Carinina plecta, Kajihara, 2006
|
publication ID |
https://doi.org/ 10.5281/zenodo.2645302 |
|
publication LSID |
lsid:zoobank.org:pub:95608DB2-F9ED-4B68-8E9E-93EE84887227 |
|
persistent identifier |
https://treatment.plazi.org/id/03825157-FF8C-5669-FB3F-936EFD66F8C0 |
|
treatment provided by |
Plazi |
|
scientific name |
Carinina plecta |
| status |
sp. nov. |
Carinina plecta sp. nov. ( Figs 3–10 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 )
Diagnosis
Carinina with rhynchocoel wall composed of inner longitudinal muscle layer and outer layer of interwoven circular and longitudinal muscle fibres; proboscis insertion originating only from rhynchocoel inner longitudinal muscle layer; interwoven layer of rhynchocoel wall continues to rhynchodaeum; a pair of rhynchocoel vessels present, communicating with lateral blood vessel via a ladderlike series of transverse connectives.
Etymology
The specific epithet, derived from the Greek plectos (= basket), refers to the nature of the rhynchocoel wall primarily composed of interwoven circular and longitudinal muscle fibres.
Material examined
Holotype, female, caudal end missing, ZIHU3123, 31 July 2003, HK coll.; 6µm transverse serial sections through the head region, the middle body, and the posterior end of the fragment, 10µm longitudinal serial sections in the two intervening portions, 116 slides in total.
External features
The body fragment obtained was 13 cm long, 1 mm wide. When alive ( Fig. 3A View FIGURE 3 ), the nemertean was uniformly a translucent white with no colour pattern; the intestine was light beige and the ovaries tinged with a khaki colour. When the animal was placed in seawater in a Petri dish its movement was very sluggish. The head is wider than the neck, rounded anteriorly, variable in shape during movement; a whitecoloured, cylindrical rhynchodaeum could be seen through the epidermis ( Fig. 3B View FIGURE 3 ).
Body wall, musculature, and parenchyma
Four major types of glandular cells are distinguishable in the epidermis by their staining affinity to the Mallory’s trhichrome method: these are identified as type G1) red granular cells with acid fuchsin positive cytoplasm; type G2) granular cells with Orange G positive cytoplasm, orange in colour with various degrees of chromatic luminosity; type G3) violet granular cells; and type G4) lightblue basophilic mucous cells ( Fig. 3C View FIGURE 3 ).
Anterior to the brain, the epidermis is 40–80 µm thick and contains all four types of glandular cells situated proximally; glandular cells are sparse at the tip of the head but gradually increasing in number posteriorly. In front of the brain, type G1 glandular cells are predominant midventrally, while the other three types appear over the rest of the circumference of the epidermis ( Fig. 3C View FIGURE 3 ).
In the brain region, the epidermal thickness increases up to 130 µm, the glandular cells occupying the proximal 2/3 to 3/4 of the epidermis; type G1 glandular cells form a median mass situated between the ventral cerebral ganglia. In front of the mouth ( Fig. 4 View FIGURE 4 ), this aci dophilic glandular mass splits into three lobes, the middle branch leading to the middorsal side of the buccal epithelium and the lateral ones remaining confined to the epidermis; these branches soon disappear into the anterior portion of the mouth opening.
In the foregut region, type G4 basophilic mucous cells predominate on all sides of the body and occupy the proximal half of the epidermis, whereas the acidophilic granular glands are scattered and mostly situated in the proximal 1/4 of the epidermis. There is an exclusively acidophilic epidermal region in front of the excretory system. Glandular cells decrease in number posteriorly, but in the intestinal region an acidophilic glandular zone is present around each gonopore ( Fig. 3D View FIGURE 3 ). E (b) = 0.25; E (i) = 0.04.
The epidermal basement membrane ( Figs 4 View FIGURE 4 , 5 View FIGURE 5 ) is up to 3 µm thick; connective tissue processes extend from the basement membrane into the epidermis in the brain region without forming a meshlike network ( Fig. 3C View FIGURE 3 ); a thin neural layer is present between the epidermis and basement membrane.
Anterior to the mouth, the bodywall musculature consists of outer circular and inner longitudinal muscle layers; a diagonal layer is situated between these two layers in all parts of the body except in the brain region ( Fig. 6A View FIGURE 6 ). Immediately posterior to the mouth, circular muscles appear lateral to the buccal epithelium and then extend dorsally and ventrally outside the lateral blood vessel. Behind the mouth, these lateral bands of circular muscles meet middorsally and midventrally to form the inner circular muscle layer of the body wall.
Immediately anterior to the ventral cerebral commissure, horizontal transverse muscle fibres appear below the rhynchocoel and above the dorsal side of the bodywall longitudinal muscle layer ( Fig. 6B View FIGURE 6 ); these horizontal fibres then connect laterally with the outer circular muscle layer of the body wall.
The longitudinal bodywall muscles below these horizontal fibres branch off ventrally from the rest of the longitudinal muscle layer to form a muscle bundle medial to each of the ventral ganglia. Just anterior to the mouth opening, this longitudinal muscle bundle diverges into a branch on each side ( Fig. 4 View FIGURE 4 ). The branches then extend lateral to the buccal cavity as the mouth opens ( Fig. 6C View FIGURE 6 ).
The horizontal transverse muscle fibres beneath the rhynchocoel remain above the buccal cavity in contact with both the circular muscles around the buccal epithelium and the bodywall circular muscle layer ( Fig. 6C View FIGURE 6 ). These horizontal transverse muscles disap pear dorsal to the buccal cavity, and the longitudinal muscles lateral to the buccal cavity fuse with the bodywall longitudinal layer in the dorsal side of the body. A longitudinal muscle plate is present between the rhynchocoel and alimentary canal ( Fig. 5 View FIGURE 5 ), emerging from the region dorsal to the buccal cavity but disappearing in the intestinal region. Unusually thick muscle fibres, up to 10 µm thick, appear in the rhynchocoel wall, bodywall inner circular muscle layer, and proximal portion of the longitudinal muscle layer ( Fig. 6D View FIGURE 6 ). No dorsoventral musculature was found postcerebrally.
Parenchymatous connective tissues, though present throughout the body, are not well developed.
Proboscis apparatus
The proboscis pore opens midventrally near the tip of the head, leading posteriorly to the rhynchodaeum, which consists of 1) an inner nonciliated epithelium up to 5–10 µm thick, 2) a longitudinal muscular stratum, 10–20 µm thick, in which a pair of rhynchodaeal nerves is situated, 3) a connective tissue layer 5–30 µm thick, and 4) an outer muscle wall composed of interwoven longitudinal and circular muscle fibres, which leads posteriorly to the rhynchocoel wall. In the region where the rhynchodaeum leads dorsally from the ventral bodywall muscle layer, just behind the proboscis pore, the rhynchodaeum is suspended in the cephalic blood lacuna. It then attaches dorsally and ventrally to the inner surface of the bodywall longitudinal muscle layer; the thickness of the rhynchodaeal outer muscle wall is only a few fibres thick except laterally, where it is up to 40 µm across.
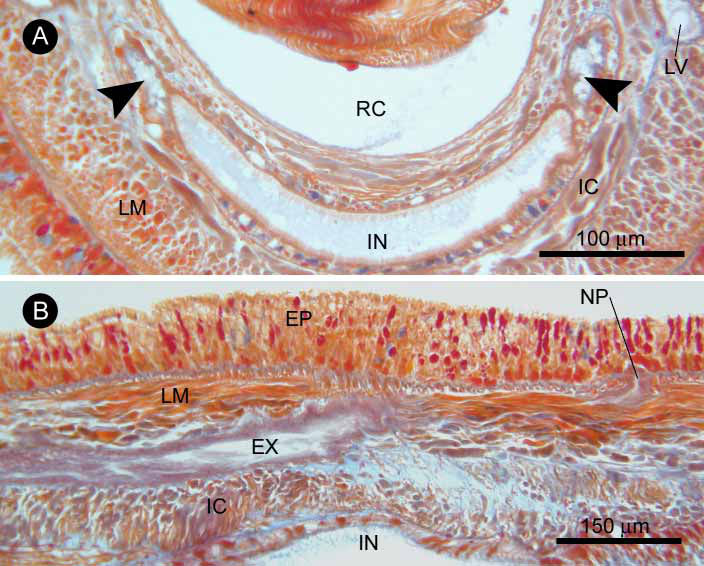
The rhynchocoel wall is composed of an outer layer of interwoven longitudinal and circular muscle fibres, a thin connective tissue layer, a sparse and incomplete inner layer of discontinuous longitudinal muscle fibres, and a fine epithelium. The posterior extent of the rhynchocoel could not be determined, since the body fragment obtained did not contain the posterior end of the rhynchocoel. Just behind the proboscis insertion, the rhynchocoel wall is thin dorsally and ventrally, thicker laterally. Postcerebrally, the rhynchocoel is generally thinnest dorsally.
The proboscis is inserted precerebrally. Anterior to the ventral cerebral commissure, the connective tissue layer of the rhynchocoel gradually increases in thickness, with its inner surface much folded ( Fig. 8A View FIGURE 8 ); the inner longitudinal muscle fibres in the rhynchocoel wall increase in number to form a continuous, distinct layer that becomes thicker, up to 7 µm, before it merges into the proboscis insertion ( Fig. 8B View FIGURE 8 ). No bodywall musculature contributes to the proboscis insertion.
The proboscis ( Fig. 8C, D View FIGURE 8 ) is composed of an outer glandular epithelium, an outer cir cular muscle layer, a middle longitudinal muscle layer, a connective tissue layer, a delicate inner circular muscle layer and an endothelium; two proboscis nerves are situated between the glandular epithelium and outer circular muscle layer ( Fig. 8C View FIGURE 8 ). As far as could be observed, the proboscis does not exhibit any significant regional histological differentia tion, although the material examined does not contain the posterior end of the organ.
Alimentary canal
The mouth opens just behind the brain. The foregut epithelium is ciliated and deeply folded, up to 90 µm in maximum thickness; basophilic glandular cells stained a bright blue dominate anteriorly, but farther back these are replaced by glandular cells stained purple with Mallory. Posteriorly the foregut epithelium decreases in thickness to 15–20 µm, becomes less folded, and has a greater number of neutrophilic cells. From the junction between the foregut and intestine, a pair of intestinal pouches extends anteriorly for at least 144 µm on both sides of the foregut ( Fig. 9A View FIGURE 9 ). There are no lateral intestinal diverticula.
Blood system
A pair of cephalic blood lacunae ( Fig. 3C View FIGURE 3 ) meets over the rhynchodaeal opening; the lacunae also communicate with each other under the rhynchodaeum just after it leaves the ventral part of the bodywall muscle layer. Throughout their length, the cephalic blood lacunae are randomly pierced by dorsoventral muscle bundles, each up to 15 µm thick, that appear to originate from either the bodywall circular or diagonal muscle layers ( Fig. 7 View FIGURE 7 ); these bundles disappear posterior to the brain. The lacunae pass through the cerebral ring along both sides of the rhynchodaeum without changing their diameters. As they traverse the vicinity of the mouth, the lacunae then gradually decrease in diameter, develop distinct walls, and lead into lateral vessels.
Farther back, the lateral blood vessels are situated dorsolateral to the alimentary canal and ventrolateral to the rhynchocoel. They first run inside the bodywall inner circular muscle layer ( Fig. 5 View FIGURE 5 ), but in the posterior foregut region gradually shift peripherally to lie embedded in the circular muscle layer; in the intestinal region they eventually come to lie outside this layer ( Fig. 9A View FIGURE 9 ).
A pair of rhynchocoel vessels is present in the foregut region; the lateral vessels repeatedly send out branches, at almost right angles, that penetrate the rhynchocoel wall to communicate with the rhynchocoel vessels ( Fig. 5 View FIGURE 5 ); in the holotype, there are 32 of these branches on the right side of the body and 25 on the left. The rhynchocoel vessel is medially exposed to the rhynchocoel lumen and appressed to the rhynchocoel wall, but extends into the wall to connect vessel ( Fig. 10 View FIGURE 10 ).
Nervous system
The brain is situated in the proximally in the epidermis; the inner halves of the lateral nerves are embedded in the bodywall musculature, whereas the outer halves remain in the epidermis ( Fig. 5 View FIGURE 5 ). There are two dorsal cerebral commissures, each about 15 µm thick, and a single ventral one 58 µm thick; the anterior dorsal commissure lies slightly anterior to the ventral commissure. There is an inner, but no outer, neurilemma. Neither neurochords nor neurochord cells were found.
A pair of buccal nerves originates medially from the ventral ganglia just anterior to the mouth, within the mass of the type G1 acid fuchsinpositive glandular cells in the epidermis. They run a short distance posteriorly before uniting into a single trunk; the trunk soon forks, giving rise again to a buccal nerve on each side, each up to 40 µm in diameter, situated laterally on the base of the buccal epithelium ( Fig. 4 View FIGURE 4 ). Each buccal nerve sends off a thin branch, about 10 µm in diameter, which runs upward and posteriorly in the basal part of the buccal epithelium near the dorsal midline, the two branches forming a pair ( Fig. 6C View FIGURE 6 ). The main buccal nerves, situated laterally, then gradually move ventrolaterally and further subdivide into several thin branches near the posterior border of the mouth; these nerves extend posteriorly in the foregut wall, but their ultimate fate could not be traced.
An upper middorsal nerve, originating from the two dorsal cerebral commissures, lies in the basal portion of the epidermis ( Figs 4 View FIGURE 4 , 5 View FIGURE 5 , 7 View FIGURE 7 ), and extends anteriorly to where the rhynchodaeal outer muscle wall attaches dorsally to the bodywall musculature. The posterior extent of the upper middorsal nerve is uncertain, but it reaches at least to the hind end of the body fragment obtained.
A lower middorsal nerve lies along the dorsal rhynchodaeal ( Fig. 7 View FIGURE 7 ) and rhynchocoel ( Fig. 5 View FIGURE 5 ) walls; it possesses frequent neural connections to the upper middorsal nerve. Precerebrally, the neural connections between the upper and lower middorsal nerves are occasionally forked.
Precerebrally, numerous thick peripheral nerves emerge anteriorly from the cerebral ring and extend between the epidermis and basement membrane; two conspicuous peripheral nerves lie ventrolaterally ( Fig. 7 View FIGURE 7 ), extending to the tip of head, and turning medially to penetrate the rhynchodaeal epithelium, eventually forming the proboscis nerves ( Fig. 8C View FIGURE 8 ).
Sense organs
The cerebral sense organs are simple, consisting of ciliated pits in the epidermis 50–65 µm deep and up to 10 µm in diameter basally. They are situated laterally to the ventral cerebral ganglia ( Fig. 4 View FIGURE 4 ). Statoliths were not found. Lateral sensory organs are absent. There are no eyes.
Frontal organ and cephalic glands There are neither a frontal organ nor cephalic glands. Excretory system
Many details of the excretory system could not be observed, since it lay in the region sectioned longitudinally. The excretory organs are situated in the anterior intestinal region on each side of the body; anteriorly, each organ is composed of a glandular mass, from which a thick collecting tubule, up to 50 µm in diameter, runs posteriorly. At the posterior end, each collecting tubule turns distally at a right angle to form an efferent duct, about 25 µm in diameter, that opens by a single nephridiopore on each side of the body ( Fig. 9B View FIGURE 9 ). The system extends for over 1.5 mm in length. The collecting tubule on each side lies immediately dorsal to the lateral blood vessel, but whether or not the tubule penetrates the blood vascular wall was unclear from the longitudinal sections.
Reproductive system
The single specimen was female. The paired ovaries are about 180 µm in maximum dimension, situated lateral to the rhynchocoel, immediately above the lateral blood vessels; each ovary contains 3– 6 eggs about 80 µm in diameter. The gonoduct leads dorsolaterally into a gonopore in the epidermis; within a radius of about 100 µm from the gonopore, the epidermis contains coarse acidophilic granules that distinguish it from the surrounding epidermis ( Fig. 3D View FIGURE 3 ).
Systematic remarks
Carinina plecta sp. nov. is anatomically similar to previously described species in the genus that all of have their nervous system situated located in the epidermis, a bodywall musculature composed of inner circular, longitudinal, and outer circular muscle layers, an excretory system with a glandlike anterior portion, and a peculiar course of the origin of the proboscis nerves. In Carinina plecta sp. nov., the rhynchocoel wall is principally composed of interwoven longitudinal and circular muscle fibres. This character state has not been reported for previously described species of Carinina , in which the rhynchocoel consists only of circular muscles. Hylbom’s (1957) generic diagnosis for Carinina is here emended to accommodate the type of rhynchocoel wall found in Carinina plecta sp. nov., rather than create a monotypic new genus for this species.
The genus Carinina currently contains sixteen species. Carinina plecta sp. nov. can be distinguished from all previously described species by the suite of characters summarised in Table 1 View TABLE 1 . The description of Carinina antarctica Bürger, 1904 is based on a posterior fragment of the body; thus states of the characters tabulated in Table 1 View TABLE 1 are unknown for this species. Carinina plecta sp. nov. differs from Carinina antarctica Bürger, 1904 in that the latter has dorsoventral muscles in the intestinal region and a ‘lateral sensory line’ in the epidermis on each side of the body ( Bürger 1904b).
Characters and character states:
A: Mid dorsal nerve(s): (1) upper only; (2) upper and lower.
B: Lateral blood vessels: (0) internal to bodywall inner circular muscle layer anteriorly but ou side these muscles posteriorly; (1)only internal to inner circular muscle layer; (2) only external to the inner circular muscle layer.
C: Rhynchocoel blood vessels: (0) absent; (1) present.
D: Statoliths in cerebral ganglia: (0) absent; (1) present.
E: Dorsal cerebral commissure(s); (1) one; (2) two.
F: Blood system in buccal/foregut region consists of: (0) two lateral vessels; (1) lateral vessels developed as vascular plexus around foregut; (2) four lateral vessels.
a
Sundberg and Hylbom (1994) regarded the name Carinina antarctica as a nomen dubium.
b
Sundberg and Hylbom (1994), Gibson and Sundberg (1999), and Senz (2000) interpret C. burgeri as having two dorsal commissures, although Joubin (1902) clearly states “Ce cerveau est pourvu d’une très large commissure dorsale, presque aussi épaisse que les ganglions euxmêmes”.
c
Sundberg and Hylbom (1994), Gibson and Sundberg (1999), and Senz (2000) interpret that ocelli are present in Carinina burgeri . However, there is no indication of ocelli in Joubin’s original description.
d
Friedrich (1970), Hylbom (1975), Kulikova (1984), and Sundberg and Hylbom (1994) place this species in Tubulanus rather than Carinina .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |