Marma Simon, 1902
|
publication ID |
https://doi.org/10.11646/zootaxa.4899.1.16 |
|
publication LSID |
lsid:zoobank.org:pub:27E67BBB-DFD0-4A96-8269-9E1CB6153B83 |
|
DOI |
https://doi.org/10.5281/zenodo.4456833 |
|
persistent identifier |
https://treatment.plazi.org/id/03403F11-FF9C-FF85-538B-FE530F5AFE7D |
|
treatment provided by |
Plazi |
|
scientific name |
Marma Simon, 1902 |
| status |
|
Genus Marma Simon, 1902 View in CoL View at ENA
Marma Simon, 1902: 376 View in CoL ( type species: Marma baeri Simon, 1902 View in CoL ).
Thysema Mello-Leit „o, 1944: 390 ( type species: Thysema dorae Mello-Leitão, 1944 ); synonymized by Galiano (1962: 36).
Paralophostica Soares & Camargo, 1948: 396 ( type species: Paralophostica centralis Soares & Camargo, 1948 ); synonymized by Galiano (1962: 36).
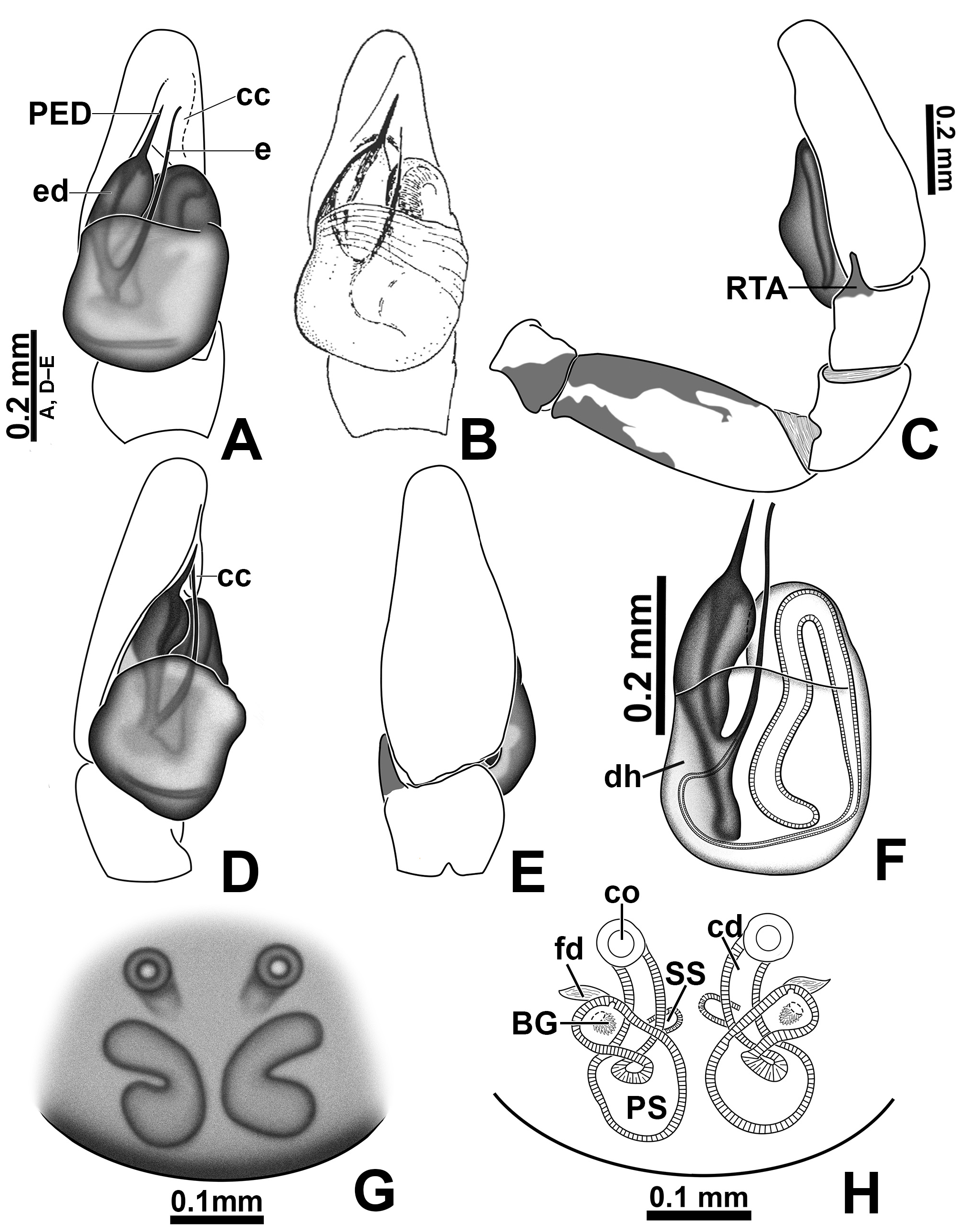
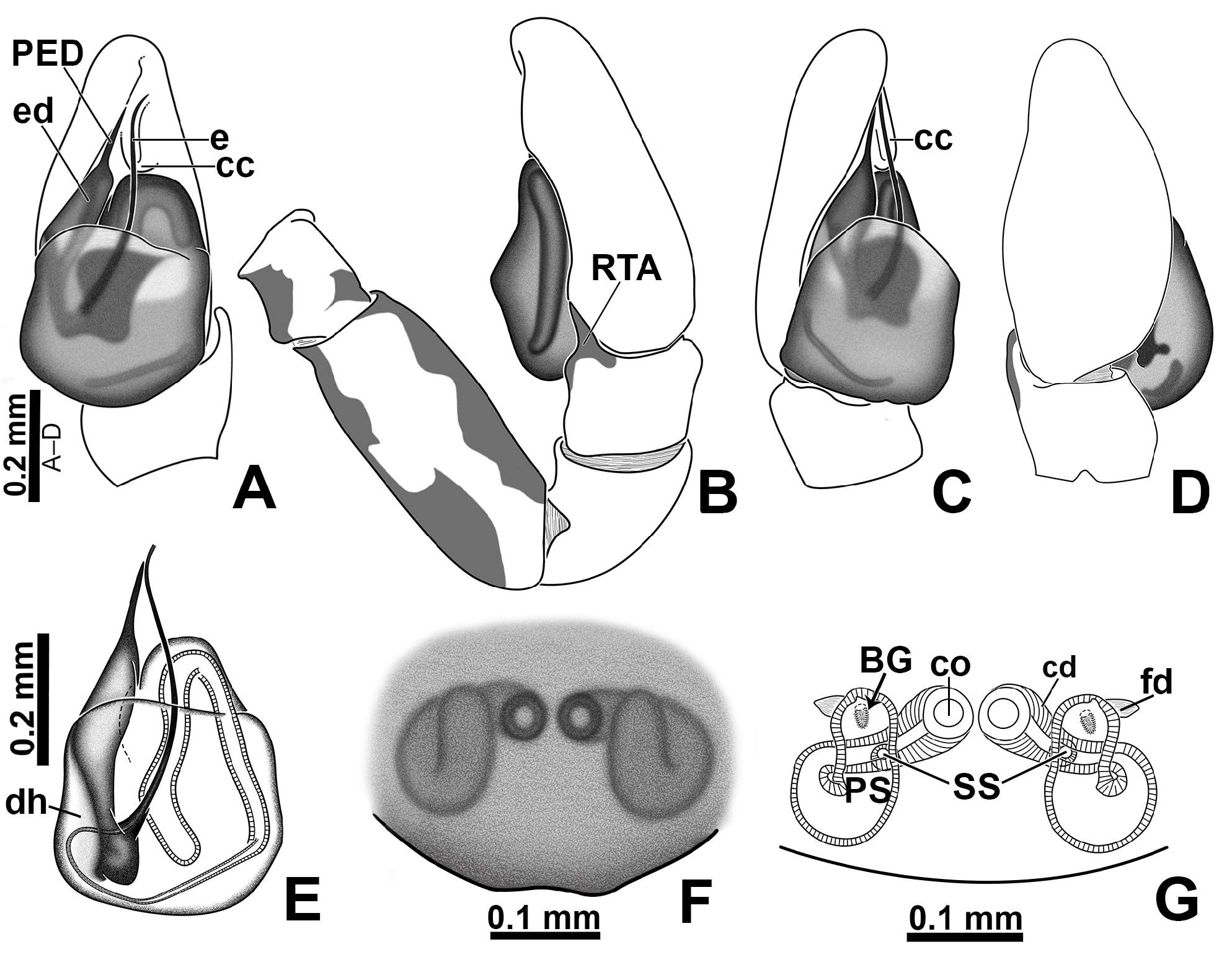
Revised diagnosis. The Amphidraus-Marma clade (which also includes genera Nebridia and Yacuitella ) is composed of small to medium-sized jumping spiders. The chelicerae in these genera have two promarginal teeth and one fissident retromarginal tooth with two or more cusps ( Figs 51A, C View FIGURE 51 ; Galiano 1999: figs 6–7; Zhang & Maddison 2015: fig. 218; Salgado & Ruiz 2019: figs 28A–C). Furthermore, the male palp has an embolic disc with projections ( Figs 5C View FIGURE 5 , 7A View FIGURE 7 , 9 View FIGURE 9 A–C, 16A, 23A–C, 28A; Galiano 1999: figs 8–9, 15; Salgado & Ruiz 2019: fig. 3A), and the epigynal plate is sclerotized and lacks the typical euophryine window structure (a pair of rounded and less sclerotized regions that allow the visualization of the spermathecae; Figs 7G View FIGURE 7 , 15F View FIGURE 15 , 22F View FIGURE 22 , 27F View FIGURE 27 , 32F View FIGURE 32 , 37H View FIGURE 37 , 46C View FIGURE 46 , 50F View FIGURE 50 , 55F View FIGURE 55 ; see Zhang & Maddison 2015: figs 39, 46, 53, 60).
Males of Marma can be distinguished from related genera by having a sclerotized cymbial conductor (cc; Figs 36C View FIGURE 36 , 40A View FIGURE 40 ), whereas it is membranous in the other members of the Amphidraus - Marma clade (see Salgado & Ruiz 2019: fig. 2E); by having part of embolus shaft hidden under the tegulum ( Figs 7A, F View FIGURE 7 , 9A, C View FIGURE 9 , 15A, E View FIGURE 15 ), whereas it is typically exposed in the tribe (see Salgado & Ruiz 2019: figs 5A, 7C); and by not having the tegular lobe ( Figs 13C View FIGURE 13 , 15A View FIGURE 15 , 25C View FIGURE 25 , 27A View FIGURE 27 ), which is developed in Amphidraus and Nebridia (see Galiano 1963: Lam. XXVII, fig. 16 and Salgado & Ruiz 2019: fig. 3A) and slightly differentiated from the tegulum in Yacuitella (see Galiano 1999: fig. 9). In addition, males of Marma and Yacuitella have an embolus composed of a shaft alone ( Figs 20C View FIGURE 20 , 22A View FIGURE 22 , 23A View FIGURE 23 , 53C View FIGURE 53 , 55A View FIGURE 55 , 56C View FIGURE 56 ; see Galiano 1999: figs 8–9, 15), whereas in Amphidraus and Nebridia the embolus opening is extended, forming a tubular piece that is less sclerotized than the shaft (see Salgado & Ruiz 2019: figs 3A, 7C). Males of Marma can be distinguished from those of Yacuitella by having a single distal process on the embolic disc ( Figs 23 View FIGURE 23 A–C, 30C, 32A, 33A, D) and a single apophysis on the male palpal tibia ( Figs 20D View FIGURE 20 , 22B View FIGURE 22 , 23 View FIGURE 23 D–E, 30D, 32B, 33E) ( Yacuitella has two processes on the embolic disc and three tibial apophyses; see Galiano 1999: figs 9–10). Females of Marma differ from those of Amphidraus by not having a coupling pocket in the epigynal plate (present in some species of Amphidraus ; see Salgado & Ruiz 2019: fig. 27E). Also, the proximal copulatory duct is longer in Marma , which places the secondary spermatheca farther from the copulatory opening ( Figs 6C View FIGURE 6 , 7H View FIGURE 7 , 10B View FIGURE 10 , 18 View FIGURE 18 C–D, 19A–B), whereas the secondary spermatheca is adjacent to the copulatory opening in Amphidraus and Yacuitella (see Salgado & Ruiz 2019: figs 17D, 25D; Galiano 1999: figs 12–13). The female of Nebridia remains unknown.
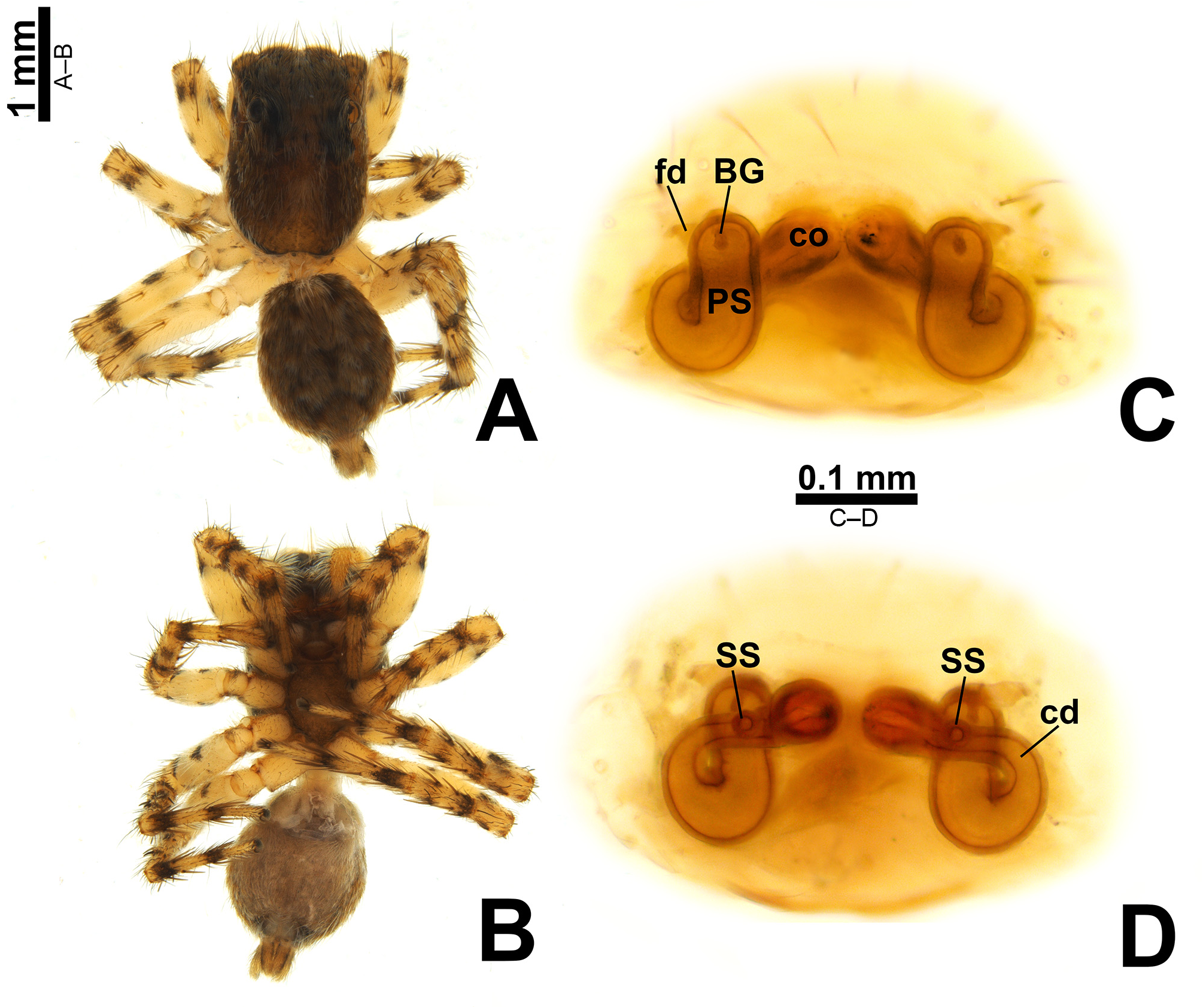
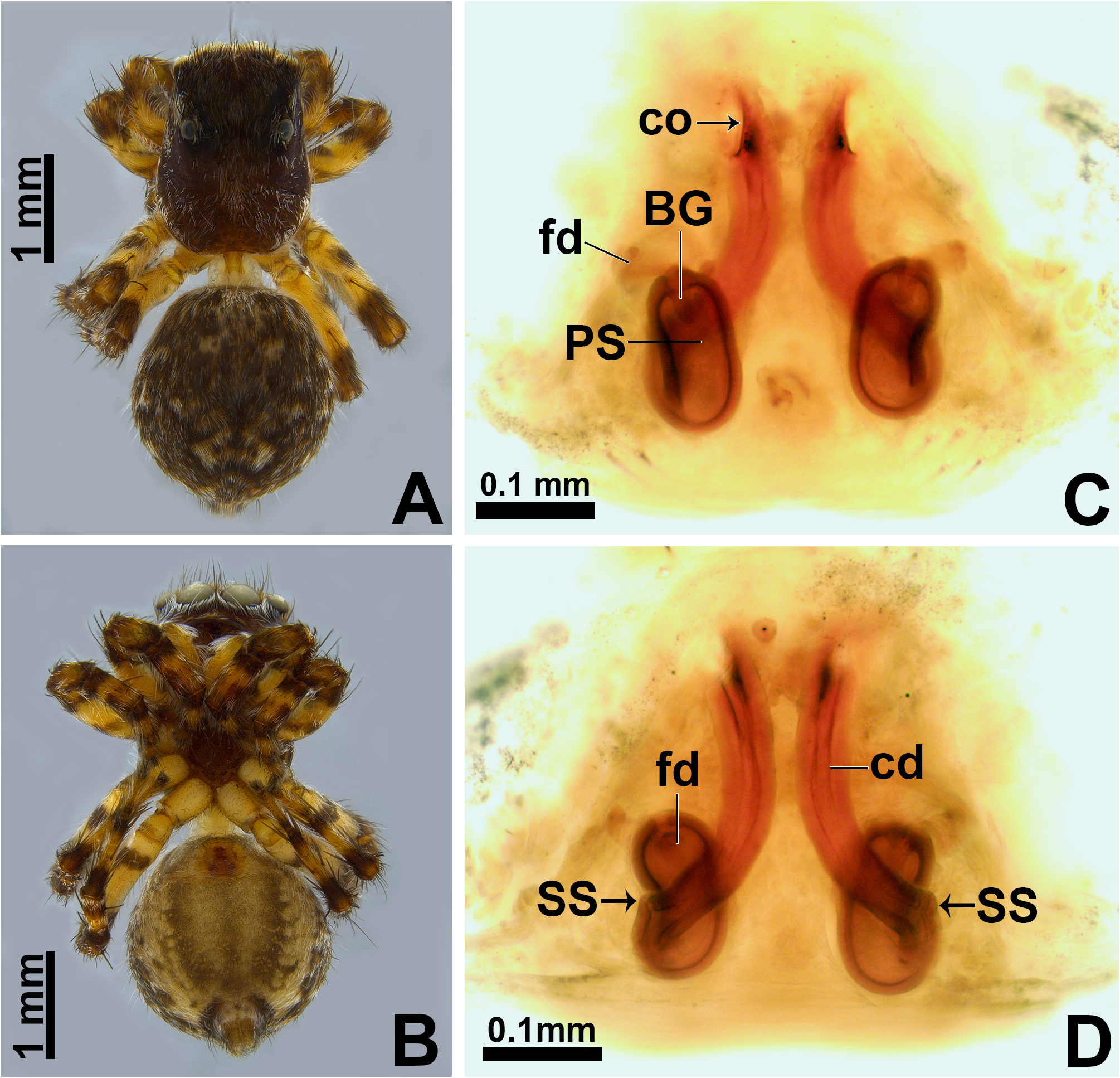
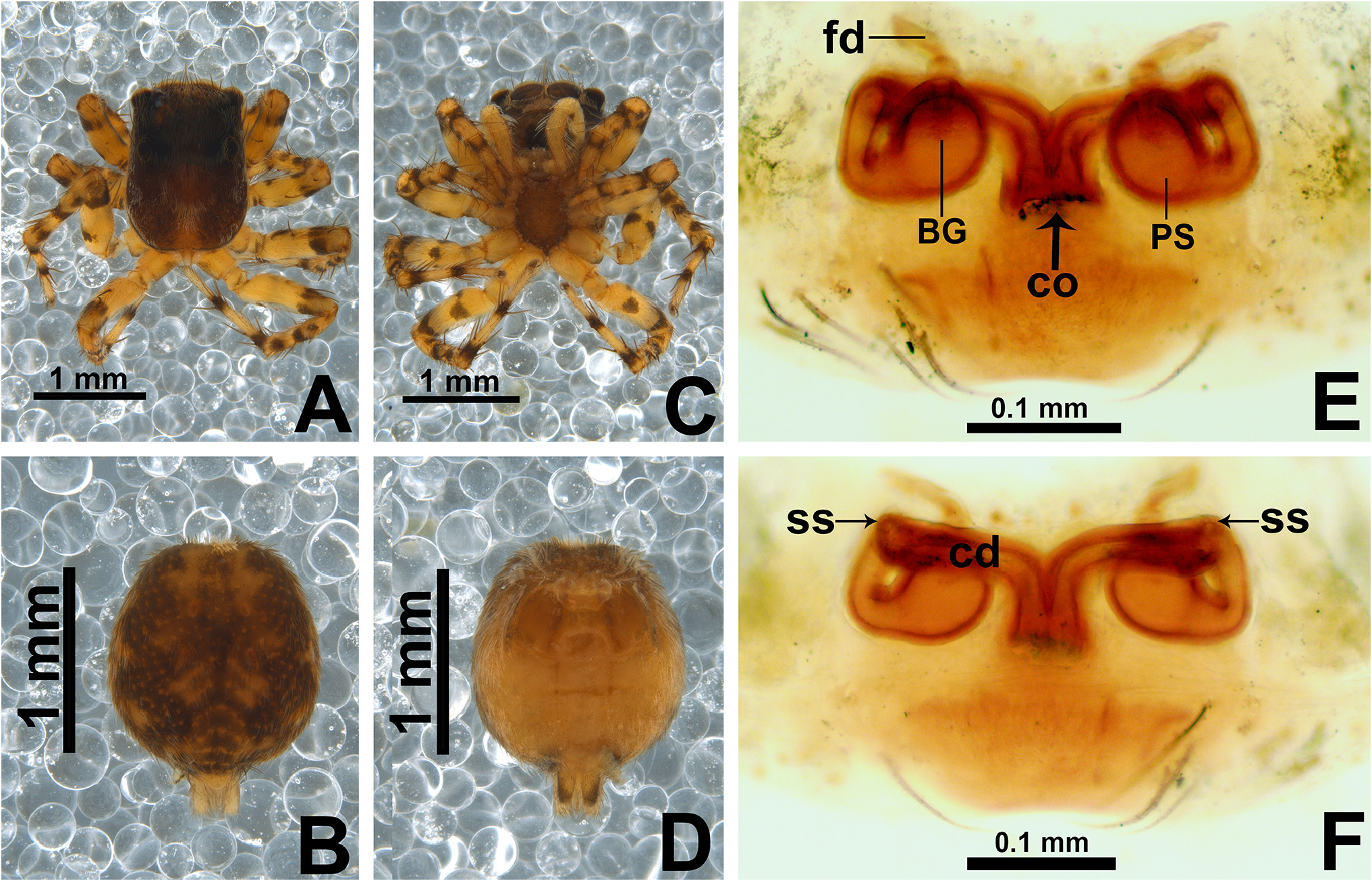
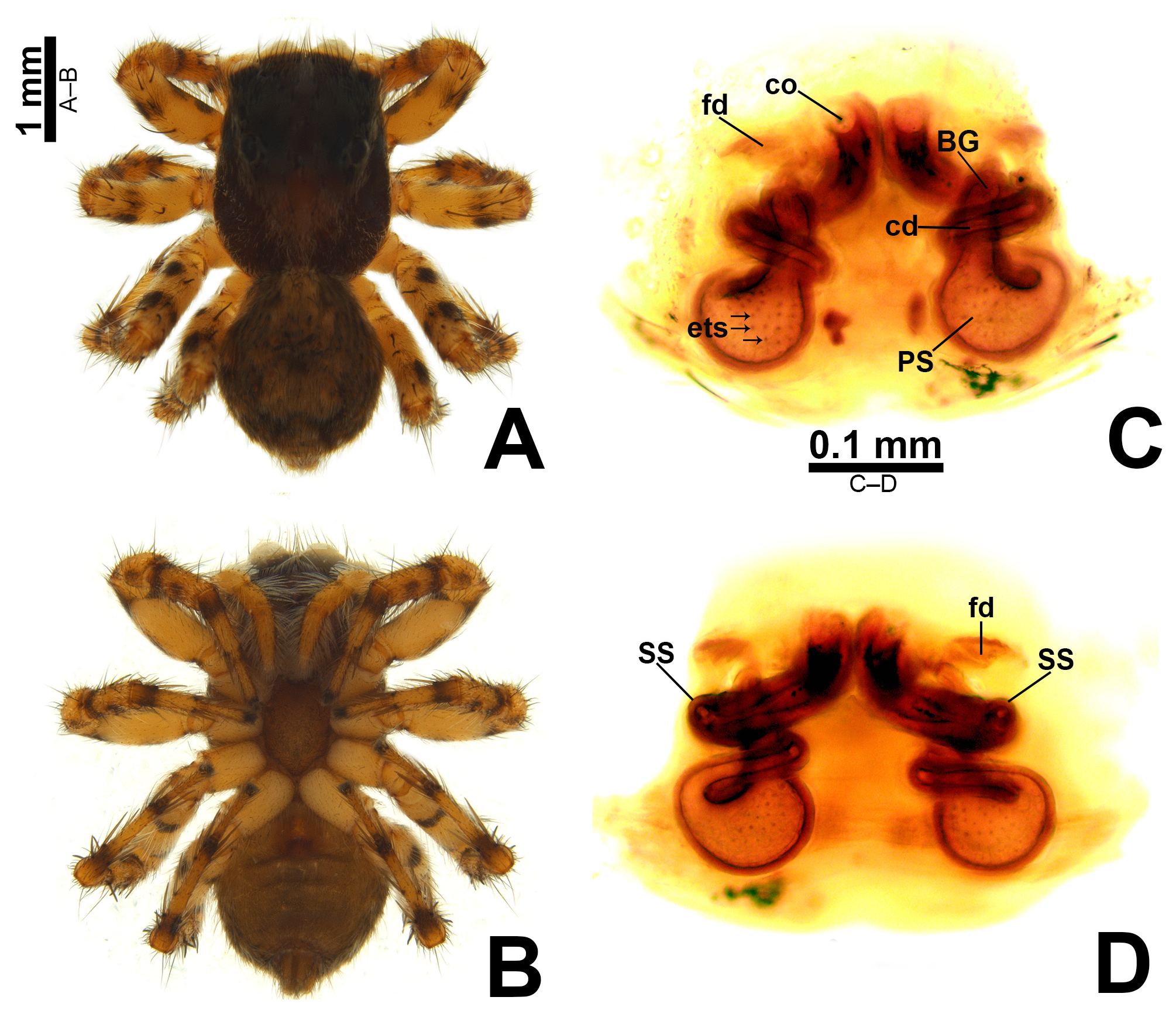
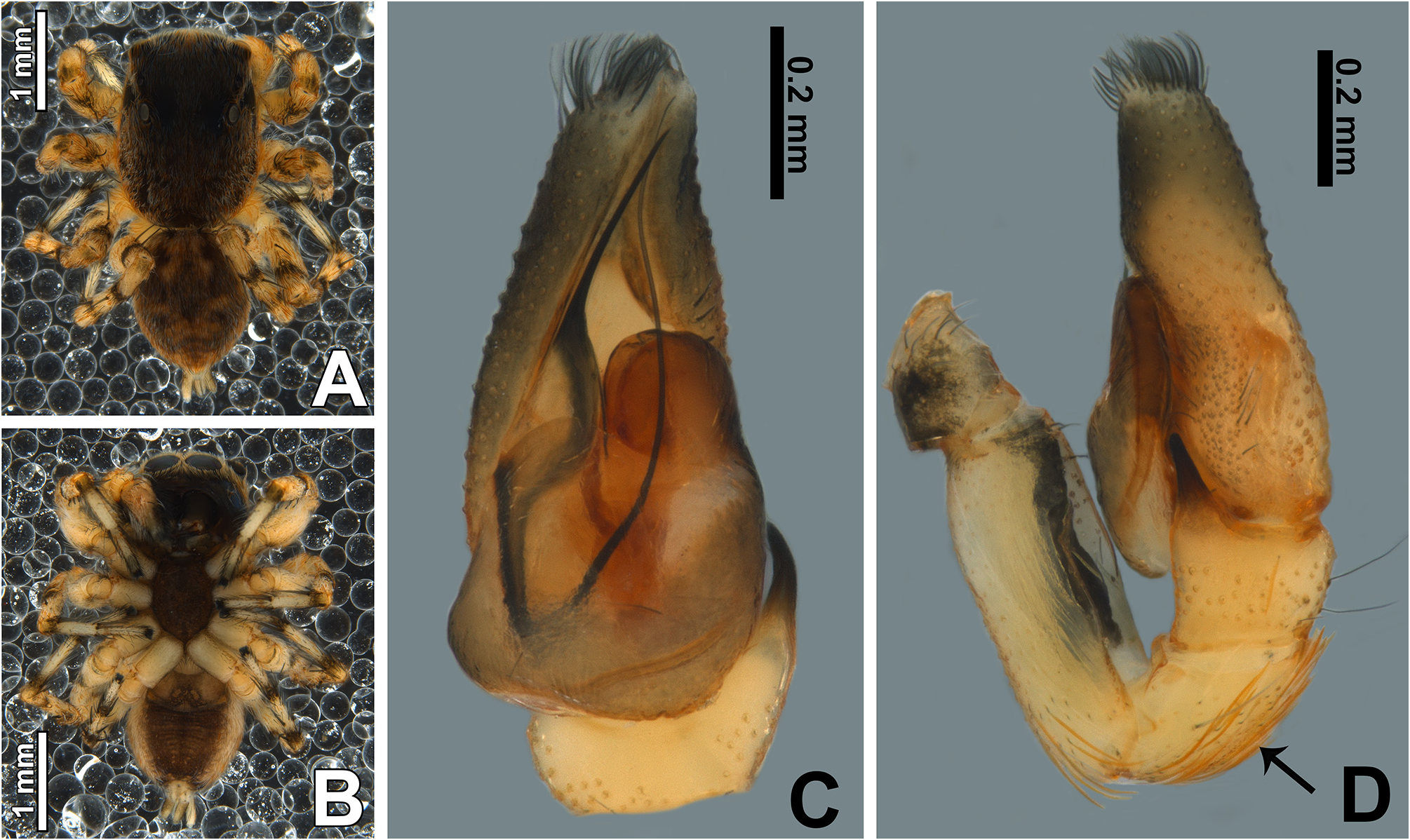
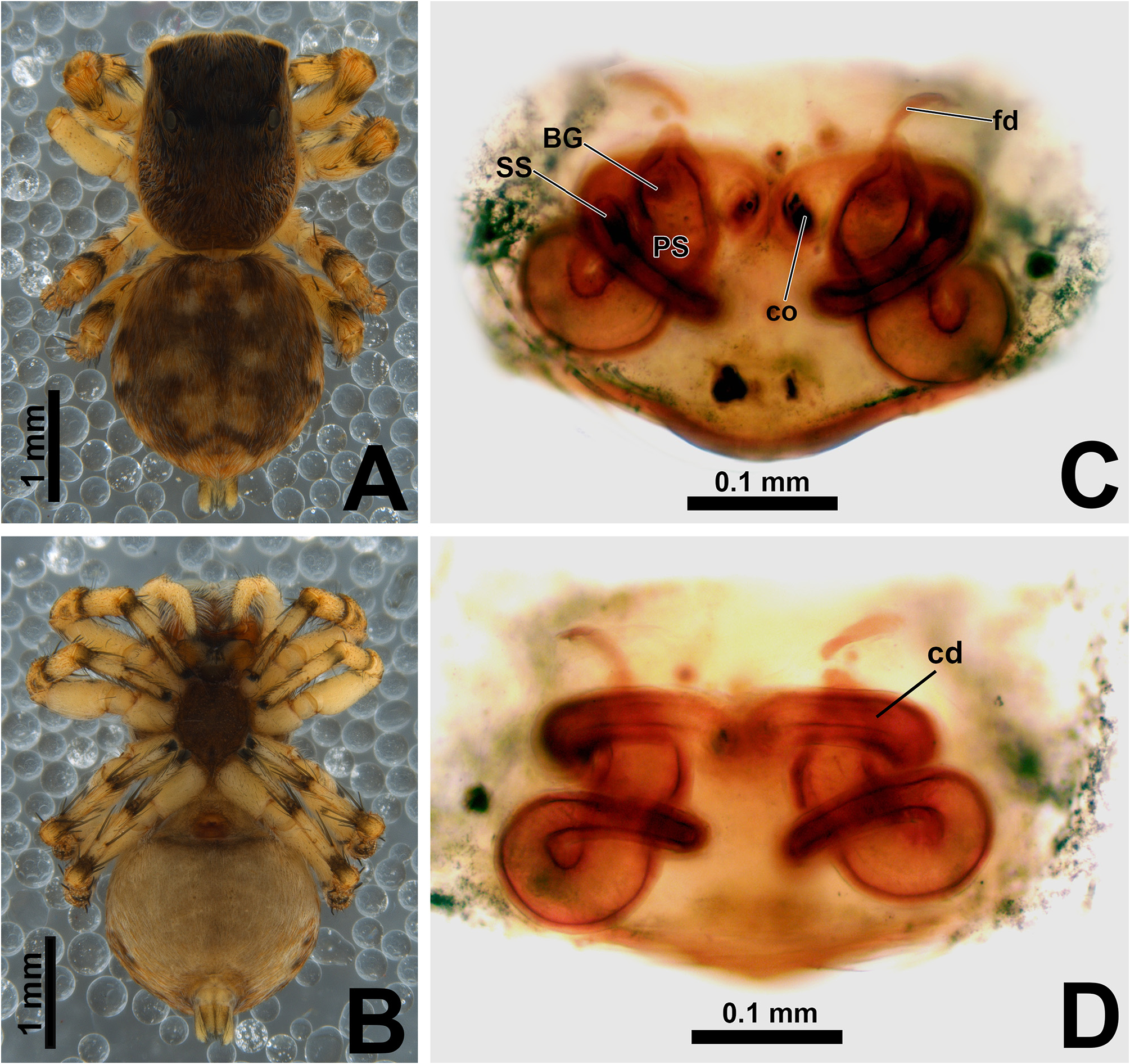
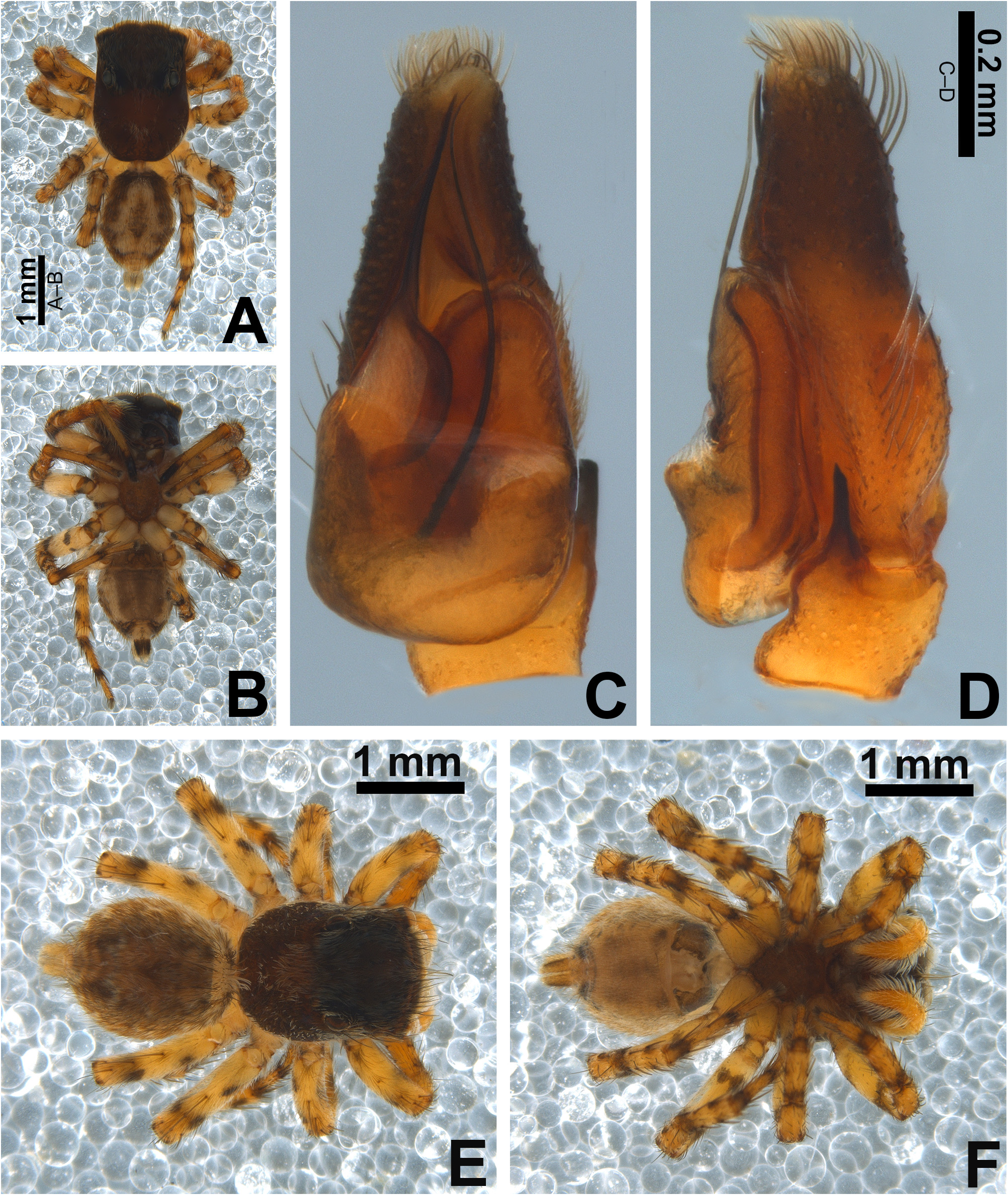
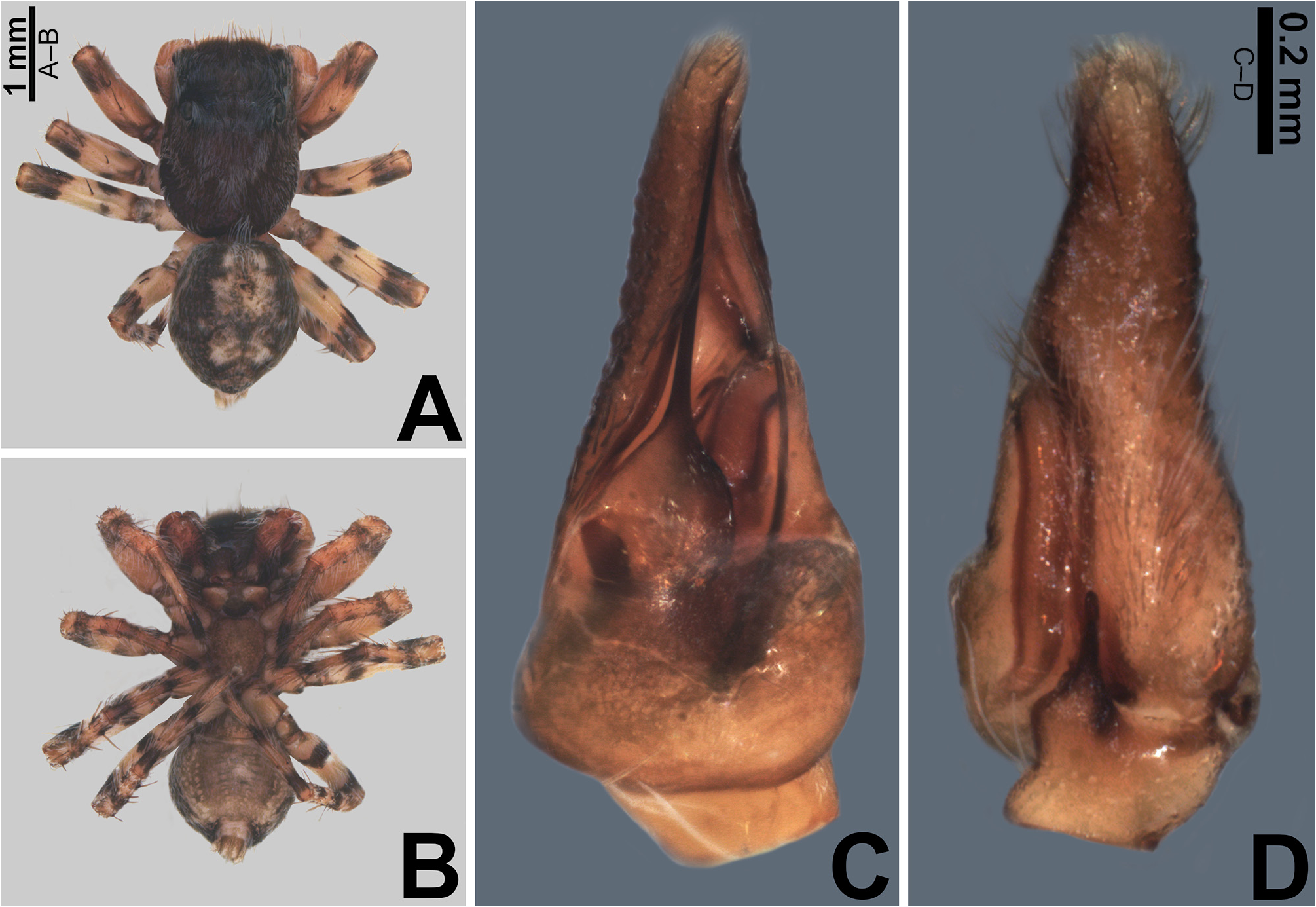
Description. Small to medium-sized ( 2.9–4.8 mm); standard jumping spider body (without striking modifications such as mimicry to ants, beetles, etc. or flattening of carapace; Figs 1 View FIGURE 1 , 2 View FIGURE 2 , 3 View FIGURE 3 , 5 View FIGURE 5 A–B, 6A–B).
Carapace: anterior region of carapace (near to anterior eyes) slightly narrower than widest section ( Figs 8D View FIGURE 8 , 13A View FIGURE 13 ); PME closer to PLE than to ALE ( Fig. 8D View FIGURE 8 ); PME slightly more dislocated to lateral side of carapace than PLE ( Fig. 8D View FIGURE 8 ); anterior eye row in frontal view wider than anterior section of carapace ( Figs 3D View FIGURE 3 , 47F View FIGURE 47 ); fovea placed slightly beyond PLE ( Figs 6A View FIGURE 6 , 20A View FIGURE 20 , 21A View FIGURE 21 ); in lateral view the posterior third with abrupt slope ( Figs 47B, E View FIGURE 47 ). Coloration: cephalic area black and thoracic area brown in both sexes ( Figs 5A View FIGURE 5 , 6A View FIGURE 6 , 30A View FIGURE 30 , 31A View FIGURE 31 ). Scale pattern: with blend of white and brown scales ( Figs 2 View FIGURE 2 A–D, 8D–E). Brown scales ( Fig. 8E View FIGURE 8 ) are longer and slenderer than white scales (about the same width throughout its length) and bear lateral barbs. White scales ( Figs 8A, E View FIGURE 8 ) are flattened and wide (at least twice the width of its base) and bear central ridge along their dorsal length; shallow, oblique grooves extending towards borders, with single lateral row of flattened barbs; venter of scales apparently also with barbs. White and brown scales are connected to the unmodified base ( Figs 8A, C View FIGURE 8 ) and rest parallel to the surface. Cephalic region also with rigid, black protective bristles connected to round, well-delimited bases ( Figs 8 View FIGURE 8 A–B, F). Two different patterns of distribution of scales on carapace can be observed (best observed in live specimens): (1) presence of a distinct triangle of scales pointing backward, with scarce scales on sides ( Figs 34 View FIGURE 34 A–B, D–E, 47A–B, D–E); (2) homogeneous distribution of scales on carapace ( Figs 1E View FIGURE 1 , 29 View FIGURE 29 A–F, 30A, 31A); anterior lateral eyes (ALE) and anterior median eyes (AME) surrounded by brown scales ( Figs 1A, D View FIGURE 1 , 3B, D View FIGURE 3 , 12C, F View FIGURE 12 , 34C, F View FIGURE 34 , 47C, F View FIGURE 47 ).
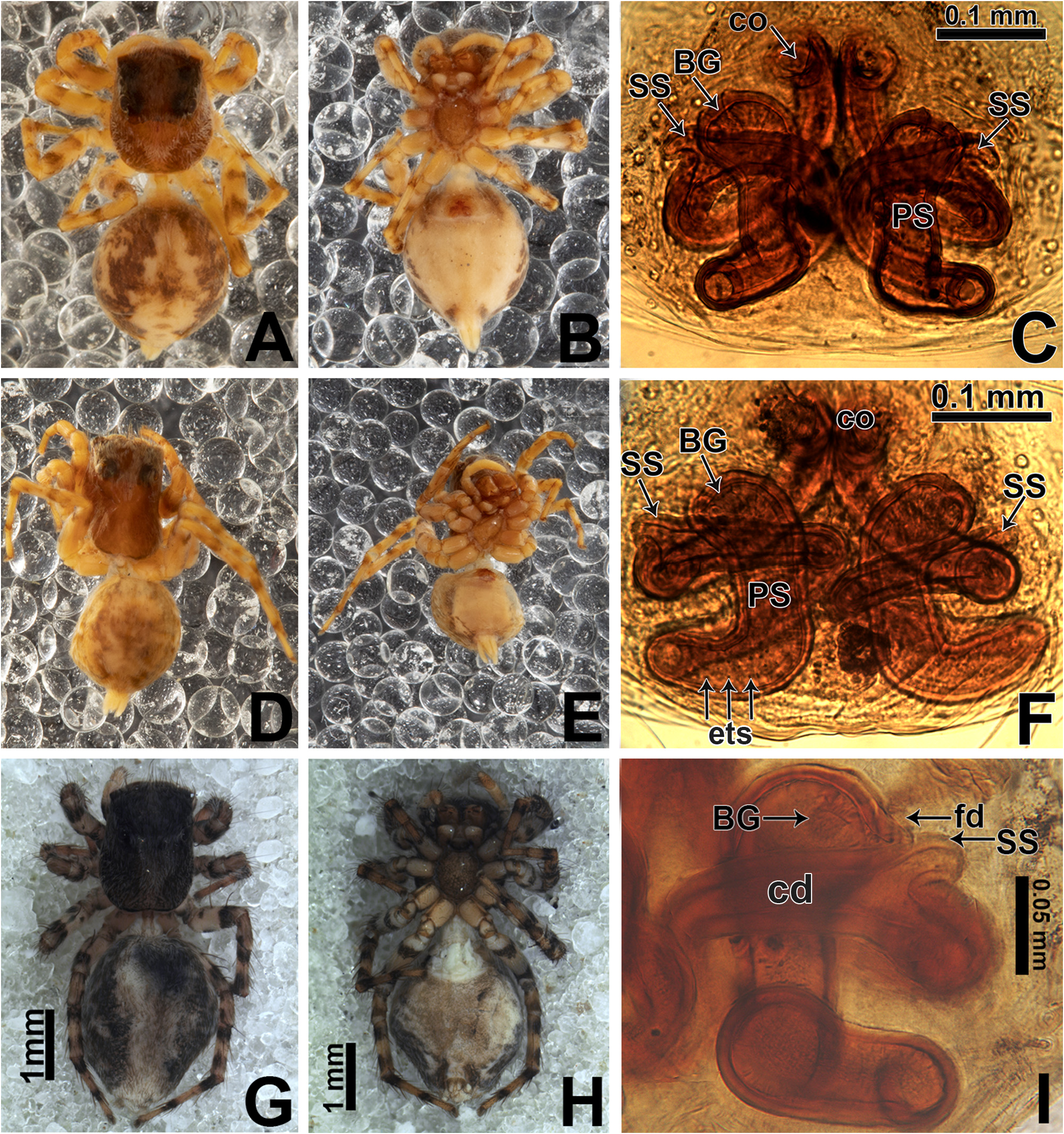
Abdomen: dorsally variegated, with brown and white scales ( Figs 5A View FIGURE 5 , 6A View FIGURE 6 ); ventrally, it can be light brown with three longitudinal, narrow dark brown stripes ( Figs 5B View FIGURE 5 , 6B View FIGURE 6 ), or with pale border and thick dark brown longitudinal stripe ( Fig. 30B View FIGURE 30 ), or without stripes ( Figs 21D View FIGURE 21 , 26B View FIGURE 26 ). Anal tubercle covered with white scales (yellow arrow in Figs 11A, G View FIGURE 11 ), like the ones present on carapace. Epiandrous region with a pair of conspicuous tufts of spigots, bearing about six long spigots each ( Figs 11 View FIGURE 11 H–I).
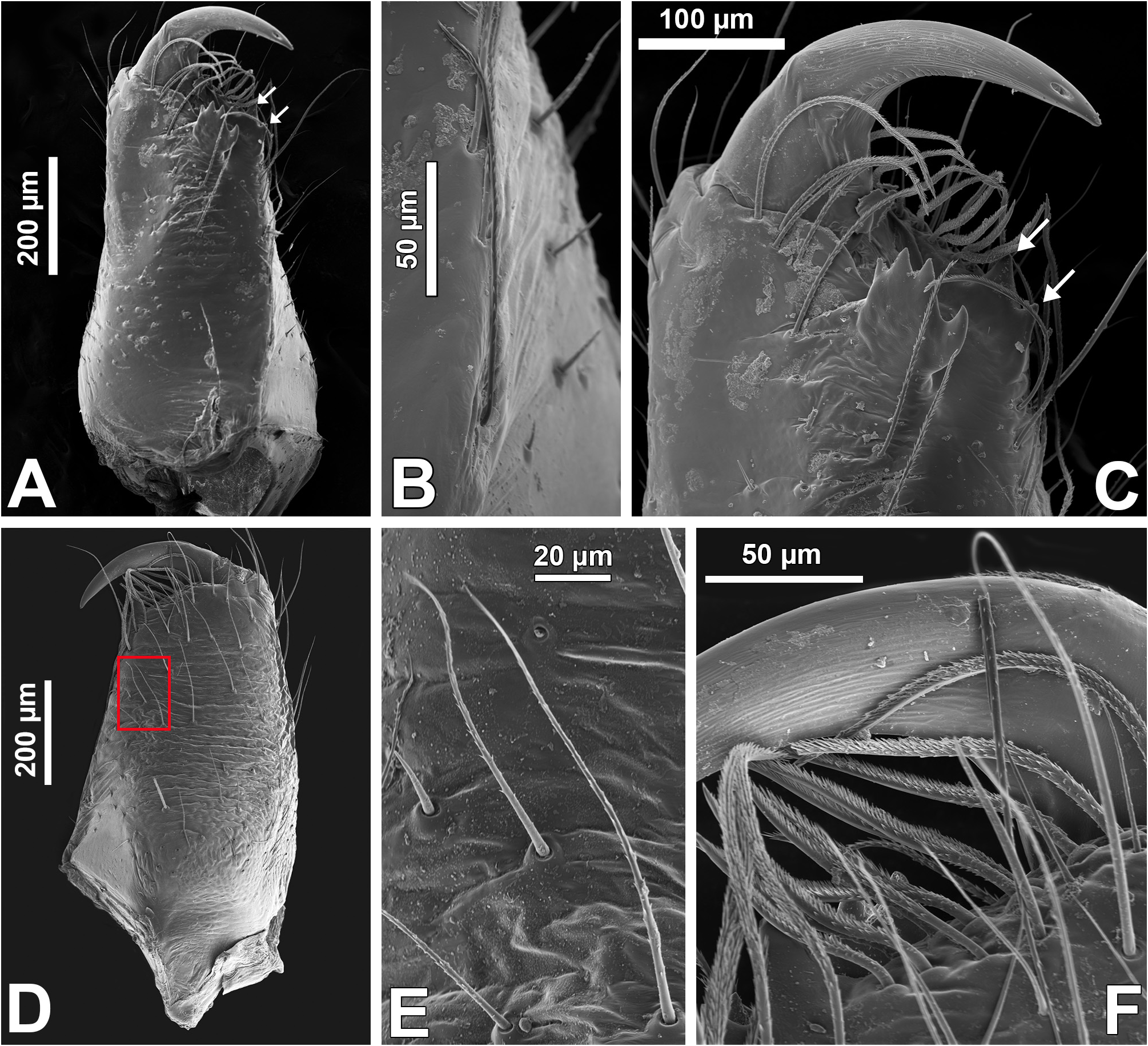
Chelicerae: with two promarginal teeth and one fissident retromarginal tooth with two or more cusps ( Figs 51A, C View FIGURE 51 ; see Zhang & Maddison 2015: fig. 218); ventral keel with long (sensitive?) setae ( Figs 51 View FIGURE 51 A–B); frontal surface with sparse long and short setae ( Figs 51 View FIGURE 51 D–E), and long, barbed setae between teeth and fang ( Fig. 51F View FIGURE 51 ).
Legs: in general, are pale with dark marks (small spots, or stripes that cover entire surface of an article, or rings that circulate an article; Figs 1A View FIGURE 1 , 3C View FIGURE 3 , 12A View FIGURE 12 ); femur I color pattern can vary within a species, being yellowish with dark marks, totally yellowish or totally dark in different males (compare Figs 12 View FIGURE 12 A–C in M. linae sp. nov. and Figs 47A View FIGURE 47 , 48E View FIGURE 48 in M. rosea ); color pattern for metatarsi is standard for all species and both sexes: metatarsi I–II with dark proximal ring, III–IV with proximal and distal dark ring ( Figs 30B View FIGURE 30 , 31B View FIGURE 31 ); all males with black tarsus on leg I ( Figs 3A View FIGURE 3 , 12 View FIGURE 12 A–B, 29A, 34A–B).
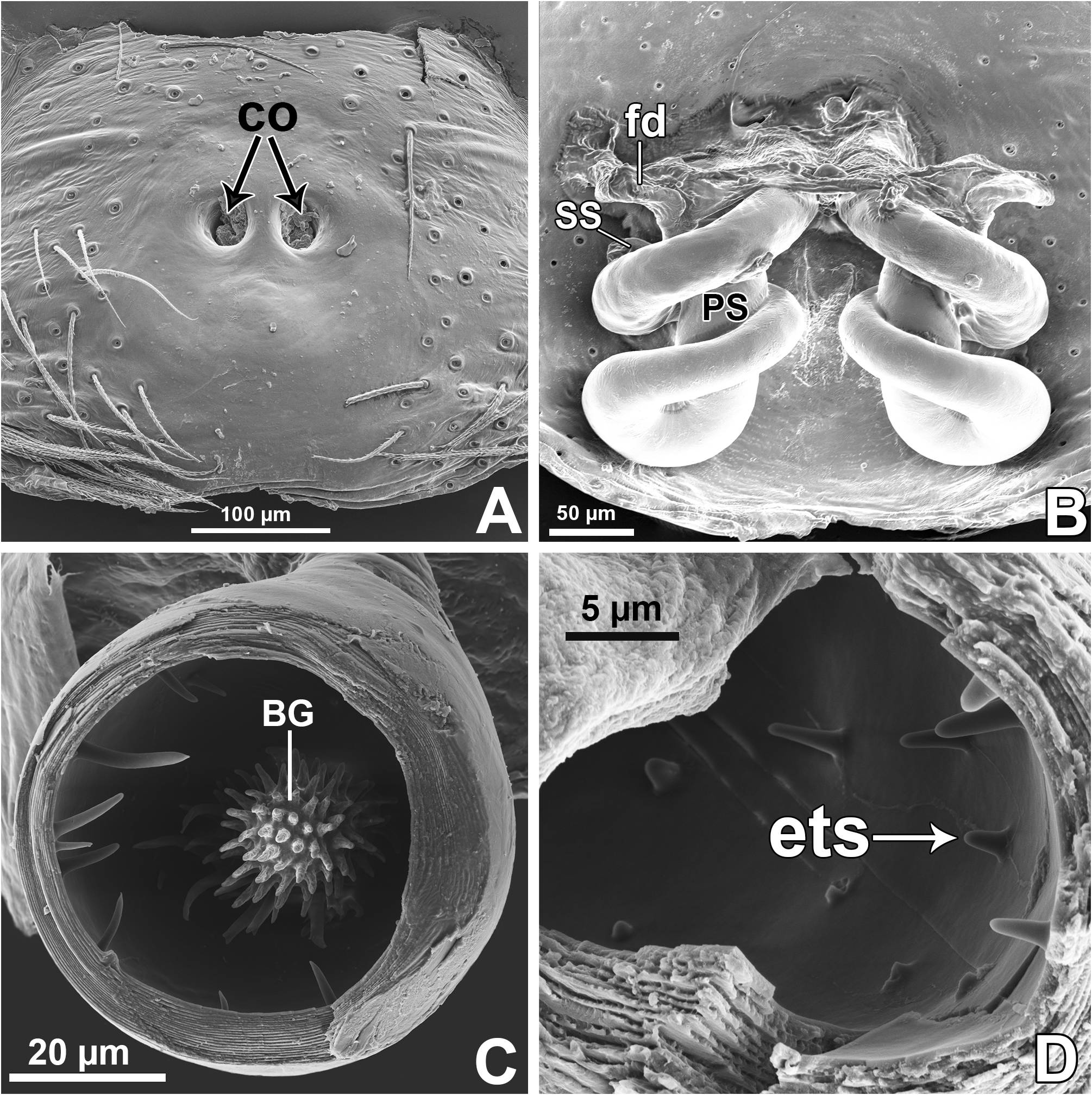
Spinnerets ( Figs 11 View FIGURE 11 A–F; examined only in M. baeri male): spigots, in general, are reduced in number in all spinnerets. In the ALS there are only four piriform spigots forming an arch anteriorly ( Figs 11 View FIGURE 11 B–C); ALS also has a single major ampullate spigot (MAP) placed posteriorly, hidden in Fig. 11B View FIGURE 11 and partially seen in Fig. 11C View FIGURE 11 (females are expected to have two MAP in ALS). In the PMS there is an unusually elongate minor ampullate spigot (mAP) right behind a large tartipore, and possibly a single lateral aciniform spigot, which is broken off in both PMS photographed ( Fig. 11F View FIGURE 11 ). In the PLS, among the tartipores, there are four unusually elongate aciniform spigots forming an arch facing mesally ( Figs 11 View FIGURE 11 D–E).
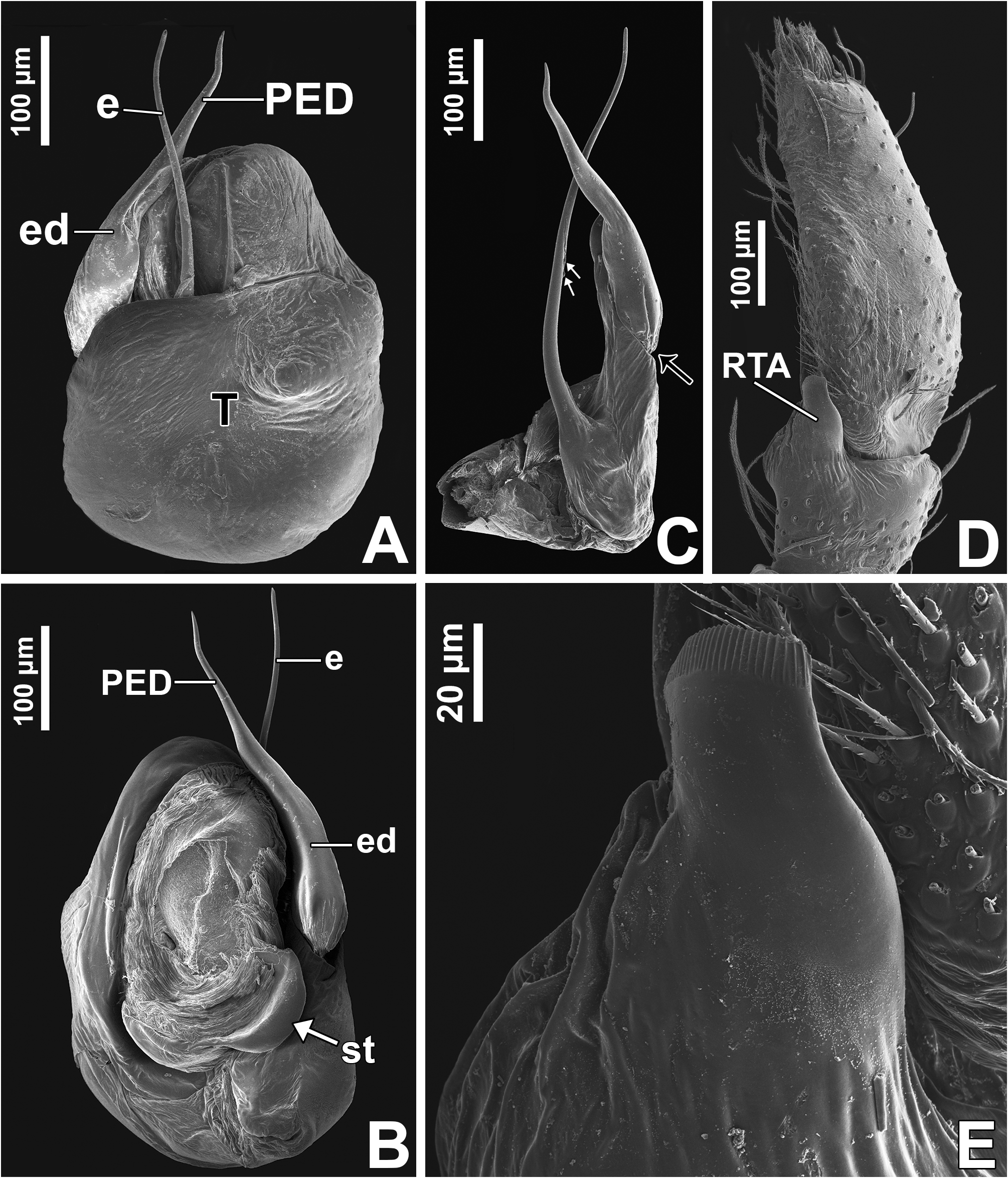
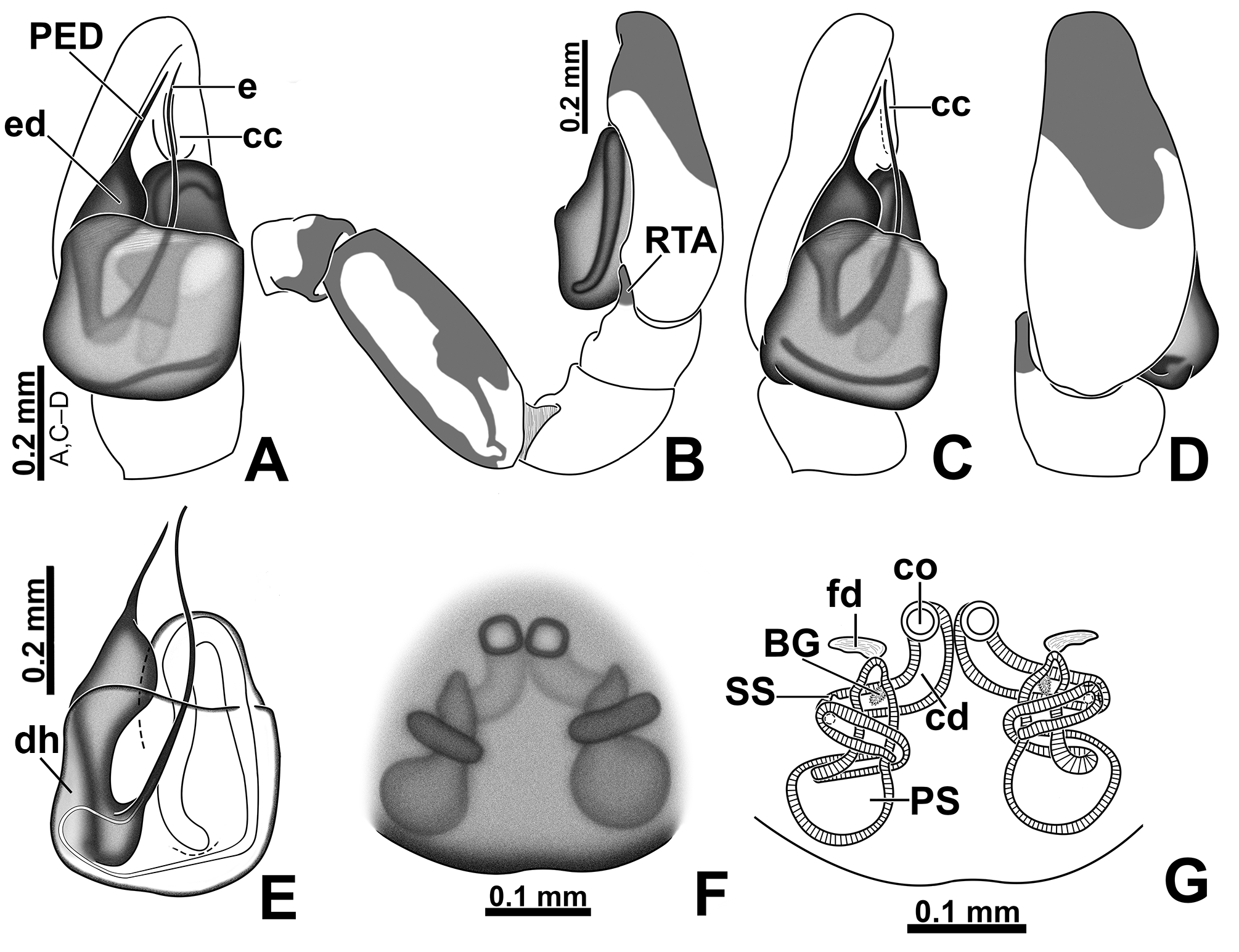
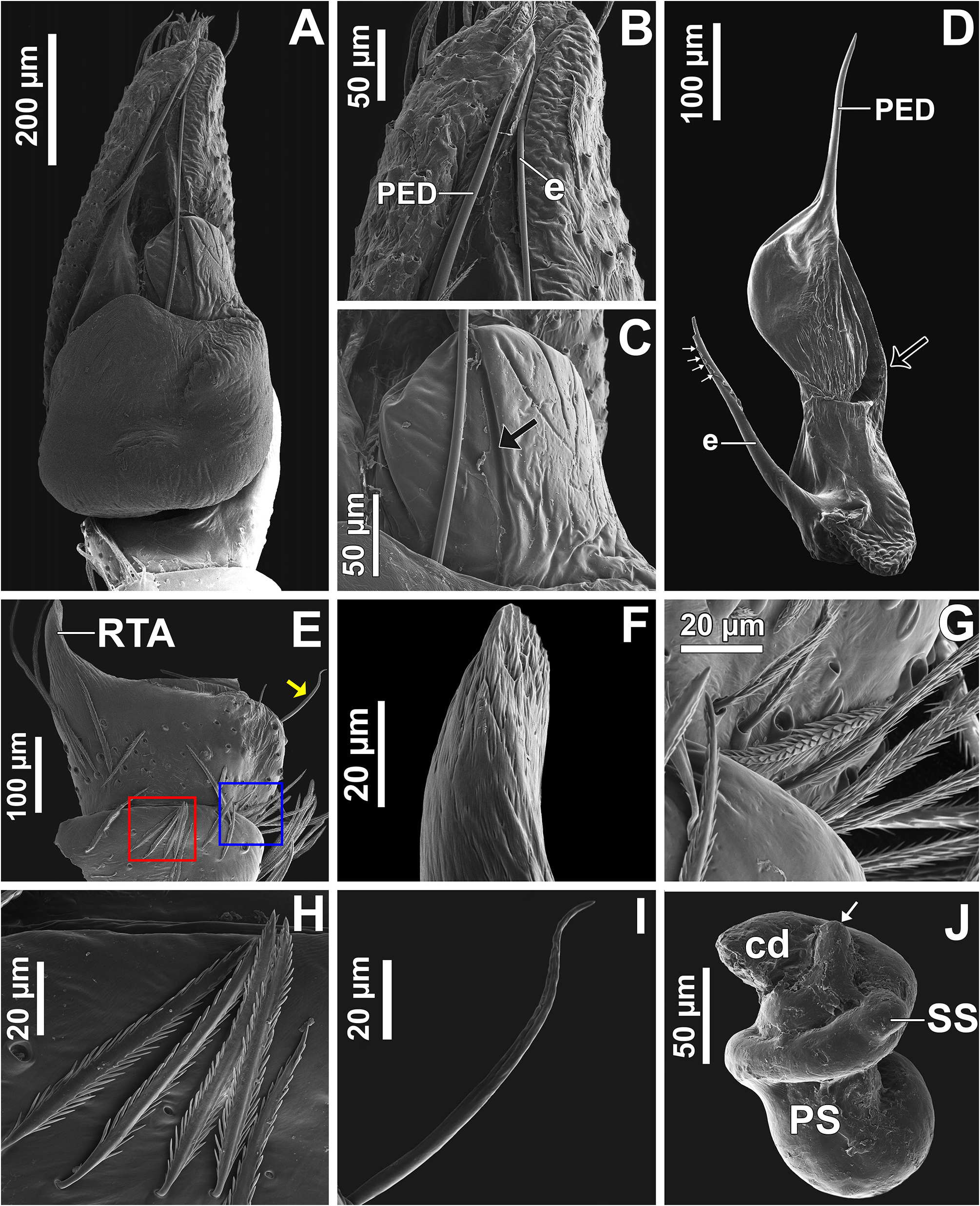
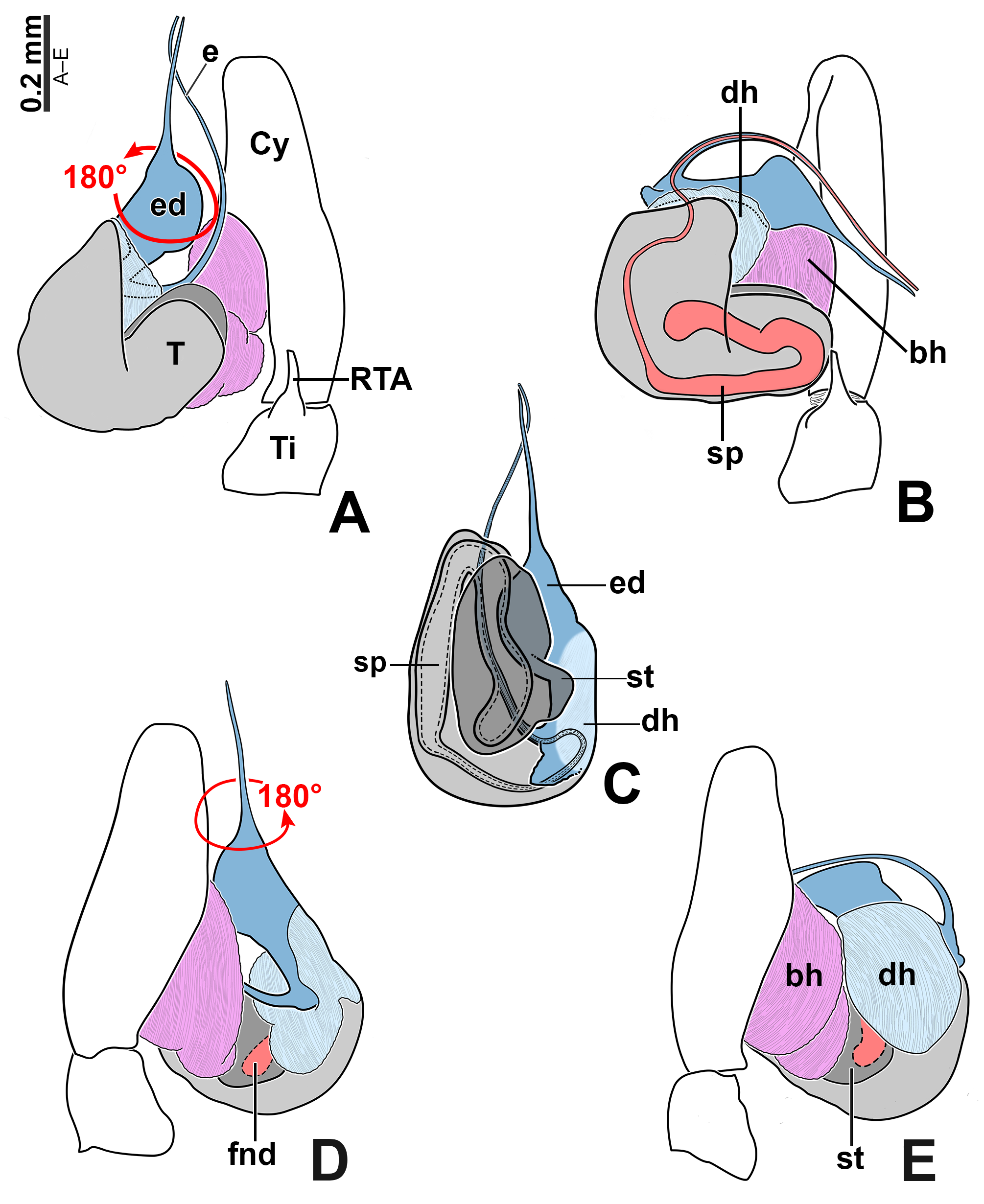
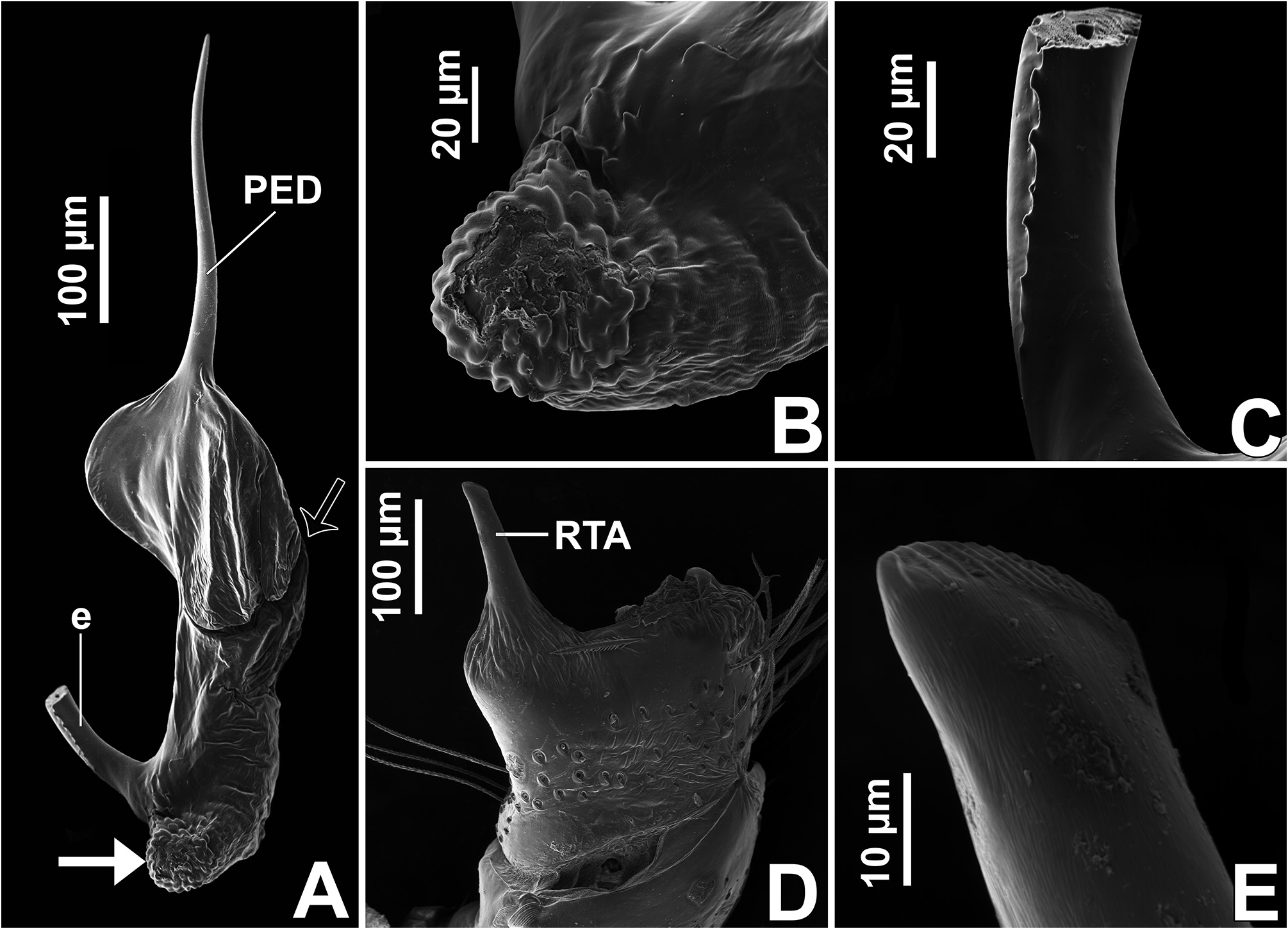
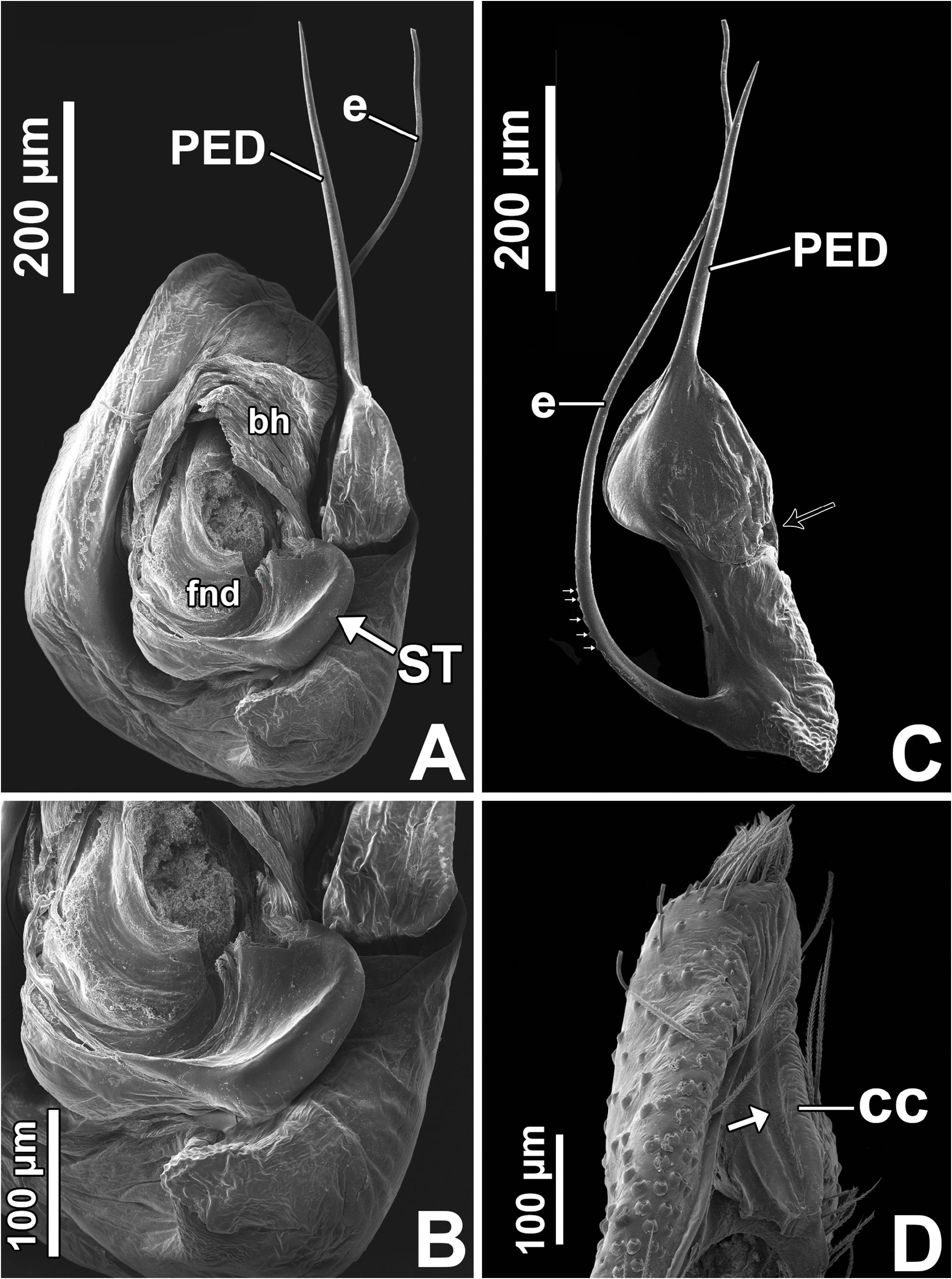
Male palp: in general, brown with some dark spots ( Figs 30 View FIGURE 30 C–D); short trochanter ( Figs 30D View FIGURE 30 , 37B View FIGURE 37 ); femur without modifications ( Figs 32B View FIGURE 32 , 37B View FIGURE 37 ); short patella without modifications ( Fig. 7C View FIGURE 7 ) and generally with white scales concentrated dorsally (orange scales in M. wesolowskae sp. nov.; Figs 30D View FIGURE 30 , 33E View FIGURE 33 , G–H); tibia with the same length as the patella and with a single apophysis placed retrolaterally (RTA) ( Figs 5D View FIGURE 5 , 7C View FIGURE 7 , 13D View FIGURE 13 , 15B View FIGURE 15 , 20D View FIGURE 20 , 22B View FIGURE 22 , 25D View FIGURE 25 , 27B View FIGURE 27 , 30D View FIGURE 30 , 32B View FIGURE 32 ); dorsally with a smooth single dark scale (without shallow, oblique grooves; Figs 33E, I View FIGURE 33 ); the retrolateral tibial apophysis (RTA) is generally finger-shaped with dark tip ( Figs 36D View FIGURE 36 , 44D View FIGURE 44 , 48D View FIGURE 48 , 53D View FIGURE 53 ); tip of RTA with parallel ridges ( Figs 23 View FIGURE 23 D–E, 28D, 33F, 52E); cymbium with tip narrower than the proximal region ( Figs 7E View FIGURE 7 , 15D View FIGURE 15 , 22D View FIGURE 22 , 27D View FIGURE 27 , 32D View FIGURE 32 , 37D View FIGURE 37 , 50D View FIGURE 50 , 55D View FIGURE 55 ); sometimes with apical dark spot ( Figs 30D View FIGURE 30 , 37B View FIGURE 37 ); with a small proximal retroventral projection (possibly part of paracymbium; indicated by arrows in Figs 9F View FIGURE 9 , 16D View FIGURE 16 , 28B View FIGURE 28 , 40B View FIGURE 40 ); with sclerotized cymbial conductor (cc, a modification of cymbial groove) placed on retrolateral ventrodistal portion ( Figs 5C View FIGURE 5 , 9E View FIGURE 9 , 13C View FIGURE 13 , 20C View FIGURE 20 , 25C View FIGURE 25 , 30C View FIGURE 30 , 40A View FIGURE 40 ); cymbial conductor with a groove on which the embolus tip rests ( Figs 9E View FIGURE 9 , 16C View FIGURE 16 , 28B View FIGURE 28 , 33B View FIGURE 33 , 40A View FIGURE 40 ; white arrow in Fig. 56D View FIGURE 56 ); subtegulum with a prolateral lobe ( Figs 23B View FIGURE 23 , 56 View FIGURE 56 A–B); tegulum without proximal lobe and with large retrolateral “shoulder” ( Figs 9A View FIGURE 9 , 23A View FIGURE 23 , 33A, C View FIGURE 33 ); tegular shoulder with a groove on which the embolus shaft rests (black arrow in Fig. 33C View FIGURE 33 ); typical euophryine retrolateral sperm duct loop is absent ( Figs 7F View FIGURE 7 , 15E View FIGURE 15 , 22E View FIGURE 22 , 27E View FIGURE 27 , 32E View FIGURE 32 , 37G View FIGURE 37 , 50E View FIGURE 50 , 55E View FIGURE 55 ); embolic disc with a pointed process (PED) projected to apical portion of the palp ( Figs 5C View FIGURE 5 , 9 View FIGURE 9 A–B, 33D, 40C, 52A); embolic disc with a dorsal groove (indicated by black arrow in Figs 9C View FIGURE 9 , 16A View FIGURE 16 , 23C View FIGURE 23 , 28A View FIGURE 28 , 33D View FIGURE 33 , 40C View FIGURE 40 , 52A View FIGURE 52 , 56C View FIGURE 56 ); base of embolus shaft with a projection of granulated appearance ( Figs 16A View FIGURE 16 , 28A View FIGURE 28 , 33D View FIGURE 33 , 40C View FIGURE 40 , 52 View FIGURE 52 A–B, 56C); part of embolus shaft hidden under the tegulum ( Figs 7A, F View FIGURE 7 , 9A, C View FIGURE 9 ); embolus shaft with small spikes, sometimes forming a spiral row ( Figs 9D View FIGURE 9 , 16B View FIGURE 16 , 23C View FIGURE 23 , 28C View FIGURE 28 , 33D View FIGURE 33 , 40D View FIGURE 40 , 52C View FIGURE 52 , 56C View FIGURE 56 ). Female with unmodified palp ( Fig. 3D View FIGURE 3 ).
Expansion of palp and copulation ( Figs 38–39 View FIGURE 38 View FIGURE 39 ): the male grabs the female’s abdomen with his leg I before the expansion of the palp ( Figs 39 View FIGURE 39 A–D); the bulb rotates to the retrolateral side when the basal hematodocha is filled with hemolymph ( Figs 38 View FIGURE 38 A–B). Later, the distal hematodocha is filled and the embolic disc rotates to the prolateral side ( Figs 38 View FIGURE 38 A–B, D–E). The subtegulum and the tegulum remain coupled during the expansion ( Figs 38 View FIGURE 38 D–E).
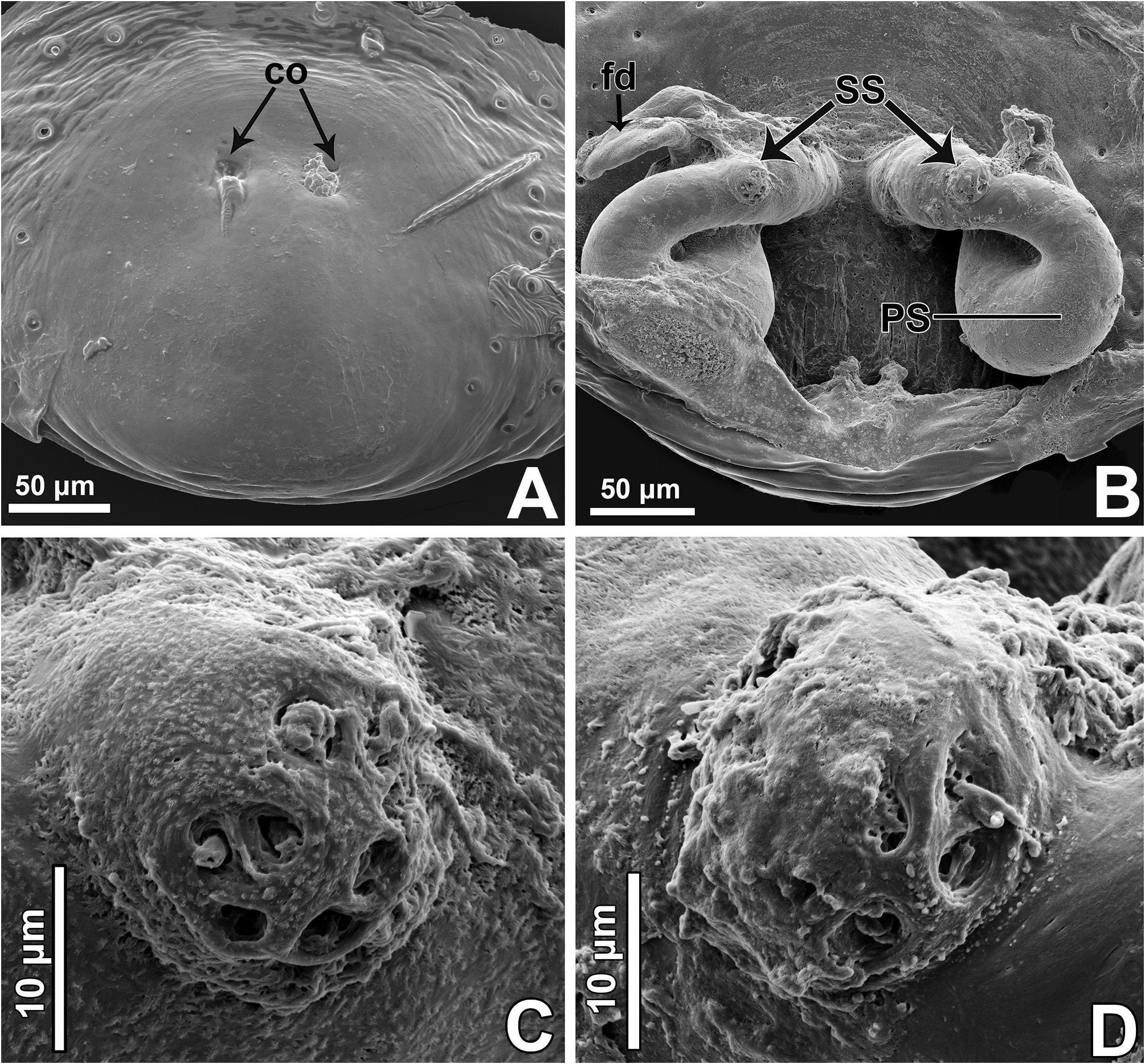
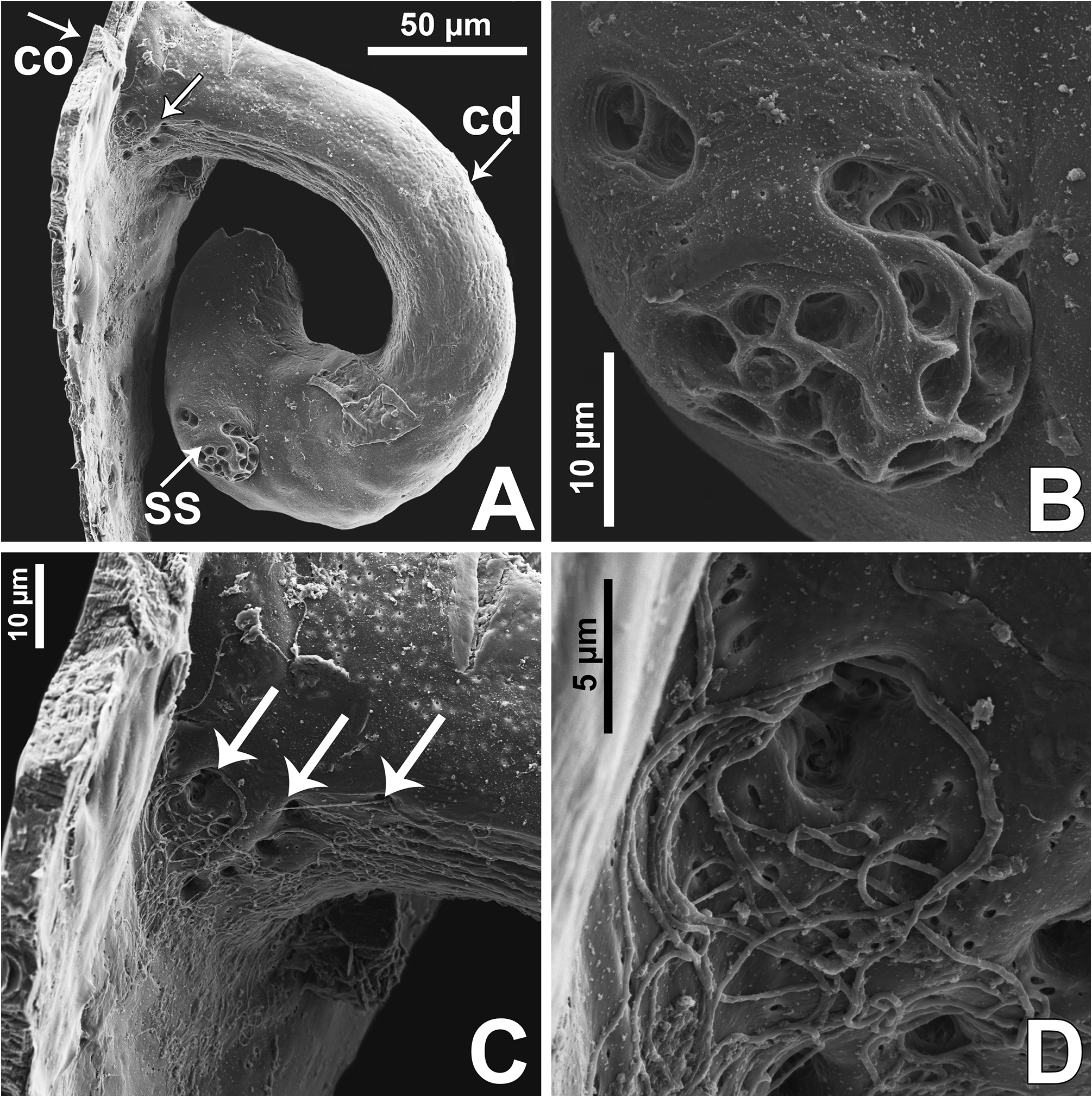
Epigyne: epigynal plate generally with two distinct copulatory openings ( Figs 6C View FIGURE 6 , 7 View FIGURE 7 G–H, 10A, 17A, 41A) ( M. sinuosa sp. nov. has the copulatory openings fused in a single opening; Figs 21E View FIGURE 21 , 22 View FIGURE 22 F–G, 24A, C); copulatory ducts with glands on their initial portion (near to copulatory openings), independent from those of secondary spermathecae ( Figs 10D View FIGURE 10 , 43 View FIGURE 43 C–D); copulatory ducts length ranges from short (e.g. M. linae sp. nov.; Figs 14 View FIGURE 14 C–D) to very long (e.g. M. argentina ; Figs 45C, F View FIGURE 45 ); internal tegument of copulatory ducts is smooth ( Fig. 42D View FIGURE 42 ); the secondary spermathecae are reduced and generally placed far from the copulatory openings ( Figs 10B View FIGURE 10 , 17 View FIGURE 17 B–D, 19A–B, 24B, F, 28E–F, 33J, 41B, 43A–B); primary spermathecae with homogeneous diameter in some species ( Figs 14 View FIGURE 14 C–D, 31C–D); in other cases, with their initial portion dilated, tapering towards the fertilization ducts ( Figs 21 View FIGURE 21 E–F, 24D, 26C–D); internally, spermathecae have spikes on the tegument (ets; Figs 41 View FIGURE 41 C–D, 42A–B); Bennett’s glands (BG) externally appear as a circular hole ( Figs 10C View FIGURE 10 , 19 View FIGURE 19 C–D, 24E); internally they are projected into the middle of the spermathecae and have a spiky appearance (spikes probably bear openings of individual glands; Figs 41C View FIGURE 41 , 42 View FIGURE 42 A–C); fertilization ducts are laterally projected ( Figs 10C View FIGURE 10 , 14C View FIGURE 14 , 15G View FIGURE 15 ).
Remarks about crypsis: Members of Marma are specialized in hunting on the ground, rock surfaces, urban constructions and tree trunks. They are not found inhabiting intertwined tree branches or leaves. The preference for open environments as those mentioned above is related to the cryptic coloration in shades of brown that provide them efficient camouflage with the substrate ( Figs 1 View FIGURE 1 , 2 View FIGURE 2 , 3 View FIGURE 3 , 12 View FIGURE 12 , 29 View FIGURE 29 , 34 View FIGURE 34 , 39 View FIGURE 39 , 47 View FIGURE 47 ). Also, no bright colors are observed in males ( Figs 2 View FIGURE 2 A–B, 3A–B, 12A–C, 29A–C, 34A–C, 47A–C).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Salticinae |
|
Tribe |
Euophryini |
Marma Simon, 1902
| Salgado, Alexandre & Ruiz, Gustavo R. S. 2020 |
Thysema
| Galiano, M. E. 1962: 36 |
Paralophostica
| Galiano, M. E. 1962: 36 |
| Soares, B. A. M. & Camargo, H. F. de 1948: 396 |
Marma
| Simon, E. 1902: 376 |