Neonesidea tenera (Brady, 1886)
|
publication ID |
https://doi.org/10.11646/zootaxa.3608.6.3 |
|
publication LSID |
lsid:zoobank.org:pub:88C9385B-1E8F-4F69-B77E-C81D9F898282 |
|
DOI |
https://doi.org/10.5281/zenodo.6153571 |
|
persistent identifier |
https://treatment.plazi.org/id/038D8671-FF89-3560-FF61-F8EA8D36F836 |
|
treatment provided by |
Plazi |
|
scientific name |
Neonesidea tenera (Brady, 1886) |
| status |
|
Neonesidea tenera (Brady, 1886) View in CoL
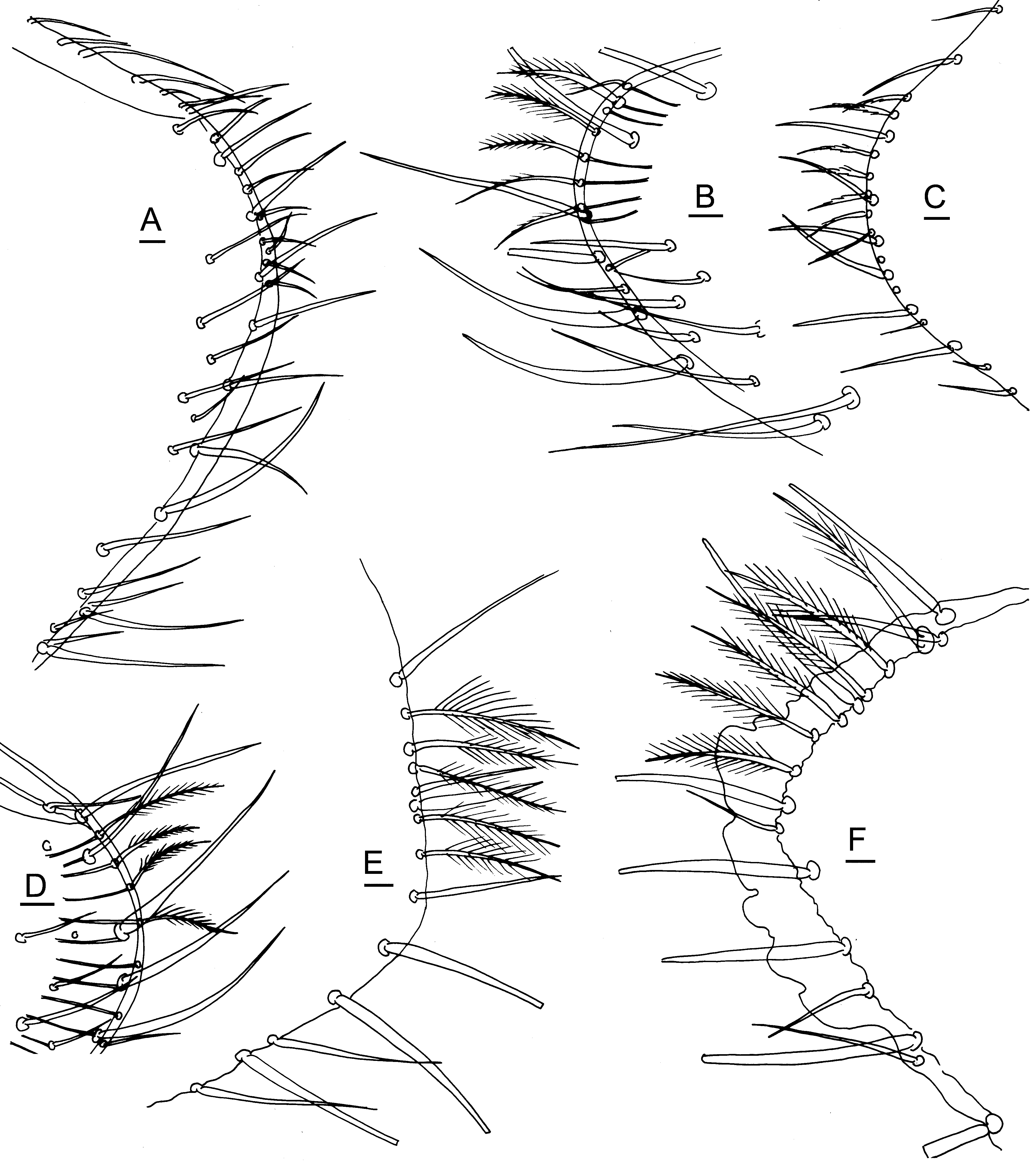
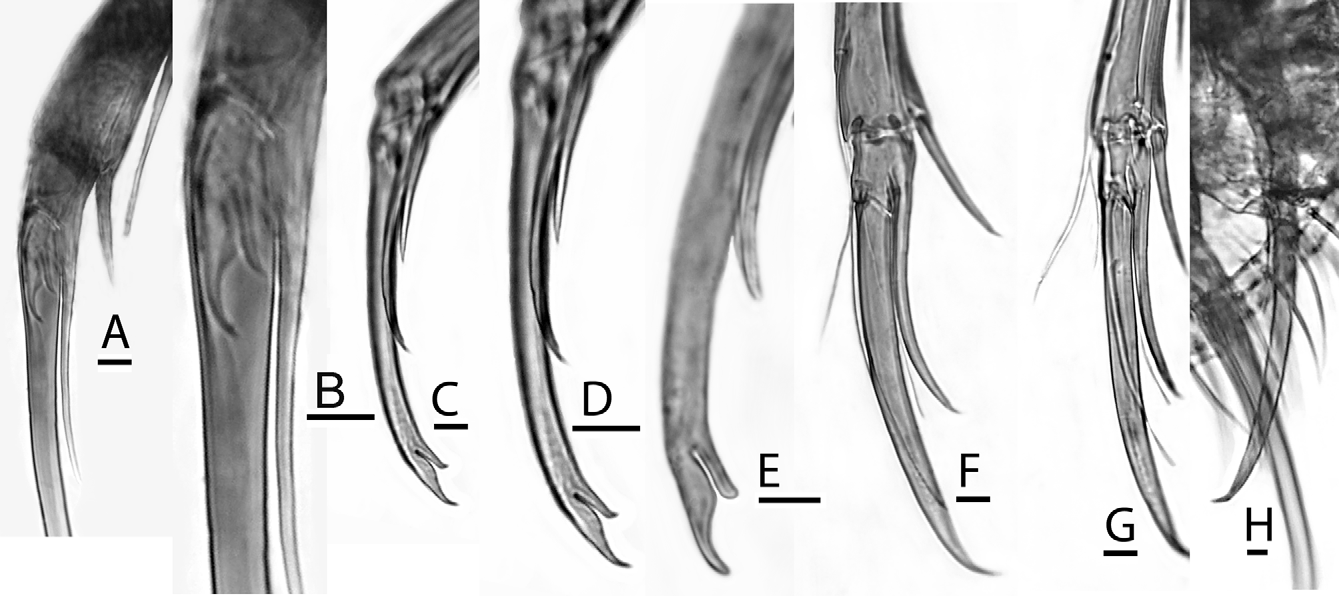
( Figs. 5 View FIGURE 5 A–D, 6–17)
1886 Bairdia tenera Brady : 304, pl. 39, figs. 13–15.
1890 Bairdia tenera Brady—Brady : 495, pl. 1, figs. 11–12. 1905 Bairdia inornata A. Scott : 372, pl. 1, figs. 11–12.
1969 Neonesidea tenera (Brady) —Maddocks: 33, pl. 2, fig. 4.
1999 Neonesidea tenera (Brady) —Whatley & Jones: pl. 1, fig. 10.
2000 Neonesidea tenera (Brady) —Whatley: Table 1.
2000 Neonesidea tenera (Brady) —Whatley, Jones & Wouters: 85, pl. 2, figs. 1, 2, 4 (not fig. 3).? 1902 Bairdia tenera Brady—Chapman : 422.
? 1983 Neonesidea n. sp. 1—Bonaduce et al.: 478, fig. 4, figs. 6–9.
? 1983 Neonesidea ? tenera (Brady) —Bonaduce et al.: 481.
? 1995 Neonesidea tenera (Brady) —Whatley & Roberts: fig. 1–1.
? 2002 Neonesidea gr. tenera (Brady) —Hoibian et al.: p. 183.
? 2012 Neonesidea tenera? (Brady) —Antonietto et al.: 35, fig. 4, figs. 16–22. Not 1894 Bairdia inornata T. Scott : 136, pl. 14, figs. 40–41 [= senior homonym of B. inornata A. Scott ].
Types and type locality. The statement by Antonietto et al. (2012, p. 35) is incorrect—Maddocks (1969, p. 33) did not designate a neotype.
The Rules (ICZN, 1999, Article 5, p. 84–85) concerning neotypes are strict. A neotype may be designated only if necessary to "define the nominal taxon objectively" and to resolve an existing "zoological problem"—not just because the original type has been lost, and never as a matter of routine. Such a designation must be accompanied by demonstration of "exceptional need," by a statement of purpose, by a differential diagnosis, and by "evidence that the neotype came as nearly as practicable from the original type locality." A type-specimen has only nomenclatural, not taxonomic, significance. It is merely the name-bearer, in case of later dispute concerning the applicability of a species name. In the absence of any dispute, it has no function, because it does not define the taxonomic concept associated with the name or describe the contents of a biological population.
There is no reason to designate a neotype for N. tenera . Although the specimen was lost (Brady 1890, p. 493), and the original description is skimpy, N. tenera has been identified globally by multiple authors with reasonable confidence.
The type locality remains as originally specified: "dredged in a depth of 2 fathoms off Calpentyn, in the Gulf of Manaar" (Brady 1886, p. 293). Contrary to Antonietto et al. (2012, p. 35), the label on the specimen subsequently identified by Maddocks (1969) is not the type locality for the species.
Material. 38 specimens in 8 samples ( Table 2). This is the most abundant of the five species of Neonesidea in this collection. Adults of both sexes and three instars are represented.
The material includes a range of preservation, from partial to total decalcification. One female (3962F) displays the dark brown color of the carapace epidermis and orange eggs. Among the many juveniles, one A- 1 male (3920MJ) was molting when captured, and the adult antennal hook is clearly visible within the juvenile claw. The juvenile carapace has split around the free margin and partly flaked off, releasing the unexpanded chitinous template for the adult carapace, including hundreds of close-packed setae.
Dimensions. Female specimen 3862F L 811 μm, H 471 μm; female specimen 3892F L 833 μm, H 500 μm; female specimen 3905F L 824 μm, H 473 μm; male specimen 3906M L 797 μm, H 453 μm. See also Figure 15 View FIGURE 15. L – H .
Description. Carapace ( Figs. 6 View FIGURE 6 A–L, 7A–I, 8A–J) moderately compressed, greatest height and greatest thickness located slightly anterior to midlength (0.46–0.49); surface smooth; epidermis or inner chitinous lining dark brown except for small clear region over eye, margins translucent white; bleached specimens with small opaque streak located centrally over AMSP. Lateral outline of left valve oblong; dorsal margin broadly arched, curving smoothly and nearly equally anteriorly and posteriorly, without any anterodorsal and posterodorsal angles; anterior margin broadly and evenly rounded, ventral margin with very weak ventral indentation or nearly straight, posteroventral margin curving smoothly upward; posterior end weakly caudate, with slight posterodorsal concavity above broadly rounded caudal process, center of caudal process located slightly above one-third of height (0.35–0.36). No anteroventral marginal denticles; posteroventral marginal denticles suspected but not clearly visible. Marginal fused zone narrow, vestibules deep and broad; false RPC numerous, of many lengths, each ending in a seta. NPC of several sizes, simple, without distinct marginal walls. AMSP rosette-shaped, with 8 polygonal scars close-packed, two by two; one small frontal scar and two small mandibular scars.
Carapace setae ( Figs. 5 View FIGURE 5 A–D, 6A–L, 7A–I, 8A–J) numerous, evenly spaced, of various lengths and thicknesses, but all fairly short, stiff, simple, tapering smoothly to sharp point; a few of the smallest setae have 2 or 3 divergent, shorter barbs or branches near base, but larger setae never have barbs. Eyelash setae simple, evenly spaced, all of the same length. Caudal setae about 6 in number, delicately feathered, thistle-like, located along posterodorsal margin of caudal process of each valve, clearly seen only in A-1 instar; caudal setae thought to be present also in adult, but too fine to see clearly.
Antennal claw of male ( Figs. 11 View FIGURE 11 A–E, 12E–F) relatively short, wide; distal edge of outer horn only slightly curved, continues smooth profile of claw; end of outer horn slightly curved, pointed; inner horn shorter, tapers sinuously, ends in sharply curved point; sigmoid groove between horns.
Hemipenis ( Figs. 10 View FIGURE 10 A–B, F–H, 14D) with wedge-shaped base, heavily muscularized; median lobe oblongsubovate in outline, consisting of D-shaped outer capsule containing three groups of muscles attached to proximal and medial chitinous ridges, plus broadly flared lamellar flange or brim; terminal segment broad-based, heavily chitinized, with a somewhat blunt, lobose, smooth termination, as well as an incised medial groove to house copulatory tube. Copulatory tube long, tapering, flexible; distal (free) part more than twice as long as proximal (fixed, arched) part.
Kauplatte ( Figs. 10 View FIGURE 10 D, 12G) of masticatory organ with 8 wide teeth of nearly equal sizes, with bluntly rounded margins; end teeth slightly larger than others and set apart by small gaps; 2 thin, straight, hairlike spines located at ends, projecting slightly beyond teeth.
Comparisons. N. tenera is poorly known in the type locality (Gulf of Mannar, Sri Lanka, northern Indian Ocean), and all three descriptions were based on single specimens. Maddocks (1969) considered B. inornata Scott to be a subjective synonym of B. tenera because of the similar dimensions and provenance. The lack of information concerning population variation, dimorphism and soft anatomy limits assessment of the validity of identifications elsewhere.
The Hawaiian specimens of N. tenera are larger than the dimensions given by Brady (1886) and Scott (1905) ( Fig. 16 View FIGURE 16. L – H ). The lateral outline has a more high-arched, symmetrically curving dorsal margin and a less exaggerated caudal process, and there are no conspicuous posteroventral marginal denticles.
The Hawaiian population agrees closely in size and shape with specimens illustrated as Neonesidea n. sp. 1 from the Gulf of Aqaba by Bonaduce et al. (1983). N. n. sp. 1 appears to be slightly larger than N. tenera from the type locality but corresponds in many details (slightly caudate lateral outline, nearly straight ventral margin, delicately punctate surface, minutely denticulate posteroventral margin of LV, gently curved contours of the dorsal view). Their mention of N.? tenera in the same paper (not illustrated) may support this identification.
The Hawaiian population is about the same size as the specimen illustrated as N. tenera by Brady (1890) from Samoa. The Hawaiian specimens have a slightly more arched dorsal margin, without distinct anterodorsal and posterodorsal angles, and lack conspicuous posteroventral marginal denticles.
Chapman (1902, p. 422) reported B. tenera from a dredging at 200 fathoms near Funafuti ( Tuvalu) but did not illustrate it. Hoibian et al. (2002, not illustrated) cited N. gr. tener a from New Caledonia.
The Hawaiian specimens are larger than those illustrated by Whatley & Jones (1999) and Whatley et al. (2000) from Easter Island but similar in LV lateral outline and absence of posteroventral denticles. The illustrated RV (Whatley et al. 2000, pl. 2, fig. 3) has a more prolonged, acute-angled caudal process and probably belongs to a different species. The LV from Henderson Island illustrated by Whatley & Roberts (1995, fig. 1-1) has an acute caudal process, and the dimensions indicate that it may be a juvenile of a different species.
Although Whatley et al. (2000) had thousands of specimens, they provided dimensions only for the four illustrated specimens and did not analyze variation. Without explanation, they excluded Brady's (1890) identification from Samoa and did not mention Funafuti (Chapman 1902). No description or taxonomic evaluation was provided for the species, only the following enigmatic remarks (p. 85):
"This is the most abundant species at Easter Island. With its laterally compressed carapace and densely hirsute or punctate surface, it not [sic] difficult to recognise. The Easter Island population, despite its isolation, does not materially differ in size or shape from other populations of the species."
These remarks are somewhat misleading: All species of Neonesidea are hirsute, several species are punctate, and all species of the N. pateriformis species-group are strongly compressed. No populations (multiple adults of both sexes plus juveniles) have been documented for N. tenera at any locality before now. We know little about sexual dimorphism, growth stages, individual variability, and regional trends in N. tenera .
The Hawaiian specimens are much larger than the supposed juveniles tentatively identified from Saint Peter and Saint Paul Rocks, central Atlantic Ocean (Antonietto et al. 2012). The SEM photos of the Saint Peter and Saint Paul specimens (kindly furnished by Dr. Claudia Pinto Machado) show recently dead, closed carapaces with abraded epicuticle, broken shafts of a few setae near the margins, and post-mortem boreholes. The lateral outline is similar to that of N. tenera except for the slightly more angulate caudal process.
The Hawaiian population of N. tenera has slightly larger carapace size than N. pateriformis at Nosy Be ( Fig. 17 View FIGURE 17. L – H ). It is less symmetrical in lateral outline, having a more broadly arched dorsal margin, a smooth (rather than denticulate) posteroventral margin, and a subtly caudate posterior end (rather than the smoothly curving, noncaudate termination of N. pateriformis ). The Hawaiian specimens are apparently smooth, whereas N. pateriformis has numerous, small, shallow, regularly spaced puncta. Posteroventral marginal denticles are suspected but not clearly seen on the Hawaiian specimens of N. tenera , perhaps because of decalcification; they are present in N. pateriformis , although small and barely visible on some specimens. Polyfurcate or basally barbed carapace setae are present in both species, although more systematic documentation of this feature is needed. The hemipenis of N. tenera is distinctive, having a broad, even, lamellar brim on the median segment, a longer copulatory tube, and a lobose, bluntly rounded terminal segment (rather than the sinuously tapering, flame-shaped termination of N. pateriformis ).
Maddocks (1969) reported other occurrences of N. pateriformis (without dimensions or illustrations) in collections from Ifalik Atoll (West Caroline Islands) and Mombasa ( Kenya). Because of this apparently broad distribution, she placed Brady's (1890, Samoa) and Chapman's (1902, Funafuti) identifications of B. tenera in synonymy. This synonymy now appears unconvincing, and all of these identifications should be re-evaluated.
N. tenera resembles N. sp. CP of Maddocks (1995, Nosy Be), which has a similarly compressed, faintly punctate to nearly smooth carapace, a weakly caudate lateral outline with nearly straight ventral margin, and no marginal denticles. N. sp. CP is much larger, however, and has more elongate proportions than N. tenera at any locality. The nearly straight anteroventral and posteroventral margins and acute-angled caudal process of N. sp. CP may also be distinctive.
N. tenera is substantially smaller and smoother than N. sp. 2 aff. pateriformis of Cabioch et al. (1986, New Caledonia). The latter species corresponds closely to N. sp. CP in lateral outline but is much larger, with deeper, better defined surface puncta than N. pateriformis or N. tenera at any locality.
N. tenera differs from N. sp. 1 of Gou (1990, South China Sea) by its smaller size and weakly caudate lateral outline. N. sp. 1 of Gou closely resembles N. pateriformis in lateral outline and proportions but is distinctly larger. The dorsal view (Gou, 1990, fig. 12b) is diamond-shaped, with greater medial thickness and more nearly straight anterior and posterior margins than N. tenera , easily distinguishable from the gentle curves shown by Bonaduce et al. (1983, fig. 4, fig. 9) and Brady (1890, pl. 1, fig. 12), though resembling the sketches by Brady (1886, pl. 39, fig. 14) and Scott (1905, pl. 1, fig. 12).
N. tenera is easily distinguished by size and details of the hemipenis from several other species of the N. pateriformis species-group, which share the weakly caudate lateral outline, brown color, and feathered caudal setae. N. anfieldingae and N. lentiphila (Hartmann, 1984, Rangiroa) are much smaller than N. tenera , have more acute caudal angles, and have large posteroventral marginal denticles on both valves. N. maddocksae Hartmann (1974, Mozambique) is about the same size but less compressed and has a much thicker, distally widened antennal hook. N. manningi Maddocks (1975, Ascension Island) is much smaller than N. tenera and smooth, with posteroventral denticles on the RV; the hemipenis has an ovate terminal segment with outer distal horn.
Geography. N. tenera does not closely resemble any of the species described by Holden (1967, 1976) from Hawaii and Midway. It is a first record for the N. pateriformis species-group for the Hawaiian Islands.
The scarcity of N. tenera in the type region is noteworthy. The early descriptions were based on single specimens. It is not listed in numerous assemblages described in recent years from the east and west coasts of India, the Gulf of Mannar, and the Andaman Islands. Although Whatley & Jones (1999), Whatley (2000), and Whatley et al. (2000) described N. tenera as "pandemic" and "widely distributed in the Indian Ocean," in actuality the species is unknown in the Indian Ocean south of the Gulf of Mannar1.
N. n. sp. 1 of Bonaduce et al. (1983) closely resembles N. tenera and may be conspecific, extending the range west to the Gulf of Aqaba. The reported occurrence of N. tenera in the Java Sea (Whatley & Roberts 1995, Antonietto et al. 2012), while plausible, is based on a never-published M.S. thesis (Watson, 1988).
On the basis of the published carapace illustrations, the identifications of N. tenera at Samoa (Brady 1890) and Easter Island (Whatley & Jones 1999, Whatley et al. 2000) are credible. The record at Henderson Island (Whatley & Roberts 1995) is less certain, and the species was not collected at Pitcairn and Oeno Islands. The occurrence of N. tenera in Chile (Antonietto et al. 2012, contradicted by Whatley & Jones 1999) is unlikely.
The tentative identification of N. tenera in the Atlantic Ocean (Antonietto et al. 2012) suggests a circumtropical distribution, although at present no representatives of this species-group are known in West Africa, the Caribbean, and the tropical East Pacific. It is intriguing that N. manningi , at another mid-Atlantic island ( Ascension), also has small carapace size. Perhaps small size favors survival on isolated, wave-swept rocks.
The Indo-Pacific occurrences of N. tenera spread across at least 3 and maybe 5 of the 13 zoogeographical provinces in the classification by Titterton &Whatley (1888b, text-fig. 1). This is a broad and somewhat spotty distribution, as the species has not yet been recognized in numerous assemblages at intervening localities. Perhaps these conspicuous gaps result in part from insufficient sampling of high-energy, hard-bottom habitats. Most faunal collecting for Ostracoda is based on sediment samples collected by dredging or coring. The diversity of Ostracoda on hard substrates is less well surveyed.
1. Munef et al. (2012, p. 154, Pl. 1, figs. 10–11) identified N. tenera from shallow marine sediments along the northern coast of Socotra Island, Yemen (east of the horn of Africa in the northwestern Indian Ocean). This occurrence should be added to the synonymy and geographic distribution described above for N. tenera .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |