Timarcha Samouelle, 1819
|
publication ID |
https://doi.org/10.5852/ejt.2020.630 |
|
publication LSID |
lsid:zoobank.org:pub:B7825899-9925-4282-80AE-B4F7C8BDF349 |
|
DOI |
https://doi.org/10.5281/zenodo.3804634 |
|
persistent identifier |
https://treatment.plazi.org/id/01513B36-7601-FFAD-9840-FA4F66928097 |
|
treatment provided by |
Valdenar |
|
scientific name |
Timarcha Samouelle, 1819 |
| status |
|
Genus Timarcha Samouelle, 1819 View in CoL
The female tarsal vestiture
Complete vestiture in females (0,0,0; 0,0,0; 0,0,0) were found in Americanotimarcha and Metallotimarcha species. Moreover, the species found in Timarcha s.str. with nearly complete vestiture were T. maroccana (0–¼,0,0; 0–¼,0,0; 0–¼,0,0) ( Fig. 1 View Figs 1–11 ) and T. scabripennis (0,0,0; 0–⅓,0,0; 0–⅓,0,0) ( Fig. 2 View Figs 1–11 ), whereas in the close T. prujai ( Fig. 3 View Figs 1–11 ) all the tarsomeres had an incomplete, narrow medial line (½–⅓,⅓–¾,⅓; ¾,¾,⅓–½; ¾,½–¾,⅓–½). Timarcha balearica ( Fig. 4 View Figs 1–11 ) showed a visible rather incomplete medial glabrous line in the pro and mesotarsomeres I and in metatarsomeres I and II (¼,0,0; ½–⅔,0,0; ⅔–1,½– ¾,0).
On the other hand, Timarcha rugosa ( Fig. 11 View Figs 1–11 ) has a fully glabrous medial line in females (1,1,1; 1,1,1; 1,1,1). An almost fully glabrous medial line was found (1,1,¾–1; 1,1,¾–1; 1,1,1) in T. intermedia ( Fig. 9 View Figs 1–11 ), T. carmelenae Petitpierre, 2013 and T. lugens Rosenhauer, 1856 . In T. lusitanica ( Fig. 7 View Figs 1–11 ) the line formula is (1,0–1,0–½; 1,½–1,½; 1,1,¼–¾). Greater variability was found in T. goettingensis ( Fig. 8 View Figs 1–11 ), with (1,1,⅓–¾; 1,1,⅓–¾; 1,4 /5–1,½–¾), T. pimelioides with (⅓–1,0–1,0–¾; ⅓–1,¼–¾,¼–½; 1,¾–1,½–¾) ( Fig. 10 View Figs 1–11 ), and T. tenebricosa with (0–¾,0,0–¼; ¼–½,0–¼,0–¼; ½–1,¼–¾,¼) ( Fig. 5 View Figs 1–11 ). The last one was similar to that of T. nicaeensis (½–⅔,0–¼,0; ½–¾,0–¼,0; ⅔–¾,0–½,0) ( Fig. 6 View Figs 1–11 ). Notably the morphology of tarsomeres III in T. rugosa is strongly emarginated ( Fig. 11 View Figs 1–11 ). Thus the morphology of tarsomeres may be useful to separate closely related species. For example, in T. scabripennis the tarsomeres I and II are not very dilated to the apex ( Fig. 2 View Figs 1–11 ), in T. prujai they are slightly dilated ( Fig. 3 View Figs 1–11 ) and in T. maroccana they are strongly widened ( Fig. 1 View Figs 1–11 ).
The sclerites of endophallus
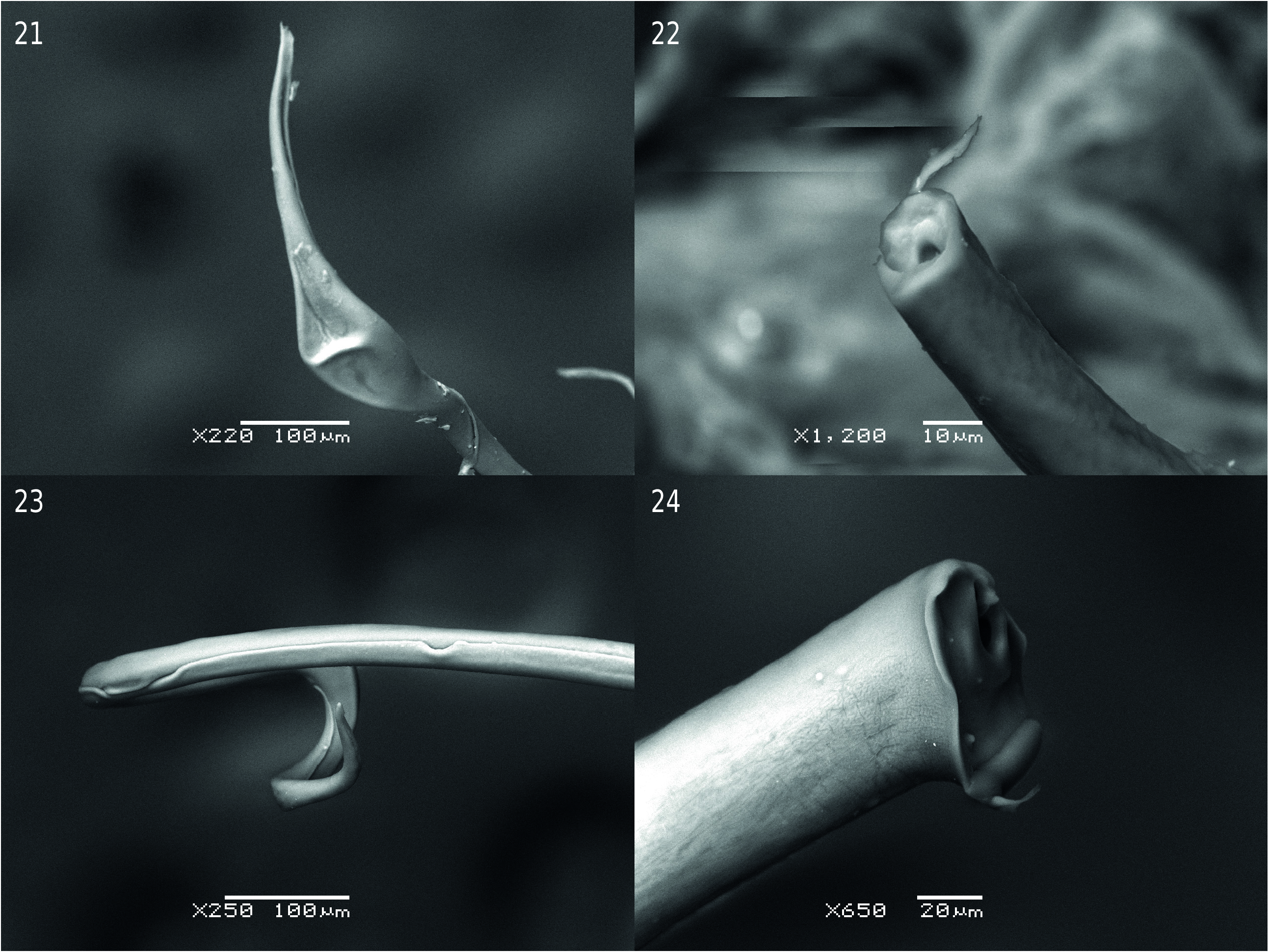
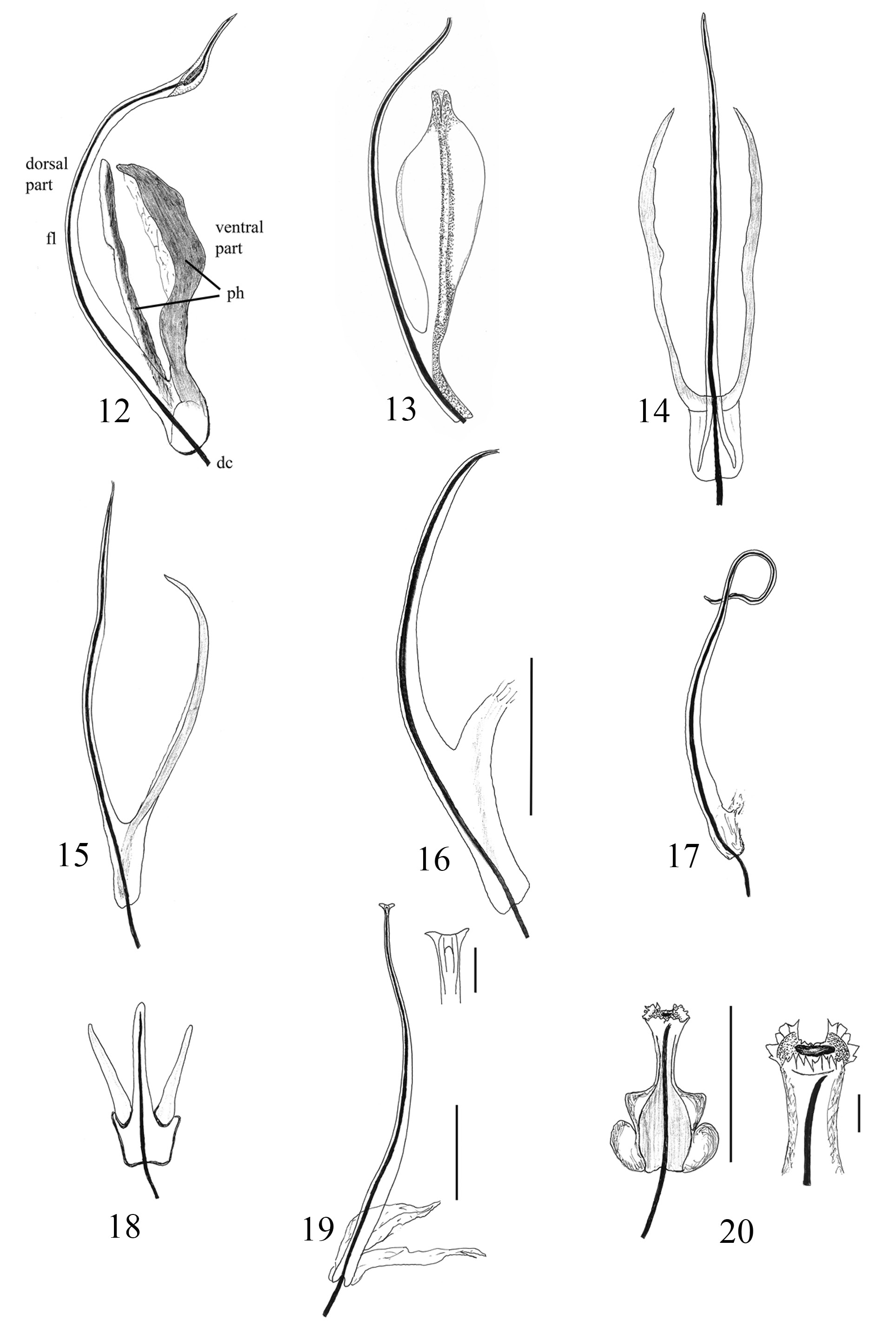
The sclerites have different patterns in the three subgenera. The species of the subgenus Timarcha have sclerites of the endophallus which generally consist of a long, fine tube called the flagellum (“tige” for Iablokoff-Khnzorian 1966), that includes the ductus. The flagellum is a long, narrow plate curved in on itself which forms a tube that directs the sperm (see Fig. 23 View Figs 21–24 ). The base of the flagellum is joined to a sclerotized piece named the phanera (Stockman 1966; manubrium for Iablokoff-Khnzorian 1966; Petitpierre 1970) which is attached to the dorso-apical part of the endophallus (see Petitpierre & Anichtchenko 2018, and Figs 25–29 View Figs 25–29 ). The phanera is usually a paired structure, with two pieces in the shape of wings. In some species, the phanera is unpaired and reduced with a simple stick shape.
The presence of a phanera with two well-developed wings occurs in Timarcha tenebricosa , where the long flagellum is interestingly curved upwards and enlarged near the apex ( Figs 12 View Figs 12–20 , 21 View Figs 21–24 ). Timarcha goettingensis has a shorter flagellum and two less sclerotized wings ( Fig. 13 View Figs 12–20 ) which, if not carefully prepared under the microscope, can turn on themselves, giving a somewhat different appearance; the apex of flagellum has a small, thin digit-like appendix next to the apical pore ( Fig. 22 View Figs 21–24 ). In T. rugosa ( Fig. 14 View Figs 12–20 ) there is a central, straight flagellum, with two rather lineal and rigid wings well separated from the base, which are observed easier dorsally than laterally ( Fig. 14 View Figs 12–20 ).
An unpaired but well developed phanera is found in Timarcha hispanica ( Fig. 15 View Figs 12–20 ), whereas the phanera is quite short in T. intermedia ( Fig. 16 View Figs 12–20 ) and T. balearica ; the latter however is very notable due to its coiling flagellum which is formed by the fusion of two symmetrical halves ( Figs 17 View Figs 12–20 , 23 View Figs 21–24 ).
The endophallus sclerite of Timarcha maroccana ( Fig. 18 View Figs 12–20 ) is quite striking with a short structure, a paired phanera, which is lineal but well sclerotized, not winged, and a flagellum only slightly longer than the phanera.
On the other hand, in Timarcha (Americanotimarcha) intricata the flagellum is very elongated, hardly curved or almost straight, bifurcated at the base, decreasing in diameter and widening in two small divergent points at the apex. At the base there is a small, paired, very weakly sclerotized phanera ( Fig. 19 View Figs 12–20 ); close up it can be seen that the apex of flagellum has a digit-like appendix ( Fig. 24 View Figs 21–24 )
In Timarcha (Metallotimarcha) metallica , the flagellum has a bifurcated base, is well chitinized, rigid, parallel sided, moderately elongated, ending in a slightly widened apex provided with a crown of denticles and an attached membrane. The phanera is paired, large, and incised at the base ( Fig. 20 View Figs 12–20 ). However, the sclerites of the endophallus are very different and characteristic in the distinct species of subgenus Metallotimarcha making it difficult to find a general pattern; although the base of flagellum always appears bifurcated, as occurs in Americanotimarcha .
The endophallus
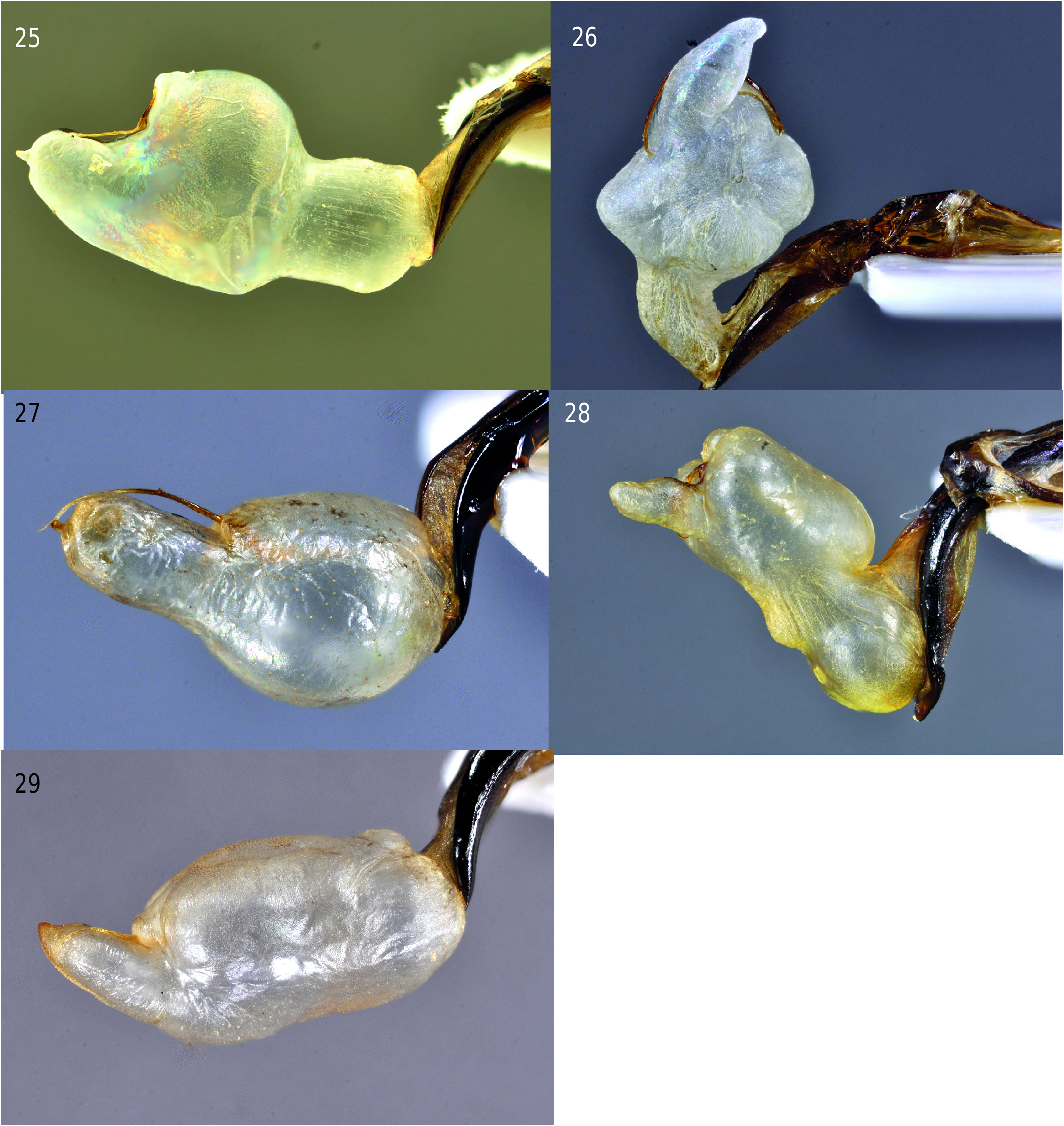
The endophallus (internal sac of penis) in Timarcha is simple, membranous, with bilateral symmetry, and is moderately variable between the species. Basically, it consists of three main lobes, in dorsal and/ or ventral position: basal, medial and distal. The basal and medial lobes may have paired protuberances; while in the majority of the species evaluated, the distal one is unpaired. Lastly, the distal lobe can end in a small apical diverticulum. Besides this, the endophallus supports the sclerites described above; the phanera is attached to the distal part of the dorsal protuberance of medial lobe. The endophallus is a fixation structure used to copulate, whereas the phanera serves to attach the flagellum which forms the sperm channel.
The endophallus is distinctive in different species. In Timarcha goettingensis ( Fig. 25 View Figs 25–29 ), there is a welldefined, long, basal lobe which opens to a widened medial lobe with a swollen dorsal protuberance and a weakly convex ventral protuberance. The distal lobe is developed and ends in a small apical diverticulum. On the other hand, T. intermedia ( Fig. 26 View Figs 25–29 ) has a narrow basal lobe, opening to a medial lobe with unpaired dorsal and paired ventral protuberances dilated with a long, tapering, distal lobe. In some species, like T. granadensis , there is not a basal lobe, but there is a swollen medial lobe and a distal lobe parallel sided, truncated at the apex, yet ending in a small distal diverticulum; interestingly, the medial lobe carries a number of tiny spines ( Fig. 27 View Figs 25–29 ; fig. 32 in Petitpierre & Anichtchenko 2018). Timarcha marginicollis ( Fig. 28 View Figs 25–29 ) has a rounded basal lobe, and a long, parallel-sided medial lobe, which has a developed dorsal proximal, two paired dorsal distal and ventral protuberances, and finally a fingershaped distal lobe; no apical diverticulum was observed. In T. strangulata ( Fig. 29 View Figs 25–29 ), the basal lobe is not differentiated from the medial lobe, which has long dorsal and slight ventral protuberances; the distal lobe is thumb-shaped and slightly turned dorsally. A well-illustrated study of the interspecific variations is found in Petitpierre & Anichtchenko (2018).
Theoretically, the ideal eversion technique should be simple, rapid, repeatable (even on the same sample, in case of initial failure), and applicable to any specimen (regardless of size); thus providing a permanent and easily studied sample. After trying various techniques, we have come to the conclusion that air filling (as suggested by Bontems 2013) is the one that better approximates the ideal technique. Techniques with other filling media, such as solution of gelatine powder, glycerine, water and Zinc Sulfate, which cause a white coloration to highlight the structures ( Meurgues & Ledoux 1966), toothpaste ( Janovska et al. 2013), K-Y gel ( Van Damm 2014), absolute ethanol ( Dang 1993; Hünefeld et al. 2013) or petroleum jelly ( Yamasako & Ohbayashi 2011) always have some application limit. The toothpaste (or even the light-cured dental composite) and the gels are limited by viscosity, which prevents their use with very thin needles (Gauge <30) and therefore with very small specimens; the absolute ethanol requires conservation of the piece in liquid, reducing the simplicity of study, and limiting the options for preservation together with the specimen. Furthermore, the problem of long-term preservation arises with fillers such as toothpaste or K-Y gel. Janovska et al. (2013) explained that some brands of toothpaste were well preserved after ten years, while in other cases after a few months the preparations were damaged by salt crystals; Van Damm (2014) does not provide information on the duration of preparations filled with K-Y gel.
In our opinion, air offers the following advantages:
1. Obviously, air flows through any needle, including the 36G needle (a needle with an external gauge of 0.127 mm and internal gauge of < 0.08 mm), allowing, in theory, the eversion of even very small specimens like Cryptocephalus Geoffroy, 1762 , Pachybrachis Chevrolat, 1837 and Alticini, for example.
2. The everted and dried piece can always be re-prepared by immersing it for a few minutes in hot water to rehydrate the tissues; this is especially true in cases where one realizes that complete eversion has not been achieved.
3. The endophallus’ membranes are almost always transparent or semitransparent, allowing an easy study of the chitinized parts.
4. We know of Timarcha endophallus preparations, using only air, made by André Vachon and Nicole Berti more than 40 years ago and still in perfect condition (specimens studied by M. Daccordi kept in coll. Jean-Claude Bourdonné).
5. The everted and dried piece, once glued on a glue-board and pinned under the specimen, is unlikely to suffer damage, except by clumsy manipulation, which is always desirable to avoid.
In reality, the only limits to the air eversion technique are the manual skills of the operator and the availability of sufficiently thin equipment. Currently 34G needles are available without great difficulty, while 36G or 37G needles (the thinnest needles produced with current technologies) are difficult to find and very expensive.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Polyphaga |
|
SuperFamily |
Chrysomeloidea |
|
Family |
|
|
SubFamily |
Chrysomelinae |
|
Tribe |
Timarchini |