Acroperus angustatus Sars, 1863
|
publication ID |
https://doi.org/10.5281/zenodo.189352 |
|
DOI |
https://doi.org/10.5281/zenodo.5686930 |
|
persistent identifier |
https://treatment.plazi.org/id/038987B8-AB55-FF9C-5596-BE8FD683FC84 |
|
treatment provided by |
Plazi |
|
scientific name |
Acroperus angustatus Sars, 1863 |
| status |
|
Acroperus angustatus Sars, 1863 View in CoL .
Sars, 1863: 217; P.E. Muller, 1867: 169, Tab. III, fig. 18, Tab IV, fig. 27; Stingelin, 1895: 240–241, Pl. VII, fig. 29; Lilljeborg, 1900: 429–432, Tab. LXIV, fig. 22–27, Tab. LXV, fig. 1–4; Bening, 1941: 254–255, fig. 10 ( harpae angustatus ); Smirnov, 1971: 406, Fig. 491–492; Flössner, 972: 284–287, Abb. 134, F–H ( harpae var. angustatus ); Chiang & Du, 1979: 203–204, Fig. 135; Negrea, 1983: 301–303, Fig. 123; Sars, 1993: 146, Pl. 102: 4–5, Pl. 103: 7–9 ( leucocephalus ); Alonso, 1996: 351–354, Fig. 157–158 ( neglectus ); Flössner, 2000: 344–345, Abb. 125.
Type locality. Lake Ostensjovand, Oslo, Norway (Sars 1963).
Type material: several parthenogenetic females from the type location, Canada balsam slides from G.O. Sars collection, Zoological Museum of Oslo University, slides F 9012, F 9013, F 9014. Type specimens not designated.
Material (* - samples, where A. angustatus coexisted with A. harpae ): over 100 parthenogenetic females from Germany, Berlin Area, Longersee Lake, 15.09.2004, coll. M.A. Belyaeva; over 50 parthenogenetic females from Germany, Berlin Area, Schampilzelsee Lake, 0 3.11.2006, coll. M.A. Belyaeva; over 30 parthenogenetic females, 12 adult and juvenile males from Germany, Berlin Area, Petersdorfersee Lake, 23.10.2006, coll. M.A. Belyaeva; 5 parthenogenetic females, 14 ephippial females, 2 adult males from Germany, Brandenburg, Dreiweibernsee Lake, 0 3.11.2006, coll. M.A. Belyaeva; over 50 parthenogenetic females from Sweden, Uppland, Erken Lake, Lake Erken, 59°51' N, 18°36' E, 07-08.2003, coll. E. Bizina; over 100 parthenogenetic females, numerous ephippial females and males from Lithuania, border of Vilnius and Moletai Regions, Lake Asveya, 19.10.1999, coll. K. Abračiauskas, AAK-1999-124; * 6 parthenogenetic females from Belarus, Vitebsk Area, Miorskii district, Lake Obsterno, 10.07.2005, coll. A.A. Palash; * 6 parthenogenetic females from Russia, Karelia Republic A rock pool near Lake Ukmozero 26.081986, coll. N.N. Smirnov, AAK-1999-039;3 parthenogenetic females from Russia, Karelia Republic, Siamosero Lake, 30.08.1960, coll. N.N. Smirnov, AAK-1999-038; * 3 parthenogentic female, ephippial female Russia, Novgorod Area, Valdai District, Lake Edrovskoje near village Edrovo, 10.10.1993, coll. A.O. Bienkovski, AAK-1999-041; * 22 parthenogenetic females from Russia, Moscow Area, Ruza District, Glubokoe Lake 55°45.217’ N, 36°30.250’ E, 0 8.2008, coll. A.Yu. Sinev; over 30 parthenogenetic females from Russia, Nizhnii Novgorod Area, oxbow Lake near Ust'e river near Otora village, 8.08.2007, coll. M. Tarbeev, AAK-2008-036; 7 parthenogenetic females from Russia, Tomsk Area, a lake near a fisherman house, 57º48.549' N, 84º11.267’ E, 13.07.2005, coll. A.A. Kotov, AAK-2005-284; * over 30 parthenogenetic females from Russia, Irkutsk Area, Barguzinka river close to the coast of Lake Baikal, 0 4.08.2005, coll. A. Evseev; over 30 parthenogenetic females from Russia, Chita Area, Chita town, Lake Kenon, 0 1.09.1971, coll. N. N. Smirnov AAK-1999-122; * over 30 parthenogenetic females from Russia, Jewish Autonomous Area, an second oxbow lake of Tunguska River, after the village of Partizanskaya, 10.09.2007, N.M. Korovchinsky, NMK-2804; * over 40 parthenogenetic females from Russia, Khabarovsk Territory, a small lake near bridge across the River Pir, 48º59,00' N, 136º24.64' E, 0 7.09.2007, coll A.A. Kotov & N. M. Korovchinsky, NMK- 2780;
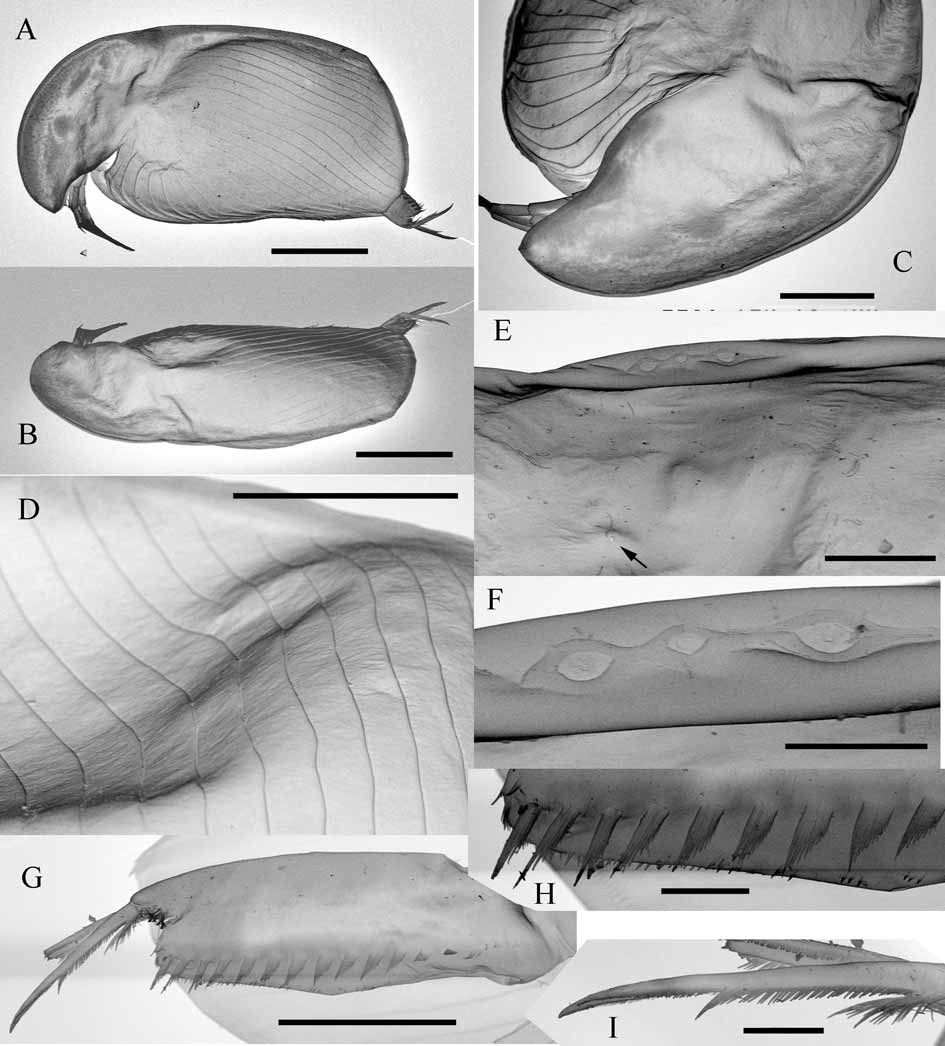
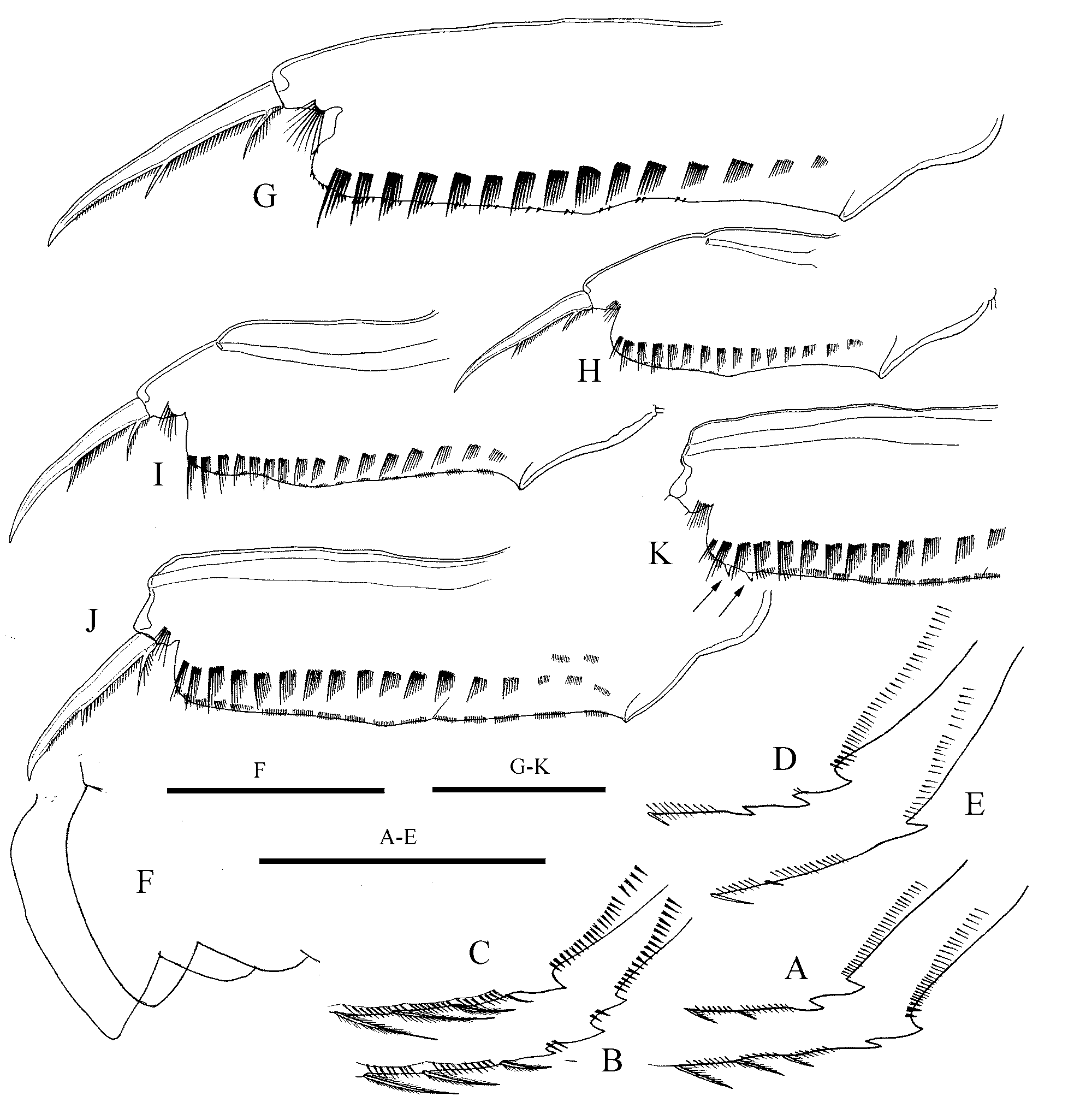
Description. Parthenogenetic female: Body of variable shape ( Fig.6 View FIGURE 6 A–C, I–K, 7A–C), usually low, subrectangular to suboval (height length ratio from 0.48 to 0.59), with maximum height before or at the midline, strongly compressed laterally. Dorsal margin from almost straight to weakly convex, in some specimens with clear depression on the border of valves and head shield. Postero-dorsal angle weakly defined to rounded, posterior margin weakly concave. Postero-ventral angles broadly rounded, provided with 1–3 triangular, saw-like denticles ( Fig 8 View FIGURE 8 A–E). Ventral setae as in previous species, but more numerous (up to 80). Sculpture of valves appears same as in previous species under optical microscope, but SEM examination revealed that the valve have no prominent lines but instead a layered surface ( Fig. 7 View FIGURE 7 D), no fine striae found.
Head keel even more variable than in previous species (see Fig 6 View FIGURE 6 ), both eye and ocellus usually bigger than in previous species, distance between eye and margin of keel varies from 0.9 to 3.2 eye diameters. Eye 2–2.5 times larger than ocellus. Head pores ( Fig. 7 View FIGURE 7 E–F) and labrum ( Fig. 8 View FIGURE 8 F) as in previous species.
Postabdomen ( Fig. 7 View FIGURE 7 G–I, 8G) as for the previous species.
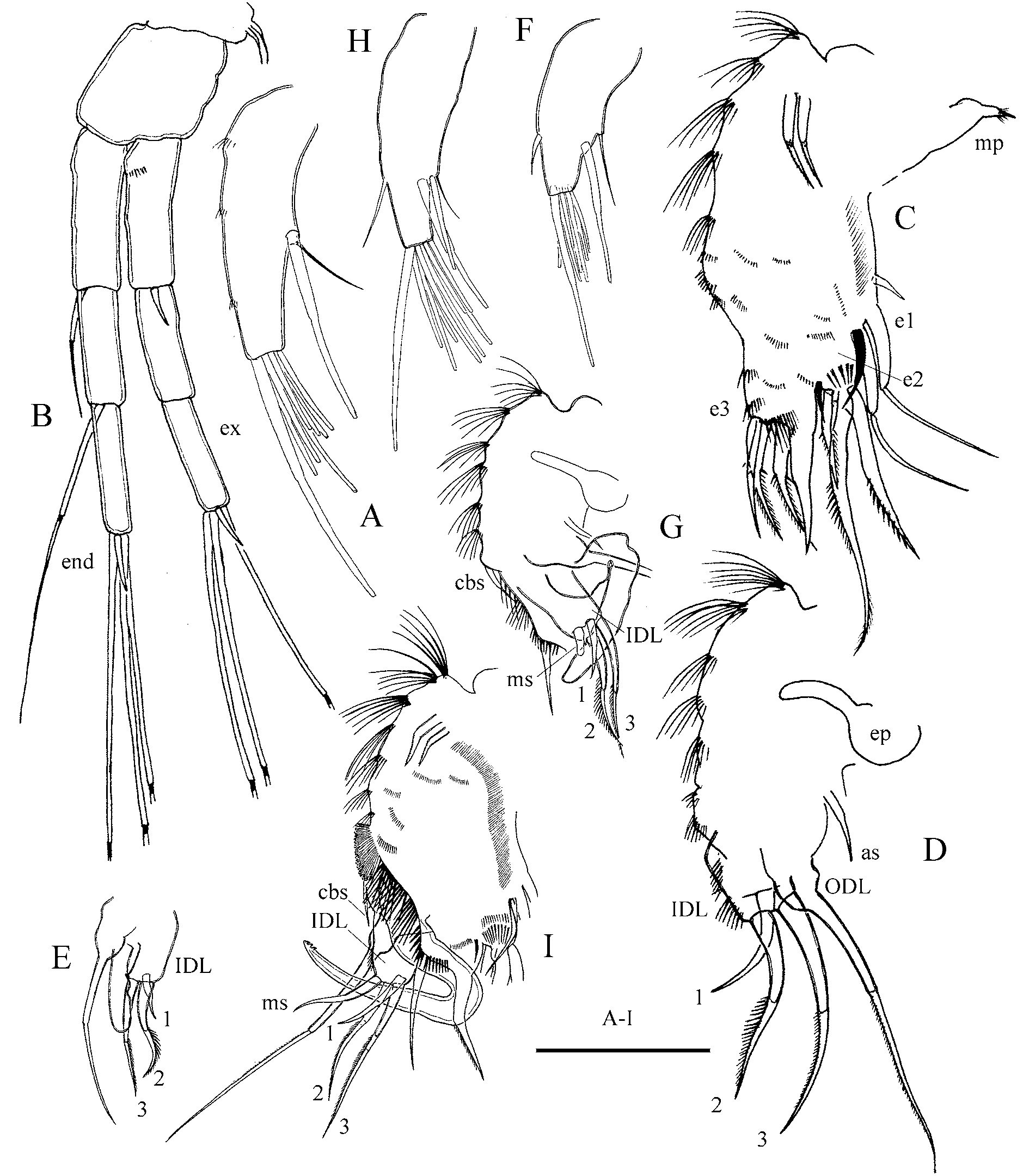
Antennule as for previous species ( Fig. 9 View FIGURE 9 A), but longest terminal aesthetasc is relatively longer than in A. harpae .
Antenna ( Fig. 9 View FIGURE 9 B) shorter than in previous species, less than 1/5 of body length. Antennal formula, setae 0-0-3/1-1-3, spines 1-0-1/0-0-1. Branches long and slender, of equal length. Seta arising from basal segment slightly shorter or equal to the middle segment. Seta arising from middle segment of endopodite 2.5 times longer than apical segment. All apical setae of same thickness and similar length. Spines same as in previous species.
Thoracic limb I ( Fig. 9 View FIGURE 9 C–D) same as in previous species, with two exceptions. Accessory seta much longer than in A. harpae . Longest seta of endite two longer than ODL seta.
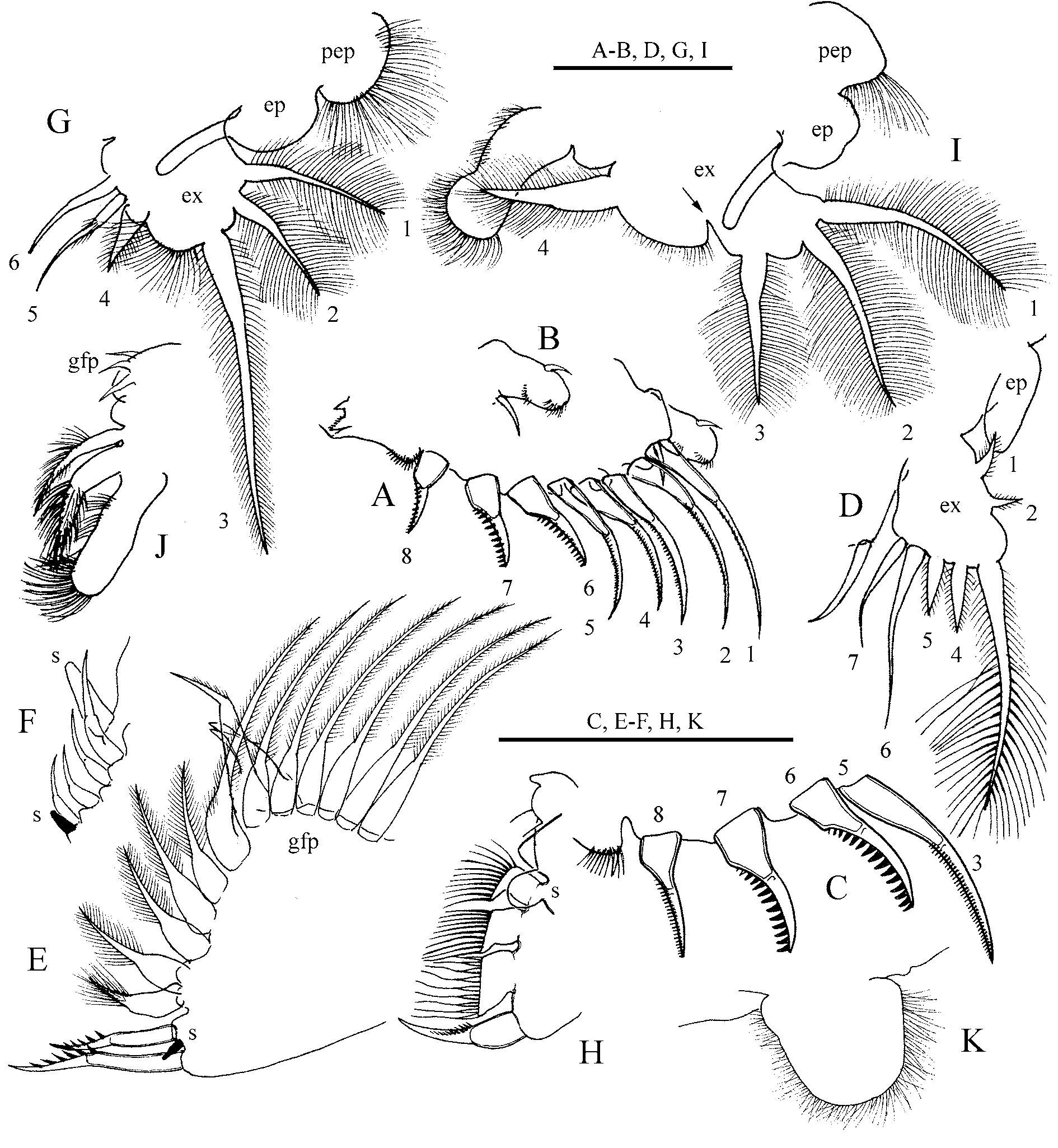
Thoracic limb II ( Fig. 10 View FIGURE 10 A–B) similar to that of previous species, but scraping spines six to seven significantly thicker than scrapers five and eight ( Fig. 10 View FIGURE 10 C) and armed with relatively thicker setules. Thoracic limb III ( Fig. 10 View FIGURE 10 D–F) as for previous species. Thoracic limb IV ( Fig. 10 View FIGURE 10 G–H) as for previous species, but exopodite seta four two times bigger than in A. harpae , seta four lack long setules.
Thoracic limb V ( Fig. 10 View FIGURE 10 I–J) same as in previous species, with two exceptions. Finger-like projection only just shorter than epipodite itself, not reaching apex of exopodite. Division in exopodite narrow, forming an acute angle or a V ( Fig. 10 View FIGURE 10 I, arrow). Exopodite with four plumose setae, their length gradually decrease from seta one to four. Seta four twice as thick as other setae. Inner limb portion a narrow lobe, with short setules on inner margin. At inner face, two distally setulated setae, subequal in length, distal one twice as thick as proximal. Filter plate with three short setae increasing in size distally.
Thoracic limb VI ( Fig. 10 View FIGURE 10 K) as in previous species.
Ephippial female. Body higher than that of parthenogenetic females ( Fig. 6 View FIGURE 6 D –E), dorsal margin, especially in smaller specimens, highly arched, height-length ratio in studied material from 0.56 to 0.62. Ephippium yellow-brown, transparent.
Juvenile male of instar I smaller than juvenile female of same instar, with lower body, ( Fig. 6 View FIGURE 6 F) with ridge in place of female head keel. Postabdomen ( Fig. 8 View FIGURE 8 H), antennule and limb I ( Fig. 9 View FIGURE 9 E) as for previous species.
Juvenile male of instar II significantly smaller than juvenile female of same instar ( Fig. 6 View FIGURE 6 G), with ridge in place of female head keel. Postabdomen similar to that of previous species ( Fig. 8 View FIGURE 8 I). Antennule ( Fig. 9 View FIGURE 9 F) similar to that of previous species, but much smaller than in adult male. Thoracic limb I ( Fig. 9 View FIGURE 9 G) similar to previous species.
Adult male. Similar in shape to juvenile female of instar II ( Fig. 6 View FIGURE 6 H), head and valves with ridge instead of the female keel. Maximum height at the second fourth of the body, height/length ratio about 0.67. Dorsal margin of valves highly arched.
Postabdomen long and narrow ( Fig. 8 View FIGURE 8 J), with parallel margins, postanal portion rectangular. Postanal angle well-defined, preanal angle not defined, distal portion 2.5 times longer than preanal. Postabdominal claws situated on small protrusion in the middle of distal margin of postabdomen. The sperm ducts open above the protrusion, posteroventral and posterodorsal angles weakly rounded. Marginal setules and lateral fascicles of setules same as in female. Postabdominal claw of much shorter than that of female, shorter preanal portion of postabdomen.
Antennule ( Fig. 9 View FIGURE 9 H) similar to that of previous species, but one of lateral aestetaschs significantly shorter and thinner than other. Limb I (Fig. I) similar to that of previous species, with a few differences. Copulatory brush seta long, longer than first IDL seta. Ventral face of the limb under the copulatory brush with two rows of very long setules, about 20 setules in one and about seven in other.
Size. Length of female of juvenile instar I— 0.39–0.43 mm, of juvenile instar II— 0.47–0.56 mm, of adult female— 0.55–0.95 mm. Length of male of juvenile instar I— 0.38–0.42 mm, of instar II— 0.42–0.46 mm, of adult male— 0.49–0.58 mm.
Main differences between A. angustatus Sars, 1863 and A. harpae ( Baird, 1834)
The validity of A. angustatus is hereby confirmed, and this species is not a form of A. harpae . Morphology reveals distinct differences in shape and armament of the antennae (see Table 1 View TABLE 1 ), A. harpae and A. angustatus clearly differs by the proportions of the branches and by the morpology of exopodite setae. It also should be noted that A. harpae have longer antenna than A. angustatus . Because crawling animals should reach the substrata with the end of the antennae for pushing (see Fryer, 1968), the species with more high body ( A. harpae ) needs longer antennae do it effectively. Thickened apical exopodite seta of A. harpae also can be used for forceful pushing, like such setae of Macrothricidae , and greatly increase effectiveness of crawling. No intermediate status of these characters was found in the studied material, even in samples where the two species coexist. The differences in antennal morphology may therefore be the main diagnostic feature for discrimination of Acroperus females, both parthenogenetic and ephippial.
Lilljeborg (1900) had already indicated these differences in antennal morphology in his monograph, but it was overlooked by following researchers. Only Alonso (1996) used these characters to distinguish these two species of Acroperus from Spain. Specimens of A. angustatus were erroneously identified as A. neglectus Lilljeborg, 1900 ; they lack, however, the main diagnostic feature of latter taxon (antennule protruding below the apex of rostrum). Specimens of A. angustatus from Spain have very short and high “ harpae - type ” body. Comparison of antennae and males with Alonso's (1996) descriptions, confirm that these are A. angustatus .
Our data further revealed differences in the shape of the postero-ventral corner of the valves (see Table 1 View TABLE 1 ), observed by Flössner (2000). Denticles of A. angustatus are more developed, saw-like, clustered together. Those of A. harpae are smaller and spaced. Our data does not confirm other differences noted by Flössner (2000).For example, we found that the end position of the marginal valve setae in A. angustatus relative to the denticles, is variable. Difference in the shape of the denticles can be particularly useful for the identification of Acroperus remains in paleolimnological studies up to species level.
Other differences in the morphology of female include the shape of exopodites V, different lengths of finger-like processes of exopodites I and V, different sizes/morphologies of scrapers of limb II and different shapes of some limb setae. Such differences are frequently observed between close species of same genus, and, of course, are of limited value for a quick identification. Nevertheless, they confirm separation of both species observed in the former characters.
No specimens with intermediate states of the above diagnostic features were revealed in samples where these two species coexisted. The possibility of hybridization is not confirmed, or at least no morphological evidence was seen.
The variability of body and head shapes/proportions between both appeared much greater than that usually observed for Aloninae . It is similar to that observed for the genera Bosmina or Daphnia ( Dumont & Negrea, 2002) . In most species of the Chydoridae , proportions of the body change with growth, but to my opinion show relatively less intraspecific variation (see Smirnov, 1971). The main source of variability is the level of head keel development. Fryer (1968) suggested that the keel on the head and body is useful for crawling through filamentous algae. But both species are frequently encountered in habitats with few filamentous algae, and I observed no correlation between the degree of keel development and presence of the algae. Variable head keel and fornix in Daphnia are an adaptation to predation ( Dumont & Negrea, 2002), and the level of their development within a species can vary greatly depending on predation pressure. I speculate that the head keel of Acroperus could be a defensive structure, increasing dimensions of animal and possibly hindering catching and handling of the animal by invertebrate predators.
TABLE 1. Main differences between Acroperus harpae and Acroperus angustatus.
| Character | Acroperus harpae | Acroperus angustatus |
|---|---|---|
| Denticles on postero-ventral corner of valves | with narrow bases, spaced | with broad bases, saw-like, clustered together |
| Antenna: | ||
| length | about 1/4 of body length | about 1/5 of body length |
| length of branches | exopodite much longer than endopodite | branches of same length |
| seta on basal segment of exopodite | less than 2/3 length of middle segment | as long as middle segment |
| seta on middle segment of exopodite | 1.5 times longer than apical segment | 2.5 times longer than apical segment |
| apical setae of exopodite | one seta much thicker than others | all setae of same thickness |
| Scrapers 6-7 of limb II | similar to other scrapers | more robust than other scrapers |
| Limb V exopodite | Incursion between lobes broad, rectangular | incursion between lobes as acute angle |
| Male postabdomen | ||
| shape | narrowing in distal part | rectangular |
| postabdominal claw | longer than preanal portion of postabdomen | shorter than preanal portion of postabdomen |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Genus |