Dinoponera Roger
|
publication ID |
https://doi.org/10.11646/zootaxa.3817.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:A3C10B34-7698-4C4D-94E5-DCF70B475603 |
|
DOI |
https://doi.org/10.5281/zenodo.5117540 |
|
persistent identifier |
https://treatment.plazi.org/id/03775906-A6F4-2CAC-FF17-FC32157BFE35 |
|
treatment provided by |
Felipe |
|
scientific name |
Dinoponera Roger |
| status |
|
Dinoponera Roger View in CoL View at ENA
Fig. 28 View FIGURE 28
Dinoponera Roger, 1861: 37 View in CoL (as genus). Type-species: Ponera grandis Guérin-Méneville, 1838: 206 (junior synonym of Ponera gigantea Perty, 1833: 135 ); by monotypy.
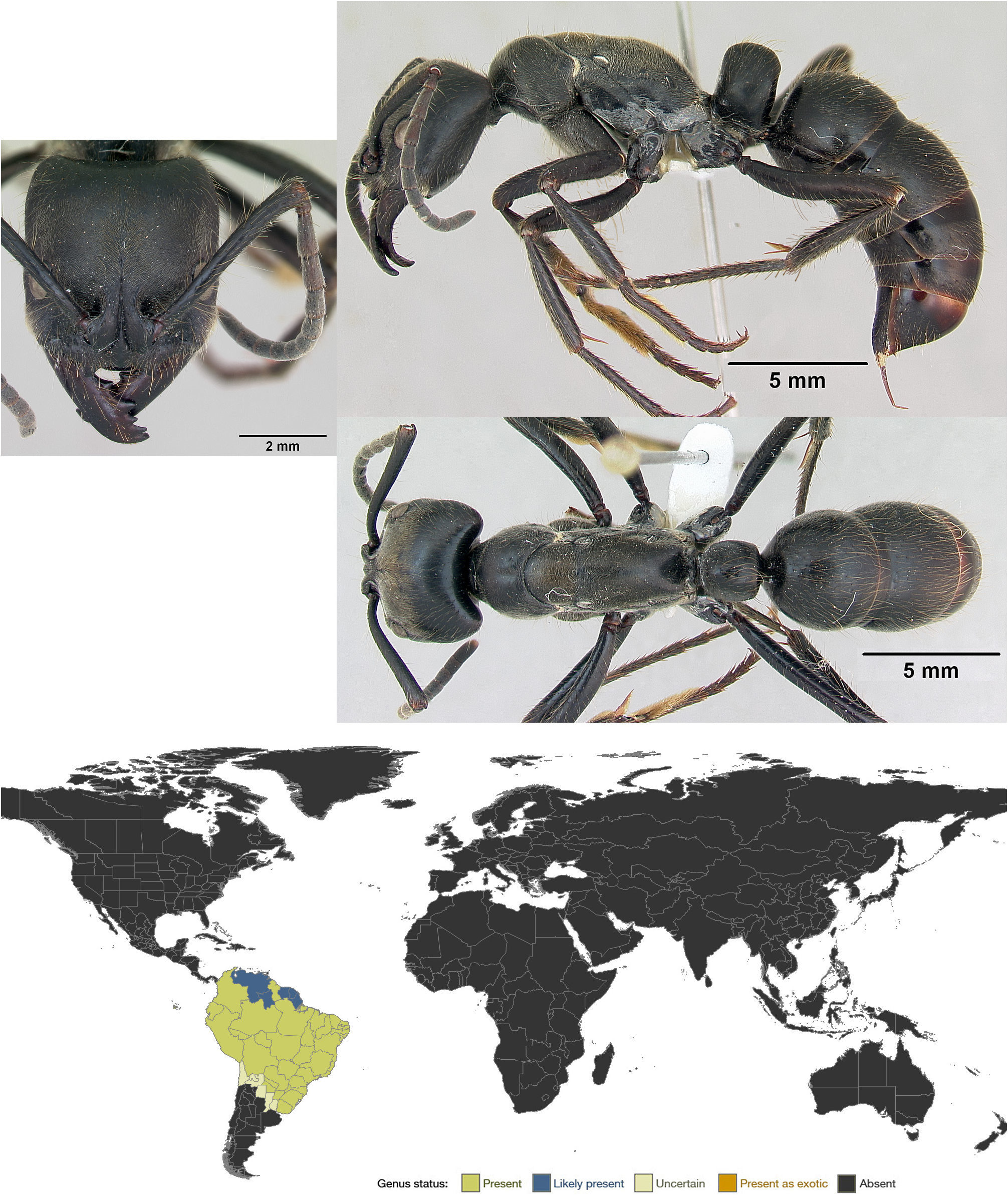
Dinoponera View in CoL is a small genus (ten described species and subspecies) found in rainforests to savannahs from southern Colombia south to Argentina and Uruguay. It boasts the world’s largest monomorphic ant workers (up to 40 mm or more). The genus is also notable for reproducing via gamergates (with the loss of the queen caste).
Diagnosis. Dinoponera workers are unmistakable due to their enormous size. Other diagnostic characters (in combination) include: subtriangular mandibles, clypeal teeth, complex metapleural gland orifice, toothed tarsal claws, and stout hypopygial spines. Some of these characters are synapomorphic with Pachycondyla , which is sister to Dinoponera and the most similar genus morphologically. Pachycondyla lacks the huge size, subtriangular mandibles, clypeal teeth, and toothed tarsal claws of Dinoponera . Streblognathus bears some resemblance to Dinoponera , given its large size, subtriangular mandibles, clypeal teeth, and forward facing eyes, but Streblognathus has a novel fin-shaped petiole and lacks the complex metapleural gland orifice, toothed tarsal claws, and hypopygial spines of Dinoponera , and is somewhat smaller.
Synoptic description. Worker. Huge (TL 25–40 mm; Kempf, 1971; Paiva & Brandão, 1995) ants with the standard characters of Ponerini . Mandibles subtriangular, with roughly five teeth and without a distinct basal angle or basal groove. Anterior margin of clypeus with a pair of long anteriorly-directed teeth. Frontal lobes moderately large. Eyes moderately large, located anterior of head midline and relatively forward-facing. Posterolateral corners of head prominent. Metanotal groove reduced to a subtle suture. Propodeum broad dorsally. Propodeal spiracles slit-like. Metapleural gland orifice with a posterior U-shaped cuticular lip and a lateral groove. Tarsal claws with a single tooth. Metatibial spur formula (1s, 1p). Petiole nodiform, with a blunt dorsal longitudinal ridge. Gaster with a strong girdling constriction between pre- and postsclerites of A4. Stridulitrum present on pretergite of A4. Hypopygium with a row of stout spines on either side of the sting. Head and body smooth to sparsely punctate, with sparse to abundant pilosity and patchy pubescence. Color black. See also description in Lenhart et al. (2013).
Queen. Absent, reproduction instead being performed by gamergates.
Male. See description in Lenhart et al. (2013).
Larva. Described for D. gigantea by Wheeler & Wheeler (1986a).
Geographic distribution. Dinoponera is a strictly South American genus, found from montane rainforests on the eastern slope of the Andes in Perú, Ecuador and Colombia to savannah and lowland rainforest in Brazil, Guyana, south through Bolivia, Paraguay and Argentina ( Lenhart et al., 2013). Lenhart et al. also provide maps showing the distribution of each Dinoponera species.
Ecology and behavior. Dinoponera is one of the best studied ponerine genera, due to its interesting social behaviors and large body size, which makes it relatively easy to work with. In particular, the papers by Monnin and colleagues document in unprecedented detail the social and reproductive behaviors of Dinoponera , especially of D. quadriceps ( e.g., Monnin et al., 1998, 2002, 2003; Monnin & Peeters, 1997, 1998, 1999; Monnin & Ratnieks, 1999, 2001; Peeters et al., 1999; Hart & Monnin, 2006; also Hart & Ratnieks, 2005). We will only briefly summarize their results here, with the caveat that much of this information stems from studies of D. quadriceps alone, and its general applicability to the entire genus is in some cases uncertain.
Dinoponera colonies generally contain fewer than 100 workers, though colony size varies among species ( Fowler, 1985; Paiva & Brandão, 1995; Fourcassié & Oliveira, 2002; Vasconcellos et al., 2004; Monnin & Peeters, 2008). Dinoponera has completely lost the queen caste and reproduction in each colony is instead performed by a single mated gamergate worker who is the top (alpha) individual in a ranked dominance hierarchy ( Haskins & Zahl, 1971; Monnin et al., 2003). Below her in the hierarchy are three to five subordinate workers who vie with one another for the opportunity to succeed the alpha once she dies ( Monnin et al., 2002; Peixoto et al., 2008). These “hopeful reproductives” perform little work in the colony and represent a drain on the colony’s resources ( Monnin & Ratnieks, 1999, 2001; Monnin et al., 2003; Hart & Monnin, 2006). The remaining workers perform most of the work for the colony and also police the colony, punishing high-ranking workers who attempt to prematurely overthrow the alpha ( Monnin & Ratnieks, 2001; Monnin et al., 2002). This policing is quite effective, as early replacement of the alpha is apparently rare ( Hart & Monnin, 2006).
A newly annointed alpha worker briefly leaves the colony and mates with a single male ( Monnin & Peeters, 1998). Subordinate workers do not mate, but sometimes lay haploid eggs which will develop into males if the alpha does not discover and cannibalize them ( Dantas de Araujo et al., 1990; Monnin & Ratnieks, 2001). Alpha workers have a different cuticular hydrocarbon profile from other workers, and this profile is transferred to their eggs, allowing them to identify the eggs of subordinates ( Monnin & Peeters, 1997; Peeters et al., 1999). The hydrocarbon profile is apparently related to ovarian activity, and allows workers to assess the rank and reproductive status of each member of the colony (Monnin et al., 1998; Monnin & Peeters, 1999; Peeters et al., 1999; Monnin & Ratnieks, 2001).
As with most ponerines lacking winged queens, colony reproduction in Dinoponera occurs via fission, with workers carrying brood and males to new nest sites and recruiting other workers via tandem running, with apparently no chemical trail ( D. australis: Fowler, 1985 ; D. gigantea: Overal, 1980 ). Nests are built into the soil, can be quite extensive, and house diverse communities of myrmecophiles including inquiline Pheidole species (Zahl, 1957; Hermann et al., 1994; Paiva & Brandão, 1995; Vasconcellos et al., 2004). Workers are not generally aggressive but can deliver a painful sting if provoked ( Allard, 1951; Zahl, 1957; Hermann et al., 1994). Dinoponera workers forage diurnally or crepuscularly on the ground, and are generalist predators of insects and opportunistic scavengers of fruits and other food sources ( Oldham et al., 1994; Hermann et al., 1994; Paiva & Brandão, 1995; Fourcassié & Oliveira, 2002; Monnin et al., 2003; Araújo & Rodrigues, 2006). Workers always forage individually (Zahl, 1957; Haskins & Zahl, 1971; Fowler, 1985; Fourcassié & Oliveira, 2002; Araújo & Rodrigues, 2006). Orientation and navigation by foraging D. gigantea workers were studied by Fourcassié et al. (1999).
The large size and interesting behaviors of Dinoponera have made them attractive model systems for histological and biochemical research, including studies of the mandibular gland ( Oldham & Morgan, 1993; Oldham et al., 1994; Hermann et al., 1994), Dufour’s gland ( Hermann et al., 1994, Morgan et al., 2003), sting apparatus and venom gland ( Hermann et al., 1994; Morgan et al., 2003), convoluted gland (inside venom reservoir; Schoeters & Billen, 1995), post-pharyngeal gland ( Schoeters & Billen, 1997), and antennal sensillae and glands ( Marques-Silva et al., 2006). Interestingly, D. lucida has the highest number of chromosomes known for any hymenopteran (2n=120), though the total number ranges from 2n=106 to 2n=120 and the significance or cause of this large number and variation is unknown ( Mariano et al., 2004; Barros et al., 2009).
Phylogenetic and taxonomic considerations. Dinoponera was erected by Mayr (1861) as a monotypic genus to house Ponera grandis Guérin-Méneville (now Dinoponera gigantea ). The enormous size and other morphological apomorphies of Dinoponera led to a stable taxonomic history for the genus. Schmidt's (2013) molecular phylogeny strongly indicates that Dinoponera is a close sister genus to Pachycondyla . Dinoponera and Pachycondyla are morphologically quite similar, and the presence of a row of stout spines on either side of the hypopygium is a strong synapomorphy linking the two genera. Ironically, though W. L. Brown (in Bolton, 1995) synonymized numerous unrelated ponerine genera under Pachycondyla , he failed to include Dinoponera , which is the only ponerine genus closely related enough to Pachycondyla to actually justify synonymy. Given the numerous morphological apomorphies of Dinoponera (including their huge body size, subtriangular mandibles, clypeal teeth, and toothed tarsal claws) and their highly derived social behaviors, we are retaining Dinoponera as a separate genus from Pachycondyla .
Dinoponera has at times been considered closely related to Streblognathus (see discussion under that genus), but neither morphological or molecular evidence supports this hypothesis. The fossil genus Archiponera was also considered by Carpenter (1930) to be closely related to Dinoponera and Streblognathus . While Archiponera does bear some superficial resemblance to these genera, and especially to Dinoponera , the purported age of this fossil genus makes it unlikely to represent an ancestor or sister group to either Streblognathus or Dinoponera .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Ponerinae |
|
Tribe |
Ponerini |
Dinoponera Roger
| Schmidt, C. A. & Shattuck, S. O. 2014 |
Dinoponera
| Roger, J. 1861: 37 |
| Guerin-Meneville, F. E. 1838: 206 |
| Perty, M. 1833: 135 |