Thecidellina leipnitzae Simon, Hiller, Logan & Mottequin, 2019
|
publication ID |
https://doi.org/10.11646/zootaxa.4613.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:A6591BD1-5729-49B2-980B-F1804FE28D3F |
|
persistent identifier |
https://treatment.plazi.org/id/038F87F8-FF88-FFC9-FF23-F6329E50FE98 |
|
treatment provided by |
Plazi |
|
scientific name |
Thecidellina leipnitzae Simon, Hiller, Logan & Mottequin |
| status |
sp. nov. |
Thecidellina leipnitzae Simon, Hiller, Logan & Mottequin View in CoL sp. nov.
Table 1 View TABLE 1 ; Text-Fig. 3 View TEXT-FIGURE 3 ; Pl. 1, Figs. 1–5; Pl. 2, Figs. 1–3; Pl. 3, Figs. 1–5
Holotype. MNHN-IB-2017-162 (Pl. 2, Fig. 1), a fully-grown adult dorsal specimen opened for observation of the internal structures.
Paratypes. MNHN-IB-2017-159 (Pl. 1, Fig. 3) , MNHN-IB-2017-157 (Pl. 1, Fig. 1) , MNHN-IB-2017-158 (Pl. 1, Fig. 2) , and MNHN-IB-2017-160 (Pl. 1, Fig. 4): complete articulated specimens . MNHN-IB-2017-163 (Pl. 2, Fig. 2) , MNHN-IB-2017-164 (Pl. 2, Fig. 3) , and MNHN-IB-2017-161 (Pl. 1, Fig. 5): complete specimens opened for the study of internal structures . MNHN-IB-2017-166 (Pl. 3, Fig. 2) , MNHN-IB-2017-165 (Pl. 3, Fig. 1) , MNHN- IB-2017-168 (Pl. 3, Fig. 4) , MNHN-IB-2017-169 (Pl. 3, Fig. 5) , and MNHN-IB-2017-167 (Pl. 3, Fig. 3): juvenile complete specimens used for the study of the ontogeny of the brachidium. Morphometric measurements of the holotype and paratypes are indicated in Table 1 View TABLE 1 .
Etymology. The species is dedicated to Heilwig Leipnitz (Uelzen, Germany) in acknowledgement of her constant help by giving us, as often as possible, very interesting brachiopod material for sustaining our studies.
Type locality. Department of Mayotte ( France). Submarine cave at 23 m depth situated at La Passe bateau off the south-west coast of the island of Mayotte in the second coral reef barrier (latitude: - 12.9776 S, longitude: + 44.9827 E).
Additional material. Collected from the sieved sediment of the submarine cave: 435 complete articulated shells, 425 isolated dorsal valves and 136 isolated ventral valves. Living specimens collected from the rocks from the ceiling of the cave: nine specimens placed immediately in ethanol for further DNA studies.
Diagnosis. Small-sized Thecidellina species, subtriangular to drop-like in outline. Flat, triangular planodeltidium with densely striated surface. Hemispondylium made of two subparallel prongs anteriorly ended by two curved pointed edges. Ventral valve floor without median ridge and covered with very rough tubercles except in the gonad pits, which have a smooth surface. Lateral ventral valve floor covered with regular subparallel rows of cuplike tubercles. Cyrtomatodont teeth short, blunt. Lid-like slightly convex dorsal valve with prominent protegulum. Very wide, thick dorsal septum with flat ventral surface. Brachial bridge and peribrachial ridge connected by two posterior outgrowths to the intrabrachial ridge. Calcitic pole variable but generally thick. Intrabrachial ridge oval, regular with smooth margins. Brachial cavities or brood pouches in adults partly covered by radially disposed, irregular spicules leaving a free space between each, rarely cemented together in their anterior part. No real canopies over the brachial cavities are observed in this species.
Diagnose. Thecidellina de petite taille, subtriangulaire ou en forme de goutte. Le planodeltidium est triangulaire, plat avec une surface nettement striée. L’hémispondylium est constitué de branches subparallèles se terminant par un apex pointu recourbé. La surface de la valve ventrale n’a pas de sillon médian et est couverte de tubercules très rugueux et irréguliers à l’exception des cavités où sont situées les gonades. Les flancs internes latéraux de la valve ventrale sont ornementés de séries parallèles de petits tubercules en forme de ventouses. Les dents sont courtes, robustes avec un apex arrondi. La valve dorsale ressemble à un couvercle faiblement convexe avec un protégulum bien individualisé et proéminent. La valve dorsale a un septum très large et épais dont la surface ventrale est plate. Le pont brachial et la crête intrabrachiale sont reliés par de fortes expansions postérieures. Le pôle calcitique est épais. Cavités brachiales partiellement couvertes par des spicules radialement disposées, assez irrégulières et très rarement cimentées entre elles dans la partie intérieure. Les cavités brachiales ne sont jamais totalement couvertes dans cette espèce.
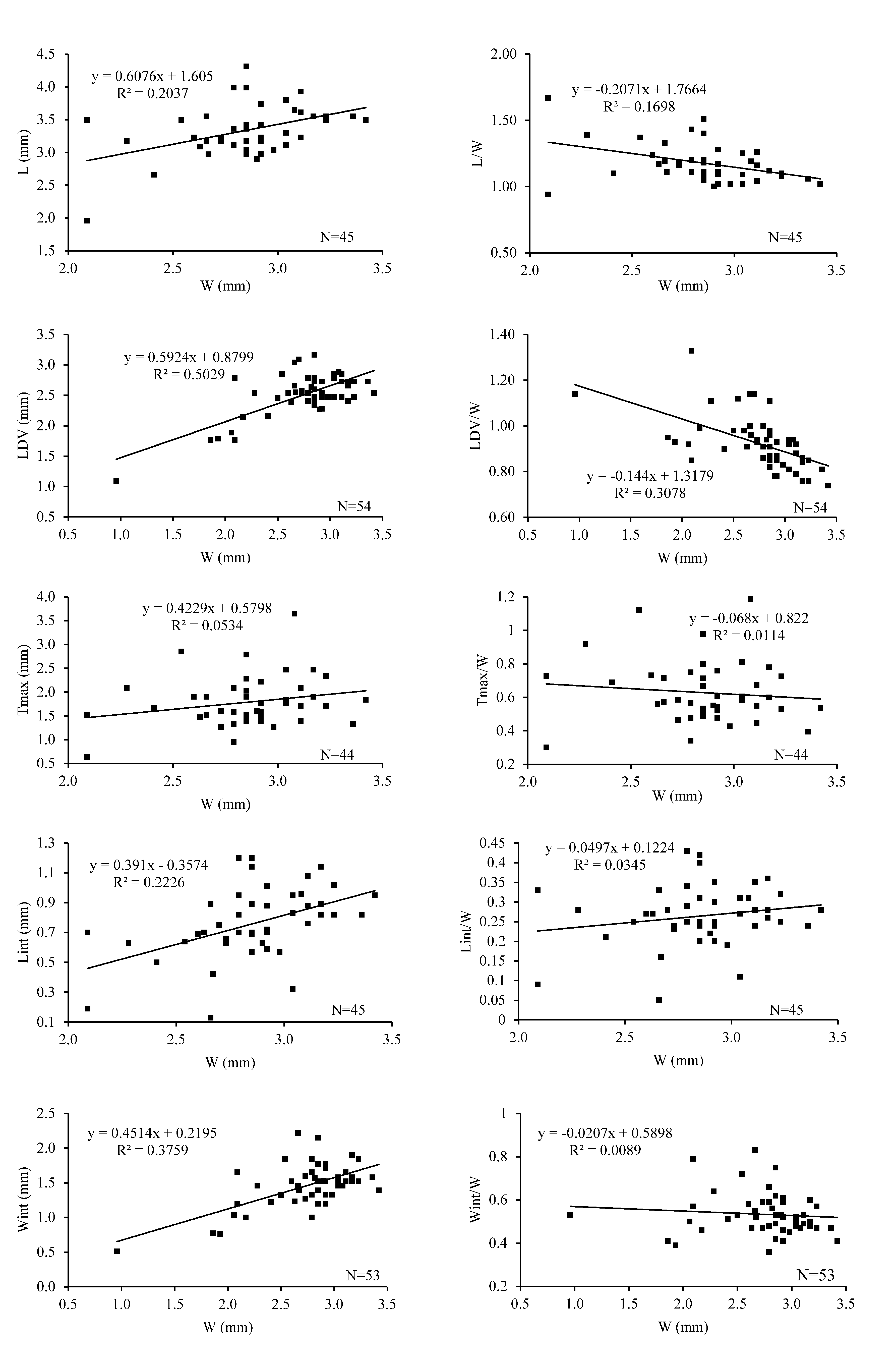
Description. External shell characters. Small-sized, whitish to light brown thecideide brachiopod ( Table 1 View TABLE 1 ), with endopunctate shell, generally with drop-like outline in adult stage (Pl. 1, Figs. 1a, 2a, 3a, 4a). The relationships between different ratios, such as length/width and width, length of dorsal valve/width and width, thickness/width and width, and length of hinge line/width and width, are illustrated in Text-Fig. 3 View TEXT-FIGURE 3 . The external surface of the ventral valve is marked by many growth lines of variable thickness (Pl. 1, Figs. 1c, 4c). On the surface of the dorsal valve, which is quite irregular, growth lines are difficult to observe (Pl. 1, Figs. 1a, 2a, 3a, 4a).
The shell is strongly ventri-biconvex, with the maximum thickness often situated at the anterior part because the growth of the ventral valve is always more important anteriorly (Pl. 1, Fig. 1b). For the dorsal valve, the maximum thickness corresponds to the position of the protegulum (Pl. 1, Figs. 2b, 3c, 4e). The lateral commissure is generally straight or very slightly concave (Pl. 1, Figs. 1b, 3b). The anterior commissure is rectimarginate (Pl. 1, Fig. 1c) or, rarely, slightly sulcate.
The deep ventral valve has a quite variable development. The thickness of the shell is the most variable morphological character ( Table 1 View TABLE 1 ). The ventral valve is cemented to the substrate by a large portion of its ventral side. The adult shell is often lifted from the substrate anteriorly (Pl. 1, Fig. 1b), its anterior part tending to be always more elevated than its posterior part. Such a development of the ventral valve is common in thecideide brachiopods as already illustrated by Pajaud (1970, p. 219, fig. 130A). During growth, the ventral valve covers all the relief irregularities present on the substrate. A very irregular aspect of the ventral exterior of the shell results from this type of growth.
The interarea is a planodeltidium ( Logan & Baker 2013) with an isosceles triangular outline and a flat (Pl. 1, Figs. 1b, 4c) or slightly concave surface (Pl. 1, Fig. 3b–c). The surface of the interarea is densely striated with parallel growth lines. The interarea represents 22–27% of the length of the shell ( Table 1 View TABLE 1 ). The planodeltidium is straight but can be curved to the left or the right sides (Pl. 1, Fig. 1a) depending on variable habitat conditions. The hinge line is straight without a notch. The interarea and the dorsal valve form a very variable angle at the level of the hinge line (Pl. 1, Fig. 4d).
The lid-like dorsal valve is much smaller than the ventral valve, with a subcircular to elongate oval outline. The weak convexity of this valve culminates posteriorly with the protegulum. The surface of the dorsal valve is quite irregular and growth lines are difficult to observe. The protegulum is subcircular, always very distinct and its external surface is covered with microgranular ornamentation (Pl. 1, Figs. 2b, 4e).
Internal shell characters. The opened ventral valve has a suboval outline (Pl. 1, Fig. 5f; Pl. 2, Figs. 1g, 3). A very narrow, concave and smooth peripheral rim is developed all along the commissure (Pl. 2, Fig. 1g). Along the internal side of this rim, a subperipheral ridge with tubercles is developed. The tubercles are slightly larger posteriorly than in the anterior part of the valve (Pl. 2, Fig. 1g).
The ventral valve floor, without a differentiated median ridge, is very roughly tuberculate with tubercles variable in outline: sometimes the tubercles are like isolated thick spines (Pl. 1, Fig. 5f), sometimes they are grouped by pairs (Pl. 2, Fig. 1g) or they can be irregularly elongate (Pl. 2, Fig. 3). The floor inside the two oval gonad pits is smooth (Pl. 2, Figs. 1g, 3). The gonad pits are very deep in this species and their length attains 30–35% of internal valve length (Pl. 2, Figs. 1g, 3). The endopunctation has a weak density between the tubercles of the valve floor but it increases in the bottom of the gonad pits (Pl. 2, Figs. 1g, 3).
The hemispondylium is made of two parallel, narrow prongs, with pointed curved tips, attached to the posterior part of the valve. The prongs are not very long in this species. Subtriangular to semicircular lateral adductor muscle scars are developed on either side of the hemispondylium and teeth (Pl. 2, Fig. 1g). Teeth are cyrtomatodont, robust, short, blunt and covered with secondary shell material (Pl. 2, Fig. 3).
In the dorsal valve interior, a flat relatively narrow, smooth marginal flange is visible along the commissure (Pl. 1, Fig. 5a). This flange is heavily endopunctate.
The external side of the peribrachial ridge is ornamented with small regular tubercles (Pl. 2, Fig. 1c) and its crest shows also more irregular tubercles till its junction with the brachial bridge (Pl. 2, Figs. 1b–c). A clearly defined peripheral rim is not present. The brachial bridge is moderately elevated and its ventral edge is ornamented with regular small indentations (Pl. 1, Fig. 5a).
In lateral profile the internal structures are weakly raised towards the posterior part (Pl. 1, Fig. 5c; Pl. 2, Fig. 1b) but this character is quite discrete. The median septum is obviously straight, very wide and very thick all along its length. It becomes broader in its anterior part. In its posterior part the septum has a pointed tip (Pl. 1, Fig. 5a; Pl. 2, Figs. 2a, e–f, 2c). In lateral view the median septum has a flat ventral profile. Its ventral surface or edge is granulated (Pl. 2, Figs. 2b, 2e).
There is a smooth lophophore groove between the peribrachial ridge and the intrabrachial ridge. This groove is heavily endopunctate (Pl. 1, Fig. 5a; Pl. 2, Figs. 1a, 2a–b).
The intrabrachial ridge is clearly defined with an oval regular outline and smooth external margins (Pl. 1, Fig. 5a). It is thin and sometimes its thickness is so reduced that the spicules of the brachial cavities are directly visible laterally (Pl. 1, Fig. 5a-c). Marsupial orifices ( sensu Zumwalt 1970) are situated in the posterior part of the intrabra- chial ridge, on either side of the septum tip (Pl. 1, Fig. 5a–b; Pl. 2, Figs. 1c, 2c). These orifices are small sub-circular rings (Pl. 2, Fig. 1a, 1c) protected by a relatively thick ventral side (Pl. 2, Figs. 1f, 2c). In the posterior part of the valve, two medium-sized lateral visceral gaps are situated between the intrabrachial ridge and the brachial bridge (Pl. 1, Fig. 5a, 5d; Pl. 2, Figs. 1a–b, 2a, 2c). The central posterior visceral gap between the brachial bridge and the intrabrachial ridge is relatively narrow (Pl. 1, Fig. 5e; Pl. 2, Figs. 1d, 2c).
The brachial bridge has a finely denticulate ventral crest (Pl. 1, Fig. 5d; Pl. 2, Fig. 1a). Clearly defined, strong, wide posterior outgrowths firmly connect the inner side of the brachial bridge and the posterior side of the intrabrachial ridge in adult specimens (Pl. 2, Fig. 2a).
The cardinal process is quite short, trilobate and relatively straight (Pl. 1, Fig. 5a, 5c). At the posterior part of the cardinal process, the diductor muscle scars are quite small (Pl. 1, Fig. 5d). Inner socket ridges are thickened and quite strong. Outer socket ridges are flat and thin. The sockets are deep. Placed on either side of the base of the inner socket ridges, the lateral adductor muscle scars are widely developed (Pl. 1, Fig. 5e).
The calcitic pole is thick and wide ventrally (Pl. 1, Fig. 5e). Seen in posterior view, it is fused with the dorsal valve floor.
The brachial cavities (brood pouches in female specimens) are ovoid, deep and partly covered with single massive canopying spicules. These spicules are relatively thin but quite high, irregular in shape and radially disposed, leaving between them a free space that has always the same size. These spicules do not fuse together and a complete closed canopy is never observed.
Comparison with other species of Thecidellina . To make comparisons between Thecidellina species it is essential to use material described accurately and well-illustrated (Simon et al. 2018a). Specimens collected from their type area are best; care must be taken with material found elsewhere, often at very far distances from the type locality. Determination of Thecidellina species is not always easy and a lot of confusion between species is found in the literature. Lee & Robinson (2003) pointed out this problem, having observed “a very wide overlapping” of distribution areas for different Pacific species in the literature. This wide zoogeographical overlapping is surprising in a genus like Thecidellina in which cryptic, allopatric speciation processes have been demonstrated ( Lüter et al. 2008).
The essential comparisons should be made between T. leipnitzae sp. nov. and other Indo-Pacific species that have a similar structure of brachial cavities. Species that have a completely fused spicular canopy can be directly distinguished. Indeed, in T. leipnitzae there is no complete canopy covering brachial cavities and radial spicules develop a characteristic openwork protection for embryos. Species like T. blochmanni Dall, 1920 and T. europa Logan et al., 2015 develop a complete spicular canopy and can be easily discarded even if the type of canopy is clearly different for each species cited. T. europa is found in the southern part of the Mozambique Channel and is distinct from T. leipnitzae sp. nov. This case further illustrates the clear propensity for the development of allopatric cryptic speciation mechanisms in Thecidellina ( Lüter et al. 2008) .
Thecidellina congregata Cooper, 1954 View in CoL from Bikini Atoll has brachial cavities incompletely covered by a canopy. The cavities are protected by subparallel spicules developed along their external margins. These spicules are of two orders: from the anterior to the middle of the valve the spicules are very thick. From the middle of the valve to the posterior part the spicules are much smaller. There are no spicules along the internal margins of the brachial cavities. However, flanges are developed along the posterior part of the internal margins. The internal side of the peripheral rim has a regular beaded ornamentation. The septum is quite narrow with a concave ventral sulcus. Posteriorly, it is limited by a small circular visceral gap (see Cooper 1954, pl. 80, figs. 9–13). The shell of T. leipnitzae View in CoL sp. nov. has brachial cavities covered only with radial spicules but these are of regular size all along the external margins of the brachial cavities (Pl. 2, Fig. 1a). There are no flanges developed along the inner margins of the brachial cavities: the lateral sides of the dorsal septum remain smooth (Pl. 1, Fig. 5a; Pl. 2, Fig. 1a). The internal margin of the peribrachial ridge is not beaded. The septum is much stronger, flat ventrally with a smooth surface and posteriorly it is limited by a larger visceral cavity, which is not circular.
In Thecidellina maxima ( Hedley, 1899) the anterior commissure is quite emarginate and the ventral valve floor is smoother with small disseminated tubercles. Flanges are developed along the inner margins of the brachial cavities and some spicules can even be developed on that side. The septum is concave ventrally and narrow. In T. leipnitzae View in CoL sp. nov. the anterior commissure is rectimarginate. The ventral valve floor is very roughly covered with strong irregular tubercles. The spicules on the brachial cavities are more spaced with a regular size and more radial. There are no flanges along the inner margins of brachial cavities. The septum is wider, thicker and its ventral edge is flat and smooth.
The material studied for Thecidellina insolita Hoffmann et al., 2009 is abundant. The calcitic pole in this species is not fused with the dorsal valve floor as it is in T. leipnitzae sp. nov. The cardinal process is more developed and less curved with a strongly developed median ridge. In T. leipnitzae sp. nov. the cardinal process is shorter and the median ridge is less developed. The canopying spicules in T. insolita are single and with a quite regular outline, and they are shorter (Hoffmann et al. 2009, fig. 2m). In T. leipnitzae sp. nov. the spicules have an irregular outline with tuberculate ornamentation on their ventral edge (Pl. 2, Fig. 1e). The posterior visceral gap is larger in T. insolita and the posterior outgrowths connecting the brachial bridge to the intrabrachial ridge are widely separated and they have a relatively weak development (Hoffmann et al. 2009, fig. 2a, 2i). In T. leipnitzae sp. nov. posterior outgrowths are strongly developed and they are placed nearer each other (Pl. 1, Fig. 5a, 5d; Pl. 2, Figs. 1a, 1b, 2a). The posterior visceral gap is thus reduced. Both species have a similar dorsal septum which is however flatter in T. leipnitzae sp. nov. and both possess cup-like tubercles on the peribrachial ridge (defined as “crater-like tubercles” by Hoffmann et al. 2009).
In Thecidellina japonica ( Hayasaka, 1938) View in CoL the dorsal septum is narrower, more ventrally concave and flares much more in its anterior part. The spicules of the brachial pouches are more fused together and they are shorter. The ventral valve of T. japonica View in CoL has a ventral valve floor with a peculiar median ridge made of three longitudinal series of irregular small tubercles (see Hayasaka 1938, fig. 2b). In T. leipnitzae View in CoL sp. nov. the dorsal septum is wider, less flaring in its anterior part and has a flat ventral surface. The spicules of the canopy are more developed, single and cover more of the brachial cavities. The ventral valve floor in T. leipnitzae View in CoL sp. nov. is roughly tuberculate and does not develop a median ridge (Pl. 2, Fig. 1g).
Thecidellina mawaliana Simon et al., 2018a View in CoL has a high, thin dorsal median septum with an acute posterior tip. Its ventral edge is convex and tuberculate or spinous. The brachial cavities are covered by thin, fragile, irregular canopying spicules that become fused during ontogeny but always leaving random gaps. The canopy apparently never completely covers the brood pouches. In T. leipnitzae View in CoL the dorsal septum is much wider with a flat ventral surface that is smooth to granulose. The spicules in the brachial canopy are regularly radially arranged with free space separating them so that a complete canopy is never formed. In addition, the floor of the ventral valve of T. leipnitzae View in CoL is much more coarsely tuberculate than that of T. mawaliana View in CoL .
Shell ontogeny. Shell ontogeny in the genus Thecidellina has already been studied and illustrated for different species, e.g. T. congregata Cooper, 1954 by Logan (2008), T. meyeri Hoffmann & Lüter, 2009 , and T. mawaliana Simon et al., 2018a . Most of the early juvenile stages of growth are quite similar in the different species. In the ventral valve the teeth, the hemispondylium and the flat interarea are immediately developed. In the earliest dorsal valve, the peribrachial ridge, the brachial bridge and the cardinal process are already developed.
A bifid spike emerges in the central part of the valve with its tips oriented posteriorly. For T. leipnitzae sp. nov., the main steps of the ontogeny described and illustrated in Pl. 3, Figs. 1–5 give a comprehensive view of the development. A peculiar aspect of the ontogeny in this species is the fact that when the intrabrachial ridge is emerging in its posterior part, the dispersed tubercles serving as basement for the construction of the whole intrabrachial ridge are not clearly developed as is the case for other species such as the new species described by Simon et al. (2018a). These tubercles are produced at a later stage of growth. At this early stage of growth, they are similar to dispersed nodules (Pl. 3, Fig. 1a–c). However, the septum is already emerging and connected with the base of the initial spikes (Pl. 3, Fig. 1b–c). The calcitic pole is undeveloped. Nonetheless a rudimentary median lobe is already visible on the cardinal process.
Later, the posterior part of the intrabrachial ridge develops and progresses anteriorly (Pl. 3, Fig. 2a). The median septum grows rapidly, increasing its height and its width (Pl. 3, Fig. 2a–b). The calcitic pole is now developed and joins the base of the median lobe of the cardinal process (Pl. 3, Fig. 2a–b).
At the next stage of growth, the brachial cavities appear as separated expansions (Pl. 3, Fig. 3a). The septum is stronger. Its ventral edge is now slightly less concave. In the posterior part, small outgrowths appear. They emerge from the base of the brachial bridge as two small pointed nodules (Pl. 3, Fig. 3b). The calcitic pole is now fused with the dorsal valve floor in front of the base of the median lobe of the cardinal process (Pl. 3, Fig. 3b).
Later, the septum reaches its mature form and it is now wide, thick and straight with a pointed posterior tip (Pl. 3, Fig. 4a). The posterior outgrowths fuse the anterior part of the brachial bridge with the posterior part of the intrabrachial ridge (Pl. 3, Fig. 4a, 4c). The calcitic pole and the base of the median lobe of the cardinal process are not fused together (Pl. 3, Fig. 4a). The intrabrachial ridge is now completely erected posteriorly and laterally but its anterior part is still missing. Canopying spicules are now appearing (Pl. 3, Fig. 4a–c). Marsupial orifices are developed at this stage of growth.
Finally, the anterior part of the intrabrachial ridge is erected. Canopying spicules are built all over the brachial cavities except in their posterior part (Pl. 3, Fig. 5a). A remarkable aspect in the ontogeny is the intrabrachial ridge, which is progressively made by erecting separate plates. At the ultimate stage of growth (Pl. 3, Figs. 4a, 5a) these separated plates are fused together.
TABLE 1. Thecidellina leipnitzae sp. nov. Morphometric measurements were taken for the holotype, the paratypes and a large number of articulated specimens collected from the sieved sediment collected in the submarine cave in Mayotte at 23 m depth. Abbreviations: N, number of specimens measured; MIN, minimum; MAX, maximum; L, length, W, width; LD, length of dorsal valve; Lint, length of the interarea; Wint, width of the hinge or interarea; T, thickness; nm, value not measured.
| Illustrated specimens | L | W | LDV | Lint | Wint | T (max) | L/W | LDV/W | Lint/W | Wint/W | T/W |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mm | mm | mm | mm | mm | mm | ||||||
| Holotype MNHN-IB-2017-162 | nm | 2.7 | 3.1 | 0.75 | nm | nm | 1.14 | ||||
| Paratype MNHN-IB-2017-157 | 3.7 | 3.1 | 2.9 | 1.0 | 1.5 | 3.7 | 1.2 | 0.9 | 0.3 | 0.5 | 1.2 |
| Paratype MNHN-IB-2017-158 | 3.2 | 2.7 | 2.6 | 0.7 | 1.6 | 1.6 | 1.2 | 0.9 | 0.2 | 0.6 | 0.6 |
| Paratype MNHN-IB-2017-159 | 3.1 | 2.6 | 2.4 | 0.7 | 1.2 | 1.5 | 1.2 | 0.9 | 0.3 | 0.5 | 0.6 |
| Paratype MNHN-IB-2017-160 | 2.7 | 2.4 | 2.2 | 0.5 | 1.2 | 1.7 | 1.1 | 0.9 | 0.2 | 0.5 | 0.7 |
| Paratype MNHN-IB-2017-161 | 2.9 | 2.9 | 2.3 | 0.6 | 1.5 | 1.6 | 1.0 | 0.8 | 0.2 | 0.5 | 0.6 |
| Paratype MNHN-IB-2017-163 | nm | 2.8 | 2.6 | nm | 1.6 | nm | 0.9 | 0.6 | |||
| Paratype MNHN-IB-2017-164 | 3.0 | 2.7 | 2.6 | 0.4 | 1.4 | nm | 1.1 | 1.0 | 0.2 | 0.5 | |
| Paratype MNHN-IB-2017-165 | nm | 1.0 | 1.1 | nm | 0.5 | nm | 1.1 | 0.5 | |||
| Paratype MNHN-IB-2017-166 | nm | 1.9 | 1.8 | nn | 0.8 | nm | 0.9 | 0.4 | |||
| Paratype MNHN-IB-2017-167 | nm | 1.9 | 1.8 | nm | 0.8 | nm | 1.0 | 0.4 | |||
| Paratype MNHN-IB-2017-168 | nm | 2.2 | 2.1 | nm | 1.0 | nm | 1.0 | 0.5 | |||
| Paratype MNHN-IB-2017-169 | nm | 2.1 | 1.9 | nm | 1.0 | nm | 0.9 | 0.5 | |||
| Total measurements | |||||||||||
| N | 45 | 54 | 54 | 46 | 53 | 44 | 45 | 54 | 45 | 53 | 44 |
| Mean value | 2.0 | 1.0 | 1.1 | 0.1 | 0.5 | 0.6 | 0.94 | 0.74 | 0.05 | 0.36 | 0.30 |
| MIN | 4.3 | 3.4 | 3.2 | 1.2 | 2.2 | 3.7 | 1.67 | 1.33 | 0.43 | 0.83 | 1.19 |
| MAX | 3.4 | 2.7 | 2.5 | 0.8 | 1.5 | 1.8 | 1.17 | 0.92 | 0.26 | 0.53 | 0.62 |
| Standard deviation | 0.3858 | 0.4391 | 0.3696 | 0.2357 | 0.3264 | 0.5338 | 0.1448 | 0.1143 | 0.0773 | 0.0979 | 0.1831 |
| Standard error (±) | 0.0575 | 0.0598 | 0.0503 | 0.0348 | 0.0448 | 0.0805 | 0.0216 | 0.0156 | 0.0115 | 0.0134 | 0.0276 |
TABLE 1. Thecidellina leipnitzae sp. nov. Morphometric measurements were taken for the holotype, the paratypes and a large number of articulated specimens collected from the sieved sediment collected in the submarine cave in Mayotte at 23 m depth. Abbreviations: N, number of specimens measured; MIN, minimum; MAX, maximum; L, length, W, width; LD, length of dorsal valve; Lint, length of the interarea; Wint, width of the hinge or interarea; T, thickness; nm, value not measured.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Thecideoidea |
|
Family |
|
|
SubFamily |
Thecidellininae |
|
Genus |
Thecidellina leipnitzae Simon, Hiller, Logan & Mottequin
| Simon, Eric, Hiller, Norton, Logan, Alan, Theuerkauff, Dimitri & Mottequin, Bernard 2019 |
T. leipnitzae
| Simon & Hiller & Logan & Theuerkauff & Mottequin 2019 |
T. leipnitzae
| Simon & Hiller & Logan & Theuerkauff & Mottequin 2019 |
T. leipnitzae
| Simon & Hiller & Logan & Theuerkauff & Mottequin 2019 |
T. leipnitzae
| Simon & Hiller & Logan & Theuerkauff & Mottequin 2019 |
T. leipnitzae
| Simon & Hiller & Logan & Theuerkauff & Mottequin 2019 |
T. leipnitzae
| Simon & Hiller & Logan & Theuerkauff & Mottequin 2019 |
Thecidellina mawaliana
| Simon 2018 |
Thecidellina congregata
| Cooper 1954 |
Thecidellina japonica (
| Hayasaka 1938 |
Thecidellina maxima (
| Hedley 1899 |