Heterophilus scabricollis Pu, 1988
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3949.4.7 |
|
publication LSID |
lsid:zoobank.org:pub:38453726-B686-4E18-9076-2E445A277578 |
|
DOI |
https://doi.org/10.5281/zenodo.5686900 |
|
persistent identifier |
https://treatment.plazi.org/id/03893070-FFAF-3518-FF17-FF45FD57D203 |
|
treatment provided by |
Plazi |
|
scientific name |
Heterophilus scabricollis Pu, 1988 |
| status |
|
Heterophilus scabricollis Pu, 1988 View in CoL
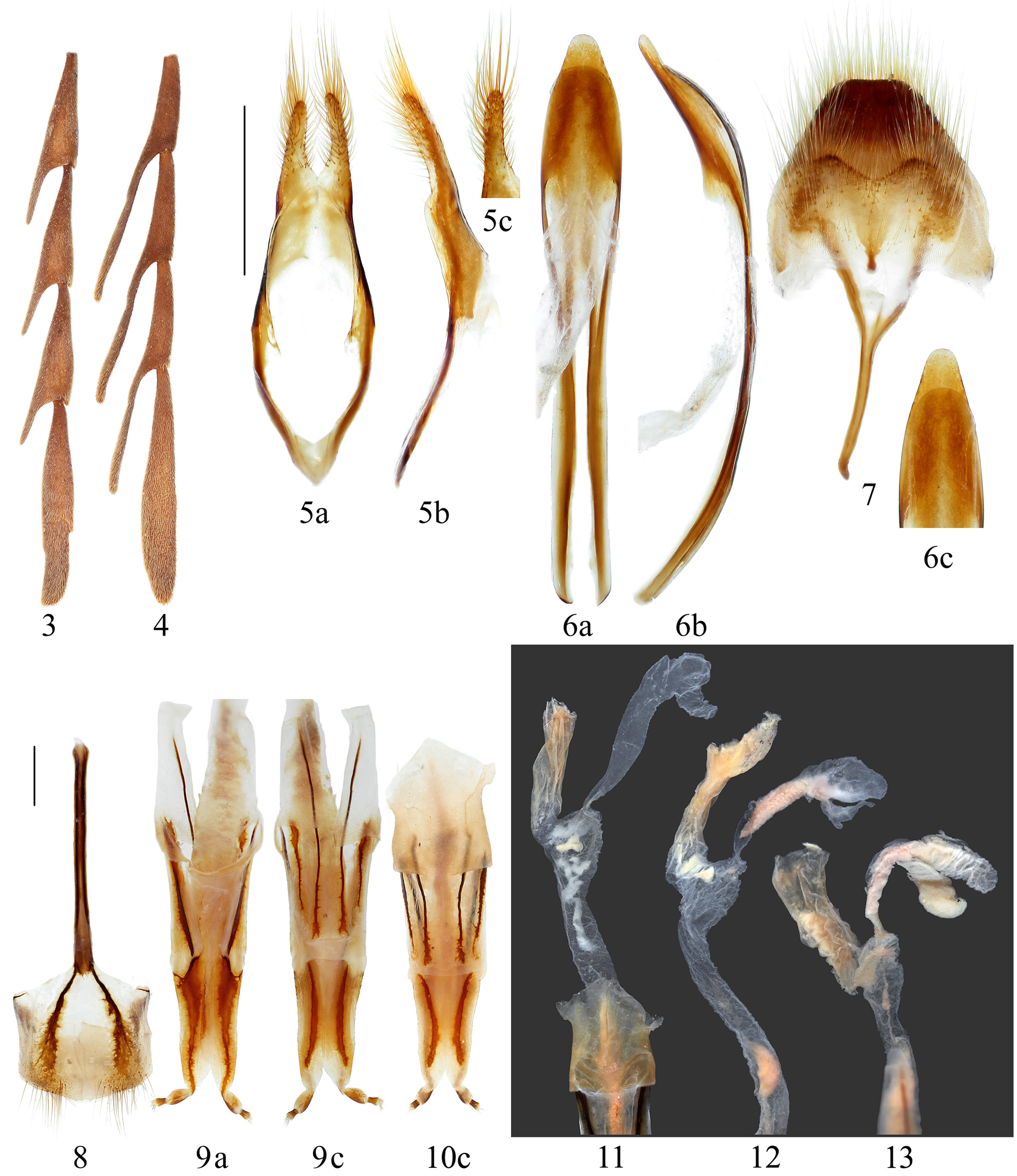
( Figs. 8–21 View FIGURES 3 – 13 View FIGURES 14 – 15 View FIGURES 16 – 21 )
Heterophilus scabricollis Pu, 1988: 294 View in CoL , 303, fig. 1.
Description (Female). Body length (from clypeus to elytral apex): 22.5–29.0 mm, maximum width (at humeri): 7.5–9.2 mm. Head and pronotum black, with some moderately long grayish pubescence. Scutellum black. Elytra, ventral surface of body and legs (femora and tibiae) black-brown to black with short grayish or brownish pubescence, antennae and tarsi usually lighter brown to red brown.
Head subvertical in front; mandibles rather long, with sharp apex, crossed when closed; maxillae relatively reduced, palpus long, four-segmented, of which third the shortest, terminal segment tapering distally (apex appears truncated or sunken in dry specimens), apex with setae; galea with dense setae, lacinia reduced; eyes moderate in size, slightly emarginated, lower lobes larger than upper lobes. Antennae short, never extending to the middle of elytra, antennomeres slightly flattened but not serrate; scape stout, third the longest; fourth slightly shorter than fifth and slightly longer than sixth; fifth to tenth very slightly diminishing, last antennomere longer than tenth; relative lengths of segments from base to apex 8: 3: 12: 9: 10: 8: 8: 7: 7: 7: 8.
Prothorax with weak lateral carinae extending from base to apex, but hardly visible in dorsal view ( Fig. 15 View FIGURES 14 – 15 ); procoxal cavities widely open posteriorly, intercoxal process broad, expanded (bearing the secondary procoxal articulation) and deeply medially impressed in its lowest part and then abruptly declivous with broadly bifurcate apex. Mesonotum without median line, stridulatory plate present. Elytra about 2.5 times as long as the maximum width of prothorax near base, widest near middle, then gradually narrowed apically with rounded apices, and slightly dehiscent apically. Hind wings strongly abbreviated.
Abdomen with ventrite V (sternite VII) wider than length with rounded apex and a small medial sinus; apical half of tergite VII exposed from elytral apex.
Legs rather long, fringed with feeble hairs; pro- and mesotibiae with short and blunt teeth (shorter than surrounding hairs) on outer side; tibial spur formula 1/2/2; tarsi with third tarsomere cleft to 1/4 of the length, first tarsomere subequal to second and third combined. Fore coxae prominent, strongly transverse.
Female genitalia ( Figs. 8–13 View FIGURES 3 – 13 ) with paraproct long, its baculi quite thick, long and almost straight; valvifer indistinct; coxite narrowed posteriorly, each baculum broadened both inwardly and laterally at the base, though tapered towards apex, about 3/4 of the length of paraproct baculi; coxite lobes short but not very narrow, scleratized except for basal and apical portions, with tactile hairs at the apices; stylus apical in position, much smaller than coxite lobes, sclerotized except for apex which bears tactile hairs; dorsal baculi short and thick, not so straight but curved outwards; proctiger long, with two pairs of thin baculi, inner pair longer than paraproct baculi, outer pair about one third the length of inner (variable, sometimes subequal to inner). Vagina long and moderate narrow, swollen at base; vaginal plates and bursa copulatrix absent; spermatheca existing as a moderate large membraneous pouch (capsule), with a spermathecal gland, of which the size and shape are variable ( Figs. 11–13 View FIGURES 3 – 13 ); spermathecal duct not clearly distinguished from membraneous spermathecal capsule, narrowed towards swollen part of vagina. Sternite VIII ( Fig. 8 View FIGURES 3 – 13 ) with two quite strongly sclerotized lateral “baculi”, which followed two patches of apical setae, and attached to speculum ventrale at base. Anterior apodeme of sternite VIII (= spiculum ventrale = tignum, Fig. 8 View FIGURES 3 – 13 ) much shorter than abdomen, ca 1/3 of abdominal length.
Distribution. China: Xizang (Tibet) Autonomous Region.
Material examined. China, Xizang (Tibet): holotype (21.0 mm long), male, Xizang, Milin, Paiqu, alt. 3000 m, 1983. VII.15, leg. Yin-Heng Han ( IOZ (E) 217595). 3 males & 4 females, Xizang, Linzhi, Milin, Pai, 3200 m, 2013. VII.9, leg. Wen-Xuan Bi ( CBWX & IZAS, IOZ (E) 1896962); 3 males & 5 females, same data but leg. Chao Wu ( CBWX & IZAS, IOZ (E) 1896963); 9 females, same data but leg. Jian Hao ( CCCC); 1 female, same data but leg. Xiao-Dong Yang ( CCCC).
Remarks. Females are considerably larger than males ( Figs. 14–15 View FIGURES 14 – 15 ) and Švácha et al. (1997) estimated that the size of females (of H. punctulatus ) may be 30 mm or more based on the size of the larvae. Body size of males examined was 19.0–21.0 mm and width (at humeri) was 5.6–6.6 mm. The largest female measured 31.5 mm from tip of mandible to elytral apex. The spermathecal gland is described for the first time for any member of Philinae in the present study, suggesting that Saito (1990) was correct to interpret the petiolate membranous sac as a desclerotized spermatheca. Sperm was observed in the spermatheca ( Figs. 12–13 View FIGURES 3 – 13 ). We dissected females of Philus antennatus (Gyllenhal), 1817 , and Mantitheus pekinensis Fairmaire, 1889 , but neither had a gland, again consistent with the findings of Saito (1990). Thus, the present of spermathecal gland might be considered a generic character of Heterophilus . The following characters of female genitalia of Heterophilus scabricollis Pu are quite variable according to our observations: 1) the length of proctiger buculi are variable, usually the outer pair shorter than inner pair but sometimes the outer pair subequal to inner pair, and begins at the same point ( Fig. 10 View FIGURES 3 – 13 ); 2) the position, size and shape of spermathecal gland are very variable ( Figs. 11–13 View FIGURES 3 – 13 ).
Biology and ecology. The habitat of this species occurs at an altitude of ca. 3000 m, near Pai town of Xizang (Tibet) Autonomous Region located on the last section of the middle reaches of the Yarlung Zangbo River. The main vegetation at the collecting site is mixed coniferous and broadleaf tree forest in alpine grasslands with the primary herbaceous species being Imperata cylindrica (Linnaeus) Palisot de Beauvois, Poaceae ( Figs. 16–18 View FIGURES 16 – 21 ) and the dominant tree species around the collecting grassland being Quercus semecarpifolia Smith , Fagaceae . Adults are predominantly nocturnal. Males ( Fig. 21 View FIGURES 16 – 21 ) are attracted by light, and females hide in the emergence holes in the ground during the daytime. One female was found walking on the ground at night. A number of females (but no males) were found in ca. 1.5 cm diameter emergence holes at depths of 10–20 cm. The soil galleries are usually vertical or slightly oblique. The expedition team searched three grasslands of similar vegetative composition, and about 60% of the holes (n= 42) of 1.5 cm diameter contained a single female. However, no beetles were recovered from holes less than 1.0 cm diameter (n>10), although spiders and wasps were often found instead. This size specificity suggests the holes which females hide in during the daytime are most likely also their emergence holes, though there were some deeper holes that could not be completely excavated. Females cannot fly because the hind wings are strongly reduced in spite of well-developed elytra. Copulation and oviposition were not observed but likely occurs at night in the grassland. They may lay eggs before July because all females collected on 9 July, 2013 did not contain eggs, presumably because they had already oviposited as this would be very late for an unmated female to be found. Larvae were not found, but are likely to be subterranean and feed on the roots of cogongrass (i.e. Imperata cylindrica ) based on the location of holes in grassland areas with cogongrass ( Figs. 16–20 View FIGURES 16 – 21 ). Larva of a congener, Heterophilus punctulatus has been recorded feeding on the roots of cogongrass (Chiang & Chen 1996; Švácha et al. 1997; Švácha & Lawrence 2014).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Heterophilus scabricollis Pu, 1988
| Bi, Wenxuan & Lin, Meiying 2015 |
Heterophilus scabricollis
| Pu 1988: 294 |