Lucernariopsis capensis Carlgren, 1938
|
publication ID |
https://doi.org/ 10.5281/zenodo.279705 |
|
DOI |
https://doi.org/10.5281/zenodo.5687541 |
|
persistent identifier |
https://treatment.plazi.org/id/038A87B6-6329-FFC1-FF0F-FB7EFBF0F830 |
|
treatment provided by |
Plazi |
|
scientific name |
Lucernariopsis capensis Carlgren, 1938 |
| status |
|
Lucernariopsis capensis Carlgren, 1938 View in CoL
Lucernariopsis capensis Carlgren 1938: 1 View in CoL −6.— Panikkar 1944: 238 −239.— Corbin 1978: 285, 289.— Grohmann et al. 1999: 386.— Zagal et al. 2011: 660 -664.
Type series and locality. East London, Eastern Cape, South Africa, Indian Ocean; 1 individual. The specimen was received from the Zoological Institute of the University of Cape Town and described by Carlgren (1938). The current location of type material is unknown. Also unknown are the collector, collection date, and substrate. The material, received by Carlgren after 1935, retained its green color in formalin ( Carlgren 1938) so was probably collected a short time before being sent to him.
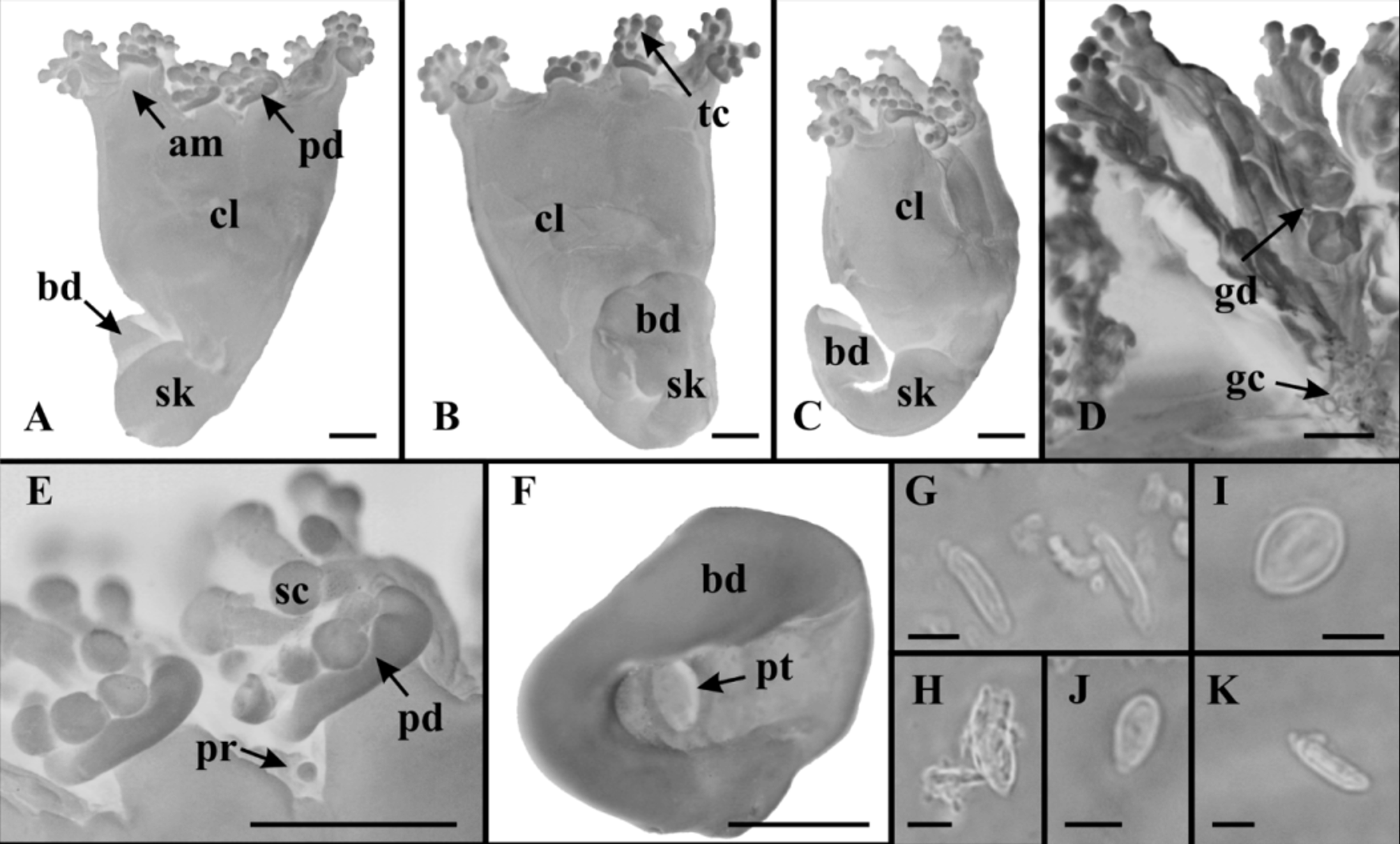
Material examined. Itanhaém, São Paulo, Brazil, Atlantic Ocean, ~ 24°11’30’’S; 46°47’30’’W. 0 8 April 1985, intertidal zone, on algae ( Sargassum sp.), formaldehyde solution, col. M.A. Haddad, det. L.S. Miranda and C.E. Mills, 1 individual ( Fig. 1 View FIGURE 1 ), MZUSP 1566. The material is not well preserved, making it difficult to observe some structures (e.g., gonads, inner part of stalk, and manubrium).
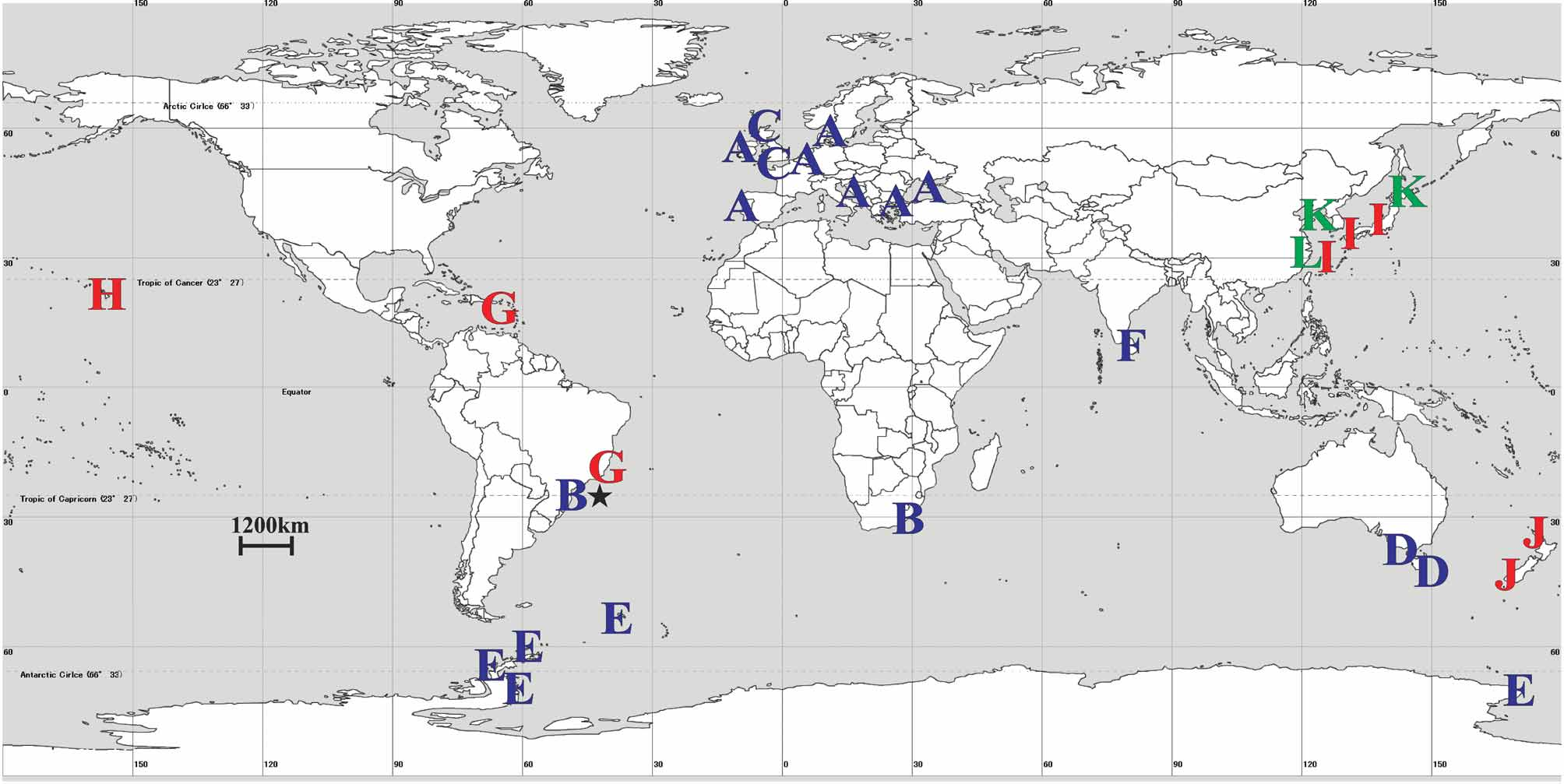
Distribution. East London, South Africa, Indian Ocean (1 specimen); Itanhaém, Brazil, Atlantic Ocean (1 specimen).
Description. Calyx (umbrella) pyramidal, narrowing aborally, height 3.34 mm (excluding arms and tentacular clusters), maximum width 3.25 mm; calyx separated from stalk ( Fig. 1 View FIGURE 1 A −C). Eight adradial arms, length 0.28 mm, width 0.39 mm (excluding tentacular clusters) ( Fig. 1 View FIGURE 1 A, B, E). Arms paired, maximum distance between arms (between base of tentacular cluster) 1.09 mm, minimum distance 0.76 mm. Distal region of each arm with 11−15 capitate tentacles. Tentacles morphologically similar, varied in length ( Fig. 1 View FIGURE 1 E); each tentacle with hollow stem and distal globular end covered with nematocysts. Anchors lacking, except one small globular knob between two of the arms, which is probably a vestigial primary tentacle ( Fig. 1 View FIGURE 1 E). Aboral stalk morphologically distinct from calyx, with one internal chamber at median region (no histological details at base of stalk), without muscles; length 3.10 mm, diameter 1.17 mm, diameter of base of stalk (swollen adhesive disc) 1.82 mm ( Fig. 1 View FIGURE 1 A −C). Base of stalk with an ovoid pit, 0.35 x 0.20 mm ( Fig. 1 View FIGURE 1 F). Abaxial cushion or pad-like adhesive organ at base of tentacular cluster, length 0.13 mm, width 0.60 mm ( Fig. 1 View FIGURE 1 A, E). Manubrium four-sided in cross-section. Numerous gastric cirri in gastrovascular cavity ( Fig. 1 View FIGURE 1 D). Eight adradial gonads extending from manubrium to distal end of arms, organized into four pairs of bands; each band consisting of elongated, nodular lobes of irregular shape ( Fig. 1 View FIGURE 1 D). Numerous nematocyst vesicles distributed at margin on subumbrellar surface. Preserved material yellowish-brown in color. Tentacles with two types of nematocysts: isorhizas (light microscopy was insufficient to distinguish spines), abundant, 11.74 x 2.56 µm (n=10) (mean size of undischarged capsules); euryteles (type I), scarce, 11.0 x 6.0 µm (n=1) ( Fig. 1 View FIGURE 1 G, H). Subumbrellar vesicles with three types of nematocysts: isorhizas (light microscopy was insufficient to distinguish spines), scarce, 10.57 x 2.36 μm (n=2); euryteles (type II), abundant, 8.36 x 6.33 μm (n=10); euryteles (type III), scarce, 6.77 x 3.35 μm (n=2) ( Fig. 1 View FIGURE 1 I −K).
The family Kishinouyeidae , encompassing the genera Kishinouyea , Lucernariopsis , and Sasakiella , is taxonomically problematic. The distinction between the genera Kishinouyea / Lucernariopsis and Sasakiella is based on absence or presence of primary tentacles, respectively, in the stauromedusa stage ( Kramp 1961). However, juvenile stauromedusae of Kishinouyea and Lucernariopsis also have primary tentacles ( Corbin 1978; Grohmann et al. 1999), so this distinction cannot be used because it only reflects developmental stages in the life cycle. The difference between Lucernariopsis and Kishinouyea / Sasakiella is the number of internal chambers in the stalk: one chamber throughout the stalk in Lucernariopsis , one in most part of the stalk but four-chambered basally in Sasakiella and Kishinouyea ( Kramp 1961) . However, detailed histological studies are not common, especially because few individuals (sometimes only one, as in this study) are available. Consequently, some misunderstandings exist concerning this character (e.g., Edmondson 1930 describing four chambers at median part of stalk in K. hawaiiensis , when in fact it has one chamber with four regions; see Edmondson 1930, fig. 6b) that hamper evolutionary understanding of the character and the suitability of using it in taxonomic discussions.
We present here the second recorded individual of L. capensis , and the first record of it from outside its type-locality. The morphology of Brazilian material matches the original description as presented by Carlgren (1938) from the South African type. However, the specimen from Brazil is smaller ( Table 1 View TABLE 1 ) and perhaps a juvenile, as corroborated by the probable vestige of a primary tentacle found between two of the arms (see Corbin 1978, p. 285) ( Fig. 1 View FIGURE 1 E).
Lucernariopsis capensis View in CoL is the second staurozoan species recorded from Brazil. The first, Kishinouyea corbini Larson View in CoL , reported by Grohmann et al. (1999), also belongs to the family Kishinouyeidae View in CoL . Compared to L. capensis View in CoL , K. corbini View in CoL has a shorter stalk, a wide-open calyx, and larger arms ( Larson 1980; Grohmann et al. 1999). The genus Lucernariopsis View in CoL includes four other species: 1) the European Lucernariopsis campanulata (Lamouroux) View in CoL ( Fig. 2 View FIGURE 2 ), intertidal, on algae, with pad-like adhesive organs on the distal arm tentacles (one to each tentacle), equidistant and larger arms (compared to L. capensis View in CoL ), and gonads embedded in the gastric pouch ( Eales 1938, as Lucernaria discoidea ; Kramp 1961; Corbin 1978; Zagal et al. 2011); 2) the European Lucernariopsis cruxmelitensis Corbin View in CoL ( Fig. 2 View FIGURE 2 ), intertidal, on algae, with a different pad structure (“the distal arms tentacles arising directly from it”, Corbin 1978, p. 289), equidistant arms, which are also larger than those of L. capensis View in CoL , and gonads embedded in the gastric pouch ( Corbin 1978; Zagal et al. 2011); 3) the South Australian Lucernariopsis tasmaniensis Zagal et al. View in CoL ( Fig. 2 View FIGURE 2 ), 2–5m depth, on algae, with a shorter stalk, wide-open calyx, equidistant arms, adhesive pad similar to L. capensis View in CoL , and gonads not embedded in the gastric pouch, with several attached nodular lobes, rather irregular in shape, similar to L. capensis ( Zagal et al. 2011) View in CoL ; 4) the Antarctic Lucernariopsis vanhoeffeni (Browne) View in CoL ( Fig. 2 View FIGURE 2 ), 32–137 m depth, on rocks, with a smaller stalk, pad-like adhesive organ on the distal arm tentacles, different from the pad on the arms of L. capensis View in CoL ( Fig. 1 View FIGURE 1 E), and gonads embedded in the gastric pouch ( Browne 1910; Zagal et al. 2011; Smithsonian online database).
Our specimen was found intertidally in Brazil during 1985 in a relatively well known area ( Marques & Lamas 2006). Despite decades of sampling there, the species has never been found again, perhaps because of gradual pollution and environmental decay along the southern coast of São Paulo (Sant’anna et al. 2007).
The disjunct distribution of Lucernariopsis capensis View in CoL , from South Africa and Brazil ( Fig. 2 View FIGURE 2 ), might be explained by a vicariant event of about 160−100 million years, at the opening of the Atlantic. If this really happened and populations have been isolated since then, one could expect highly derived DNA, but molecular data are currently unavailable for the species. Another hypothesis to account for this distribution would be active or passive locomotion of at least some stage of the life cycle. However, Staurozoa have a limited capacity for movement: stauromedusae and stauropolyps spend most of their lives attached to a substrate by their stalk (although they can detach themselves, see Wietrzykowski 1912; Mills & Hirano 2007); and their slow-moving benthic planulae do not possess cilia, unlike other Medusozoa ( Otto 1976). Passive transport, such as by rafting, is a more likely means for these animals to cross oceanic barriers. Nevertheless, there are no data concerning the occurrence of such long-range transport in literature on Staurozoa. Finally, members of the class Staurozoa have been little-studied, and the reported distribution of L. capensis View in CoL may not reflect its actual range.
The family Kishinouyeidae View in CoL is widespread, and the only staurozoan family with species in shallow tropical waters ( Fig. 2 View FIGURE 2 ).This distribution is biogeographically and evolutionarily interesting because most species of Staurozoa inhabit cold waters. New records with morphological and intraspecific data are crucial for solving taxonomic problems in the class Staurozoa and improving knowledge about the diversity and evolution of the group.
TABLE 1. Comparison of measurements between Lucernariopsis capensis from South Africa (SA) (Carlgren, 1938) and Brazil (BR) (this study).
| L. capensis (SA) | L. capensis (BR) | |
|---|---|---|
| Length of arms | 1.8 mm | 0.28 mm |
| Length of calyx | 6.5 mm | 3.34 mm |
| Diameter of calyx | 5.5 mm | 3.25 mm |
| Length of stalk | 5.5 mm | 3.10 mm |
| Diameter of stalk | 2.0 mm | 1.17 mm |
| Base of stalk (swollen adhesive disk) | 3.5 mm | 1.82 mm |
| Tentacles per cluster | 11–27 | 11–15 |
| Isorhiza nematocysts of tentacle | 12.0–14.0 x 2.0–2.5 µm | 11.1–12.4 x 2.2–3.0 µm |
| Isorhiza nematocysts of subumbrellar vesicles | 12.0 x 2.5 µm | 10.3–10.8 x 2.2–2.5 µm |
| Euryteles of subumbrellar vesicles | 7.0–10.0 x 4.0–4.5 µm | 8.1–8.6 x 5.8–6.9 µm |
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Lucernariopsis capensis Carlgren, 1938
| Miranda, Lucília S., Haddad, Maria A., Mills, Claudia E. & Marques, Antonio C. 2012 |
Lucernariopsis capensis
| Zagal 2011: 660 |
| Grohmann 1999: 386 |
| Corbin 1978: 285 |
| Panikkar 1944: 238 |
| Carlgren 1938: 1 |