Caulophacus (Caulophacus) chilense, Reiswig, Henry M. & Araya, Juan Francisco, 2014
|
publication ID |
https://doi.org/10.11646/zootaxa.3889.3.4 |
|
publication LSID |
lsid:zoobank.org:pub:EB84D779-C330-4B93-BE69-47D8CEBE312F |
|
DOI |
https://doi.org/10.5281/zenodo.5685621 |
|
persistent identifier |
https://treatment.plazi.org/id/039387AB-8B4F-FFA0-58BF-FE58FE987047 |
|
treatment provided by |
Plazi |
|
scientific name |
Caulophacus (Caulophacus) chilense |
| status |
sp. nov. |
Caulophacus (Caulophacus) chilense View in CoL sp. n.
( Figs. 1 View FIGURE 1 & 2 View FIGURE 2 , Table 1 View TABLE 1 )
Material examined. Holotype: MNHNCL-POR100, FV Juan Antonio, 0 7 January 2014, about 50 km NO of Caldera, Atacama Region, Chile. 26°44’00” S; 71°07’00” W, 1300–1800 m, dry.
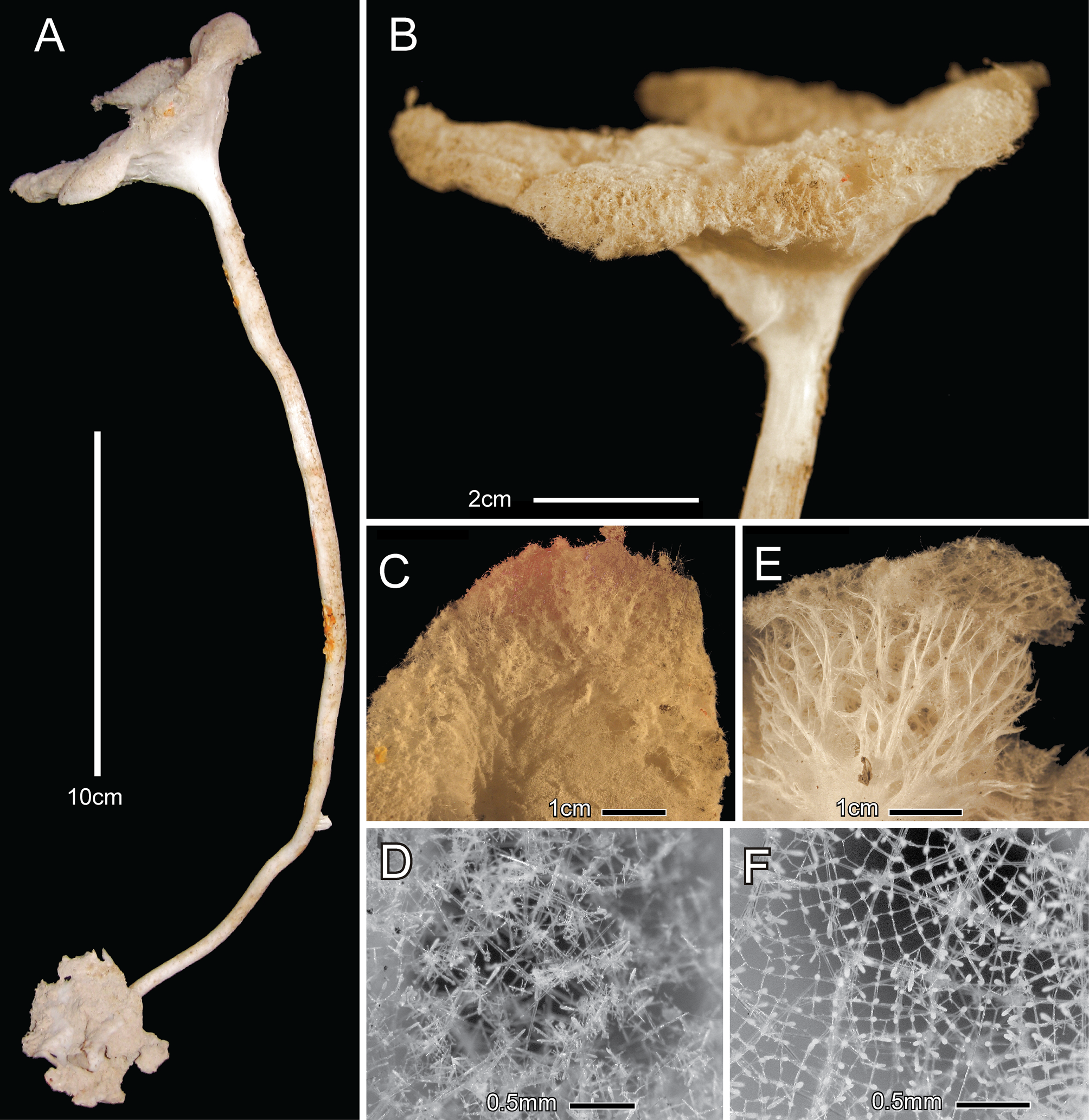
Description. The holotype ( Fig. 1 View FIGURE 1 A) is 320 mm in total length composed of an intact basal plate, 30 x 51 mm in widths, which was attached to hard substratum, a thin hollow curved stem 277 mm long, surmounted by a body 28 mm in height. The stem varies in diameter from 5.0 mm near the basal attachment to 8.4 mm just below the body; the lumen at the broken point 9 cm above the base is 2.0 mm in diameter and the wall is 1.5 mm in thickness. Loose spicules are absent from a few mm below the body attachment to its base. The body ( Fig. 1 View FIGURE 1 B) is a shallow funnel, 81 x 65 mm in diameters with a central depression of 10.4 mm. It is two mm thick at its rounded margin and 20 mm thick at the center. The upper (atrial) body surface ( Fig. 1 View FIGURE 1 C) is relatively smooth, covered by a very fine, inconspicuous, felt-like lattice of atrial spicules ( Fig. 1 View FIGURE 1 D). The lateral (dermal) surface ( Fig. 1 View FIGURE 1 E) is more transparent, with a conspicuous network of white strands of upward radiating choanosomal diactins surrounding large inhalant canals seen through the surface lattice ( Fig. 1 View FIGURE 1 E). The overlying lattice of dermalia and hypodermalia ( Fig. 1 View FIGURE 1 F) is thin and quite regular in arrangement. A marginal fringe is not evident at the junction of dermal and atrial surfaces. Color of the dry specimen is very light tan.
Megascleres are dermal pinular hexactins and rare pentactins, atrial pinular hexactins and rare pentactins, choanosomal and stalk diactins, hypodermal and hypoatrial pentactins, and choanosomal hexactins. Spicule dimensions are given in Table 1 View TABLE 1 . Dermalia are pinular hexactins and rare pentactins ( Fig. 2 View FIGURE 2 A) with bushy spindleshaped distal ray and entirely rough tangential and proximal rays. The pinular ray is quite variable in shape with a few ovoid in profile. Maximum thickness occurs at 65% of the distance from the axial cross. About 4% of these are pentactins. Atrialia ( Fig. 2 View FIGURE 2 B) are similar but slightly smaller than dermalia in mean dimensions and especially in maximum width of the pinular ray. The pinular rays of both dermalia and atrialia lack apical spines, their tips being gently rounded in profile. Choanosomal diactins of the body ( Fig. 2 View FIGURE 2 C) are slightly curved and have rounded, occasionally inflated, rough terminal ends with smooth caps. The central swelling is generally inconspicuous. The diactins of the stalk are fused by synapticula to form a rigid framework; they are larger than the free body diactins but otherwise similar. Hypodermalia ( Fig. 2 View FIGURE 2 D) are large regular oxypentactins, some of which bear macrospines near the base of their tapered rays: of 42 spicules examined, 45% were entirely smooth, 19% bore such spines only on proximal rays and 36% had spines on both tangential and proximal rays. Rays end with subterminal roughness behind a smooth rounded cap. Hypoatrialia ( Fig. 2 View FIGURE 2 E) are much smaller, mean tangential rays are 62% of those of dermalia. These spicules have macrospines on all tangential and proximal rays. Tangential ray ends are sharply pointed and rough. Choanosomal hexactins ( Fig. 2 View FIGURE 2 F) are larger than the hypodermalia and hypoatrialia. Their rays generally all bear macrospines (96% of 168) but a few have them restricted to one short ray (1%) and some bear no macrospines at all (2%). Ray tips are rough and abruptly pointed.
Microscleres are discohexactins (91% of 200), hemidiscohexasters (5.5%) and two forms of discohexasters (together 3.5%). The discohexactins ( Fig. 2 View FIGURE 2 G) are generally regular with thorned rays ending in large semianchorate tips with 5–6 marginal claws. Occasionally one ray is bent back and very rarely the terminal disc is reduced to a single pointed hook. The hemidiscohexasters ( Fig. 2 View FIGURE 2 H) are similar to the discohexactins but one or more rarely two or three rays are divided near their origin into two terminal rays. Discohexasters A ( Fig. 2 View FIGURE 2 I) are as robust as the discohexactins but rare (0.2%); each primary ray is divided near its origin into two or three terminal rays. Discohexasters B ( Fig. 2 View FIGURE 2 G) are much smaller and thinner than all other microscleres, are rare (3%) and are restricted to the atrial side of the body. They have short primary rays divided into 2–5 straight terminal rays ending in small discs with 4–6 marginal claws. This spicule was not located in SEM preparations hence a light micrograph ( Fig. 2 View FIGURE 2 J) is used to document their occurrence and form.
Etymology. The species name, chilense , refers to the location of collection: Chile.
Remarks. The new specimen described here lacks onychoidal and oxyoidal microscleres and thus is excluded from the subgenera Caulodiscus Ijima 1927 , Oxydiscus Janussen et al. 2004 and Caulophacella Lendenfeld 1915 ; it is assignable only to subgenus Caulophacus Schulze 1885 . Within the subgenus, it cannot be assigned to C. antarcticus Schulze and Kirkpatrick 1910 , C. basispinosus Lévi 1964 , or C. galathea Lévi 1964 since these three species lack discohexasters. It is excluded from 13 species which possess lophodiscohexasters characterized by having four or more terminal rays on each primary ray: C. arcticus (Hansen, 1885) , C. cyanae Boury-Esnault and De Vos 1988 , C. discohexactinus Janussen et al.2004 , C, discohexaster Tabachnick and Lévi 2004, C. elegans Schulze 1885 , C. hadalis Lévi 1964 , C. instabilis Topsent 1910 , C. latus Schulze 1886 , C. oviformis Schulze 1886 , C. pipetta ( Schulze 1886) , C. schulzei Wilson 1904 , C. scotiae Topsent 1910 and C. variens Tabachnick 1988 . Since the discohexactins of the new form are considerably less than 200 µm, it cannot be a member of the two species C. adakensis Reiswig and Stone 2013 or C. agassizi Schulze 1899 . Finally, it is separated from C. abyssalis Tabachnick 1990 by the pinular rays of dermalia differing drastically in shape in the two forms and having a single length range instead of two size classes of those rays (126–299 µm in the new form vs 100–180 µm and 320–400 µm in C. abyssalis ). Based upon these and many other differences from the presently recognized members of the subgenus, it is clear that the form described here is a new species designated here as Caulophacus (Caulophacus) chilense sp. n.
Associated fauna only include the verrucid cirripedian Gibbosaverruca sp., with three specimens growing on the stalk of the new Caulophacus species; these barnacles are currently under description (Araya & Newman in preparation).
TABLE 1. Spicule dimensions of Caulophacus chilense, sp. nov., MNHNCl-POR 101, from Caldera, Region of Atacama, Chile (dimensions in µm).
| parameter | mean | s.d. | range | no. |
|---|---|---|---|---|
| Dermal pinule, pinule ray length | 205 | 31 | 126–299 | 50 |
| pinule ray basal width | 12.8 | 2.4 | 7.8–17.2 | 50 |
| pinule ray maximum width | 37.7 | 5.5 | 26.5–48.4 | 50 |
| tangential ray length | 116 | 14 | 82–143 | 50 |
| tangential ray width | 9.9 | 1.3 | 7,1–12.7 | 50 |
| proximal ray length | 113 | 10 | 89–138 | 48 |
| proximal ray width | 10.0 | 1.5 | 6.6–12.8 | 48 |
| Atrial pinule, pinule ray length | 191 | 41 | 77–316 | 50 |
| pinule ray basal width | 10.8 | 1.7 | 7.1–15.4 | 50 |
| pinule ray maximum width | 27.9 | 5.5 | 16.8–42.4 | 50 |
| tangential ray length | 105 | 14 | 79–152 | 50 |
| tangential ray width | 8.8 | 1.2 | 6.3–11.6 | 50 |
| proximal ray length | 99 | 16 | 57–139 | 47 |
| proximal ray width | 9.2 | 1.5 | 6.1–13.6 | 47 |
| Choanosomal diactin length | 2112 | 408 | 1043–2879 | 50 |
| width | 10.3 | 2.3 | 6.8–15.6 | 50 |
| Stalk top diactin length | 3996 | 821 | 2603–5156 | 27 |
| width | 13.1 | 3.8 | 7.4–24.2 | 27 |
| Hypodermalia tangential ray length | 528 | 111 | 313–728 | 50 |
| width | 27.6 | 6.3 | 14.0–43.0 | 50 |
| proximal ray length | 623 | 182 | 251–1018 | 46 |
| width | 26.9 | 5.4 | 16.8–39.0 | 46 |
| Hypoatrialia tangential ray length | 327 | 118 | 164–721 | 50 |
| width | 17.3 | 4.5 | 9.3–27.4 | 50 |
| proximal ray length | 334 | 124 | 171–732 | 50 |
| width | 18.0 | 4.8 | 11.6–32.6 | 50 |
| Choanosomal hexactin ray length | 816 | 190 | 357–1194 | 50 |
| width | 36.8 | 7.8 | 16.4–50.6 | 50 |
| Discohexactin diameter | 190 | 27 | 114–249 | 50 |
| terminal disc diameter | 17.7 | 2.9 | 10.8–22.7 | 50 |
| minimum ray width | 6.6 | 1.0 | 4.2–8.7 | 50 |
| Hemidiscohexaster diameter | 154 | 28 | 104–204 | 50 |
| primary ray length | 6.5 | 3.2 | 1.6–19.2 | 50 |
| secondary ray length | 69.3 | 14.1 | 31.4–93.9 | 50 |
| Discohexaster A (thick ray) diameter | 111 | na | 93–132 | 3 |
| primary ray length | 3.8 | na | 3.3-4.1 | 3 |
| secondary ray length | 51.8 | na | 44.2–63.3 | 3 |
| Discohexaster B (thin ray) diameter | 81 | 19 | 38–124 | 50 |
| primary ray length | 4.4 | 1.0 | 1.9–6.7 | 50 |
| secondary ray length | 36.6 | 9.3 | 15.5–57.2 | 50 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Rossellinae |
|
Genus |
|
|
SubGenus |
Caulophacus |