Stroganova 1958) and bird eggs and nestlings ( Ognev 1966; Etemad 1978) also are eaten.
Diseases and parasites. —Leptospirosis antibodies were reported for 6 of 57 Sciurus anomalus in Caucasia; antibodies for the following serological groups were identified: L. pyrogenes , L. autumnalis , L. australis , and L. bataviae ( Kakabadze et al. 1978) . Leishmania infantum was not detected in 3 individuals examined in Iran ( Fallah et al. 2006). Endoparasites identified include Cateaotaenia pusilla ( Enukidze 1960) , Taenia martis , Echinococcus granulosus (Enukidze and Nikolaishvili 1980) , Eimeria serbica ( Couch et al. 2005) , Syphacia , and Ascaris ( Enukidze 1960) . The ectoparasites, Ixodes ricinus (Acari— Enukidze 1960; Anderson and Magnarelli 1993), I. redikorzovi (Acari) , Ceratophyllus sciurorum (Siphonaptera—Stroganova 1958; Albayrak and Arslan 2006), C. cusimilis (Siphonaptera— Enukidze 1960), Enderleinellus krochinae (Anoplura—Durden and Musser 1994) , Enderleinellus nitzschi (Anoplura—Durden and Musser 1994) , Neohaematopinus syriacus (Anoplura— Durden and Musser 1994) are known to infest S. anomalus . A rich fungal community exists in the pelage. Dermatophytes most frequently isolated from skin lesions were Microsporum canis (3.3%), M. gypseum (3.3–9.5%), M. persicolor (3.3%), and Trichophyton mentagrophytes (3.3–14.3%). All samples from skin lesions and pelage showed the growth of saprophytes to include Mucor (38.3%), Penicillium (28.3%), Aspergillus (26.7%), Alternaria (23.3%), and Fusarium (20%). Malassezia , Rhodotorula , and Candida species were the most important yeasts isolated (Khosravi and Mahmoudi 2003; Rostami et al. 2010). Alopecia in a yearling female from Iran was attributed to infection by the dermatophyte Epidermophyton floccosum ( Hosseininejad et al. 2010) .
Pelts are harvested in significant numbers in Caucasia ( Ognev 1966; Vereshchagin 1967) and are used for fur clothing and ornamentation ( Dahl 1954). However, Kolosov et al. (1965) considered the number of pelts to be commercially insignificant relative to S. vulgaris , likely because the quality of S. anomalus pelts is considerably less than that of the S. vulgaris (Vinogradov and Gromov 1952; Enukidze 1960; Bobrinskii et al. 1965). Recent harvest rates are not available. Carcasses of S. anomalus are used to feed other domestic animals ( Stroganova 1958). S. anomalus is consumed by humans in northeastern Iraq ( Harrison 1972) and Jordan (Mendelsshon and Yom-Tov 1999). Young squirrels are sold as pets in Turkey ( Osborn 1964), Iran ( Rostami et al. 2010), and Jordan (Mendelsshon and Yom-Tov 1999). S. anomalus is often fed in the wild to increase viewing opportunities in Turkey ( Masseti 2012). It is considered a pest in some regions of Caucasia where it invades nut and fruit orchards ( Vereshchagin 1967).
Miscellaneous. —Surveys for discarded pine cones that have had seeds removed can provide information on occupancy by Sciurus anomalus ( Amr et al. 2006) . S. anomalus was the least common of 9 mammals trapped in the Zagros Mountains of Iran (1.2% of 198 individuals—Shayan et al. 2008); however, the species can be livetrapped successfully ( Gavish 1993). Camera traps were used to detect S. anomalus ( Soyumert et al. 2010; Abi-Said et al. 2014; İlemin 2014). Individuals have been maintained in captivity for> 2 weeks and can be sedated using a xylazine/diazepam intramuscular cocktail (xylazine 5 mg /kg, diazepam 30 mg /kg—Veshkini et al. 2011). S. anomalus is displayed in Iran’s Tehran Zoo ( Asadi et al. 2008).
BEHAVIOR
Although Sciurus anomalus is most often solitary, groups of
≤ 9 individuals can feed in seed-laden trees ( Amr et al. 2006). The vocalization is a high-pitched, metallic sounding “chit-chitchit” ( Ognev 1966) and is used in response to the presence of a potential predator ( Kolosov et al. 1965). The call resembles that of the green woodpecker ( Picus viridis — Stroganova 1958). Calling is rare in spring but during other seasons can serve as an index to squirrel abundance ( Stroganova 1958). Individuals mark their home ranges with urine and feces (Hecht-Markou 1994, 1999).
Sciurus anomalus is diurnal ( Ognev 1966; Harrison and Bates 1991) and active year-round with a morning activity period from sunrise to 1100 h (Abi-Said et al. 2014), and often a midday lull, and evening activity lasting until about 1800 h in Israel ( Gavish 1993) and Jordan ( Amr et al. 2006), extending to 1900 h in Greece (Hecht-Markou 1994). During winter, activity peaks during midday ( Enukidze 1960). Activity is reduced in cloudy and rainy weather ( Stroganova 1958). Juveniles may remain inactive during cold periods (Hecht- Markou 1994).
Sciurus anomalus forages on the ground as well as in trees. Many nuts are harvested from trees and eaten prior to falling ( Stroganova 1958). S. anomalus appears to larderhoard seeds within cavities or clefts in trees ( Hatt 1959; Ognev 1966), in middens ( Osborn 1964), or in abandoned stone buildings ( Stroganova 1958). One larder was located> 300 m from the nearest nut tree ( Stroganova 1958). Larders may contain 5–6 kg of seeds ( Enukidze 1960). Conversely, S. anomalus in Lesbos caches food in the leaf litter and loose soil. The cached food is pressed into the ground with 5–10 repeating movements of the head and the food is covered with earth or leaves using the forelegs and paws (Hecht-Markou 1994). Nuts are held in the paws and the lower incisors are used to gnaw and enlarge a hole in the shell; the nut meat is retrieved by the lower incisors ( Stroganova 1958; Enukidze 1960).
Sciurus anomalus is a highly terrestrial relative to S. vulgaris ( Kolosov et al. 1965) but does spend time in trees (Harrison and Bates 1991; Gavish 1993), although leaps between trees are rare ( Stroganova 1958; Kolosov et al. 1965). Although an excellent swimmer, S. anomalus avoids water ( Ognev 1966).
Major postures ( Gavish 1993) include an attentive (vigilant) posture in which the individual sits or stands erect with forepaws against chest, sitting in the crotch of a tree with body against the trunk while eating with feet drawn forward for support, and crouching on a branch with head and tail rested upon limbs during rest or sunbathing. S. anomalus often forages in trees in an inverted position and can descend trees head first with extreme rotation of the crurotalar joint ( Fig. 4 View Fig ). Another characteristic posture is crawling on its abdomen with legs spread (Hecht- Markou 1994). Tail may be held above head with guard hairs erect and sometimes curved upward away from the posterior dorsum. The tail can be held folded on the back or stretched as far as the ears. The tail lies flat when resting and drags behind when running.
Sciurus anomalus runs to the nearest tree and escapes through the canopy when threatened. Autogrooming is accomplished with the mouth; 2 instances of face-wiping against branches forested areas in Turkey (Yiğit and Çolak 1998; De Marinis and Maseti 2009); however, habitat degradation and fragmentation are a concern (Albayrak and Arslan 2006) and the species is considered uncommon in the Artvin Province of northern Turkey ( Gokturk et al. 2011). S. anomalus is locally common in protected areas in Azerbaijan ( Guliyev 2014). Population decline is noted in many peripheral areas of the species range, dangerously so in Lebanon and Syria, where hunting pressure and habitat loss continue ( Lewis et al. 1967; Yiğit et al. 2008). S. anomalus syriacus is considered endangered in Jordan ( Amr 2000). It is also protected due to imperiled status on Lesbos (Karandinos and Legakis 1992). Climate change has the potential to negatively impact 98% of the habitat of S. anomalus through the 21st century ( Harrison et al. 2006).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Sciurus anomalus Güldenstädt, 1785
| Koprowski, John L., Gavish, Leah & Doumas, Sandra L. 2016 |
Sciurus fulvus
| Blanford 1875: 311 |
Macroxus syriacus var. pallescens
| Gray 1867: 285 |
Sciurus historicus
| Gray 1867: 273 |
Sciurus russatus
| Wagner 1843: 155 |
Sciurus syriacus
| Ehrenberg 1828 |
Sciurus caucasicus
| Pallas 1811 |
Sciurus anomalus : Güldenstädt, 1785:781
| : Guldenstadt 1785: 781 |
Sciurus persicus
| Erxleben 1777 |